1 CHAPTER 9 CHEMICAL NAMES AND FORMULAS Sections






















































- Slides: 130

1 CHAPTER 9: CHEMICAL NAMES AND FORMULAS Sections 1 -5 (Read pages 252 -285)

Chapter 9 Vocab Acid Base Binary compound Law of definite proportions Law of multiple proportions Monatomic ion Polyatomic ion 2

Section 9. 1: Naming Ions Read pages 252 -259

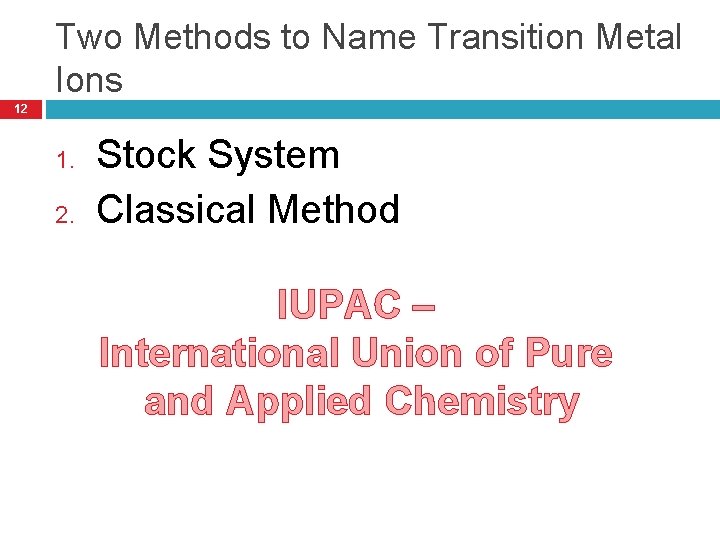
Monatomic Ions 4 Ions that consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons Two Types � Positive Ions Cations � Negative Anions Ions

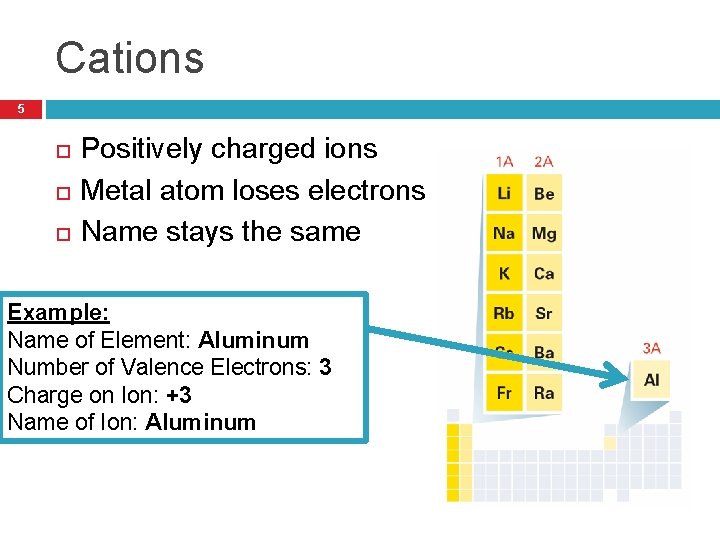
Cations 5 Positively charged ions Metal atom loses electrons Name stays the same Example: Name of Element: Aluminum Number of Valence Electrons: 3 Charge on Ion: +3 Name of Ion: Aluminum

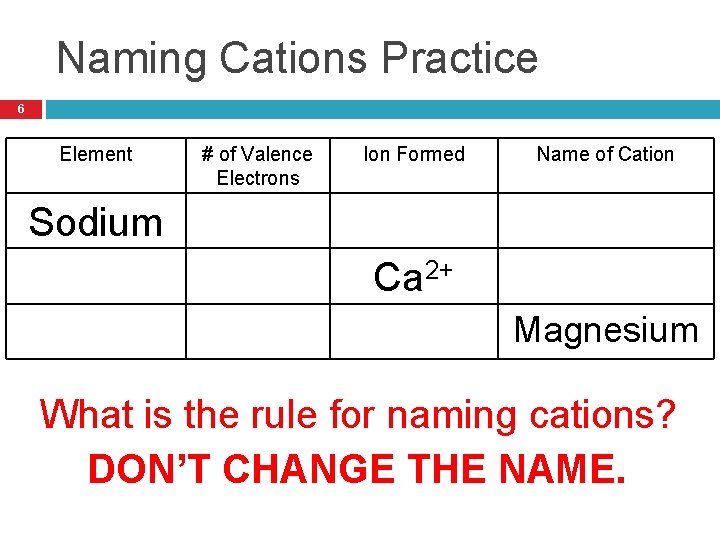
Naming Cations Practice 6 Element # of Valence Electrons Ion Formed Name of Cation Sodium Calcium 1 2 2 Na+ Ca 2+ Mg 2+ Sodium Calcium Magnesium What is the rule for naming cations? DON’T CHANGE THE NAME.

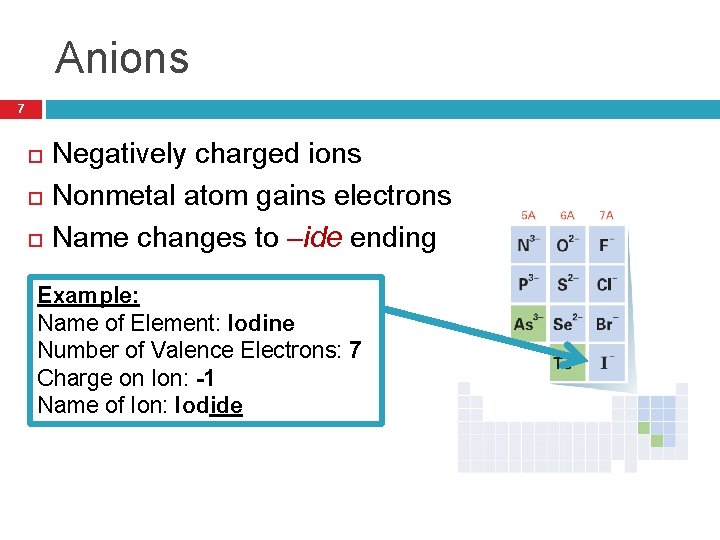
Anions 7 Negatively charged ions Nonmetal atom gains electrons Name changes to –ide ending Example: Name of Element: Iodine Number of Valence Electrons: 7 Charge on Ion: -1 Name of Ion: Iodide

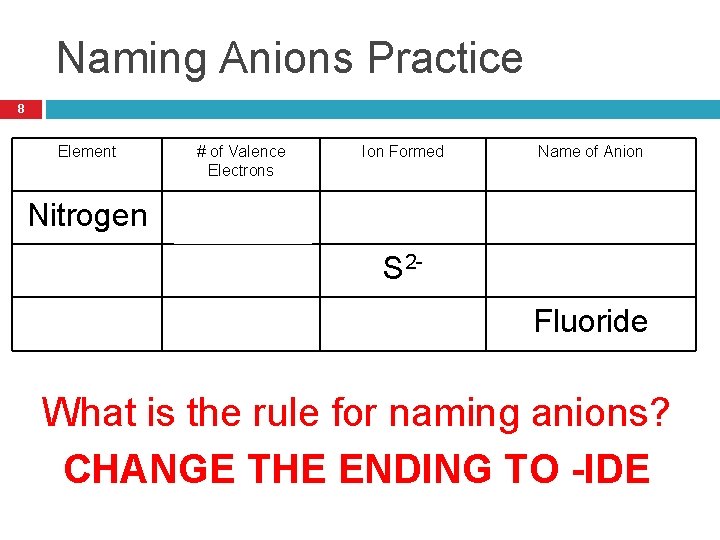
Naming Anions Practice 8 Element # of Valence Electrons Ion Formed Name of Anion Nitrogen 5 N 3 - Nitride Sulfur 6 S 2 - Sulfide Fluorine 7 F- Fluoride What is the rule for naming anions? CHANGE THE ENDING TO -IDE

Note: 9 Groups 14 (4 A) and 18 (8 A) usually do not form ions

10

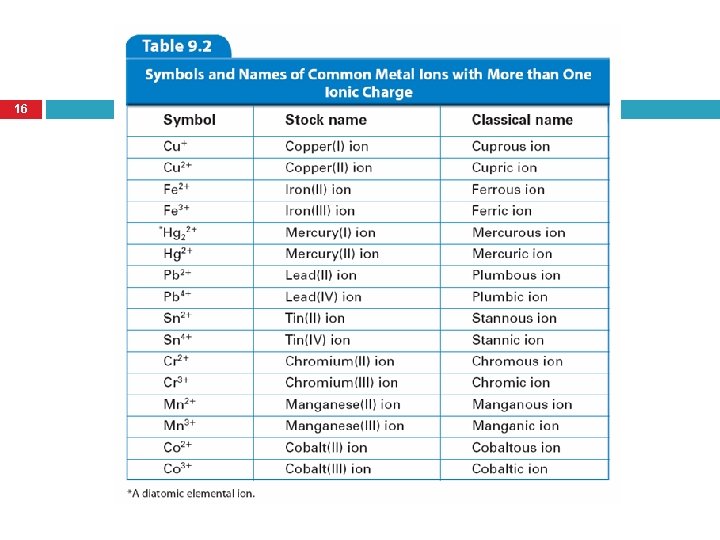
Ions of Transition Metals 11 Many of the transition metals form more than one cation with different ionic charges � Not all transition metals have more than one charge Charges must be determined from the number of electrons lost These colorful solutions contain the transition metal ions Co 3+, Cr 3+, Fe 3+, Ni 2+, and Mn 2+.

Two Methods to Name Transition Metal Ions 12 1. 2. Stock System Classical Method IUPAC – International Union of Pure and Applied Chemistry

Stock System 13 Preferred method of naming A Roman numeral in parentheses is placed after the name of the element to indicate the numerical value of the charge Number Roman Numeral 1 I 2 II 3 III 4 IV 5 V 6 VI

Stock System Naming Practice 14 Ion Formed # Electrons Lost Stock Name Cu+ 1 Copper (I) ion Cu 2+ 2 Copper (II) ion Pb 2+ 2 Lead (II) ion Pb 4+ 4 Lead (IV) ion

Classical Method 15 In the past, scientists used the Latin names of elements. Example: Ferrum is Latin for Iron. Ferr- is the root of this word. Iron forms two ions: Fe 2+ and Fe 3+ Ion names: Fe 2+ is the ferrous ion Fe 3+ is the ferric ion

16

Practice Problems 17 a) Selenide (anion) b) Barium (cation) c) Phosphide (anion)

Polyatomic Ions 18 What does the prefix poly mean? � MANY Polyatomic ions are composed of more than one atom Tightly bound groups of atoms that behave as a unit and carry a charge The names of most polyatomic ions end in –ite or –ate � Different Endings Ammonium NH 4+ Cyanide CNHydroxide OH-

19

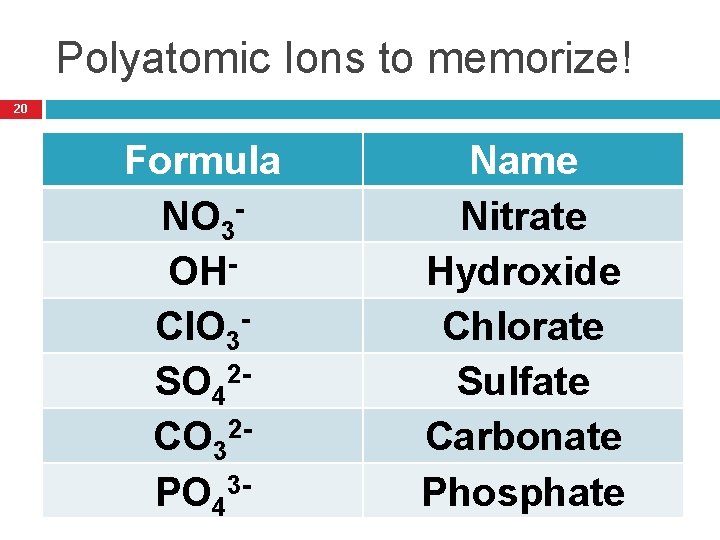
Polyatomic Ions to memorize! 20 Formula NO 3 OHCl. O 3 SO 42 CO 32 PO 43 - Name Nitrate Hydroxide Chlorate Sulfate Carbonate Phosphate

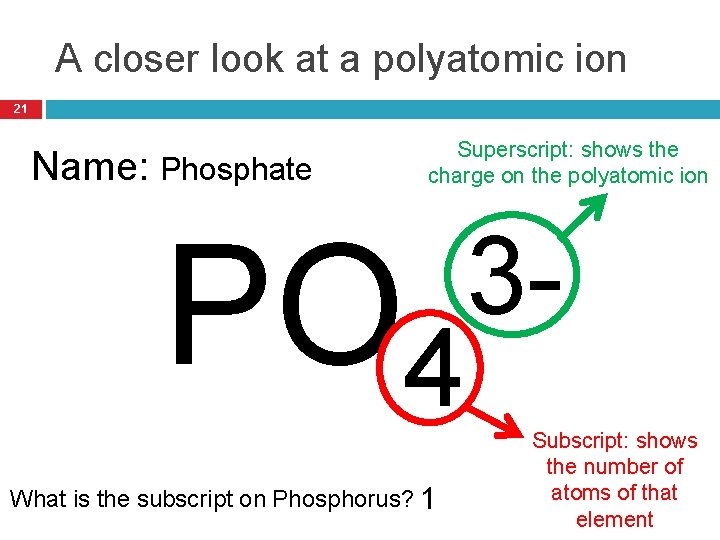
A closer look at a polyatomic ion 21 Name: Phosphate Superscript: shows the charge on the polyatomic ion PO 4 What is the subscript on Phosphorus? 1 3 Subscript: shows the number of atoms of that element

An important note: 22 Pay close attention to the names! Example: = S 2� Sulfate = SO 42� Sulfite = SO 32� Sulfide

Quick Quiz Section 9. 1

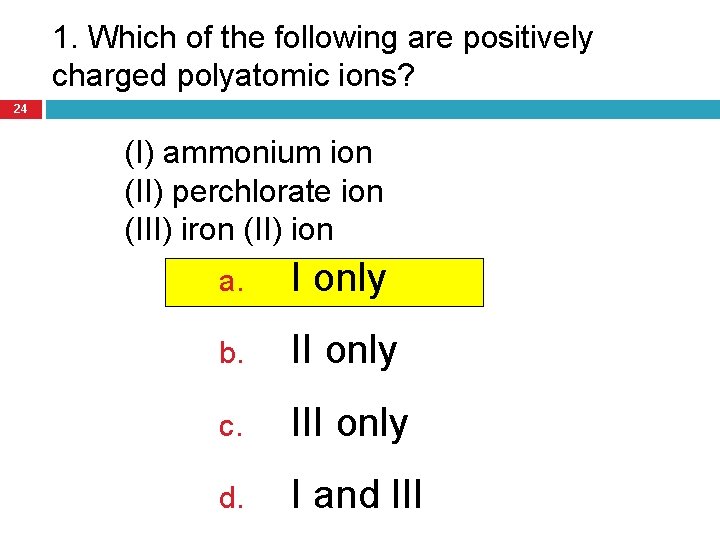
1. Which of the following are positively charged polyatomic ions? 24 (I) ammonium ion (II) perchlorate ion (III) iron (II) ion a. I only b. II only c. III only d. I and III

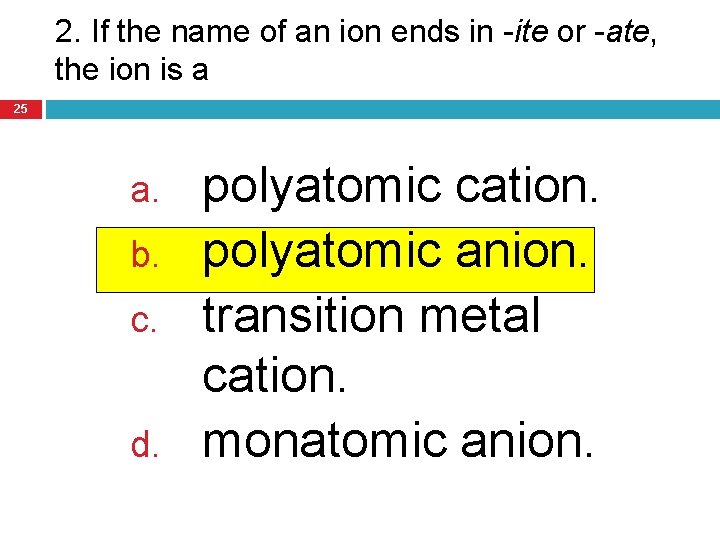
2. If the name of an ion ends in -ite or -ate, the ion is a 25 a. b. c. d. polyatomic cation. polyatomic anion. transition metal cation. monatomic anion.

Section 9. 2: Naming and Writing Formulas for Ionic Compounds Read pages 260 -266

Different Types of Ionic Compounds 27 Simple Ionic Compounds (last chapter!) Ionic Compounds Involving Transition Metals 1. 2. 3. Transition metals with only 1 charge Transition metals with more than 1 possible charge Ionic Compounds Involving Polyatomic Ions

Binary Ionic Compounds 28 What does the prefix bi mean? �TWO A binary ionic compound is composed of two elements - a metal and a nonmetal

Naming Binary Ionic Compounds 29 If you know the formula of the ionic compound, you can write the name To name any binary ionic compound, place the cation name first, followed by the anion name.

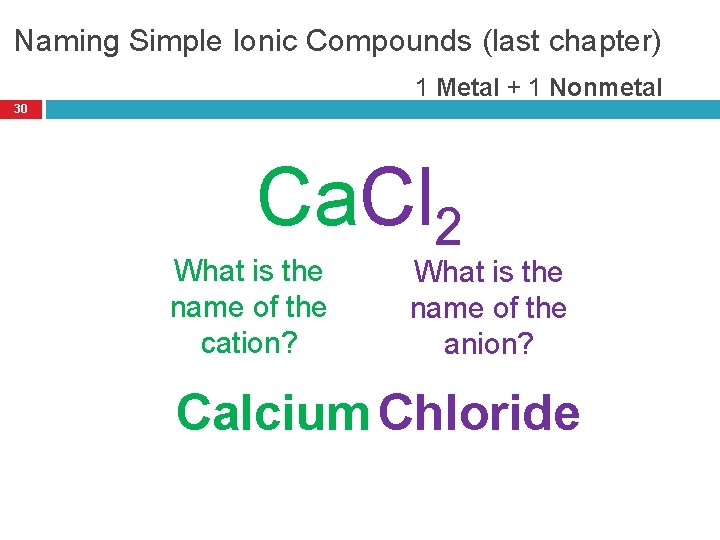
Naming Simple Ionic Compounds (last chapter) 1 Metal + 1 Nonmetal 30 Ca. Cl 2 What is the name of the cation? What is the name of the anion? Calcium Chloride

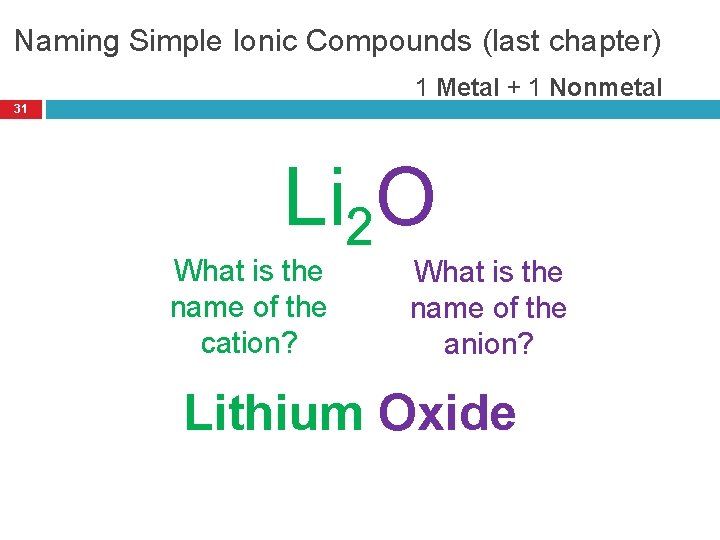
Naming Simple Ionic Compounds (last chapter) 1 Metal + 1 Nonmetal 31 Li 2 O What is the name of the cation? What is the name of the anion? Lithium Oxide

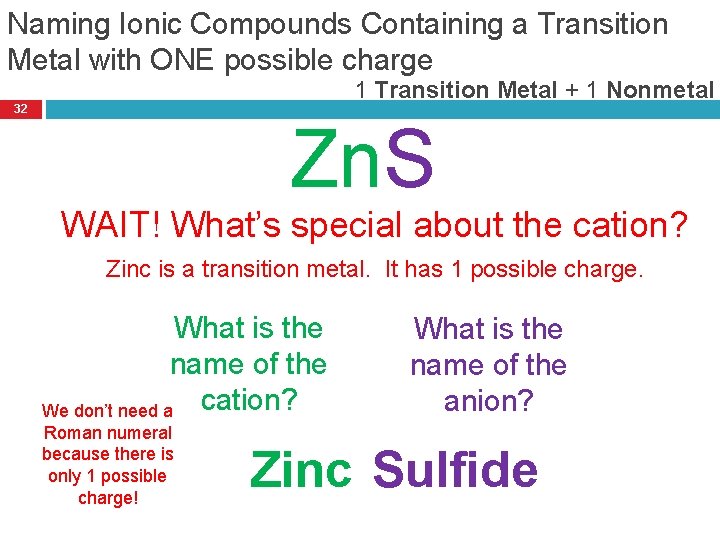
Naming Ionic Compounds Containing a Transition Metal with ONE possible charge 1 Transition Metal + 1 Nonmetal 32 Zn. S WAIT! What’s special about the cation? Zinc is a transition metal. It has 1 possible charge. What is the name of the cation? We don’t need a Roman numeral because there is only 1 possible charge! What is the name of the anion? Zinc Sulfide

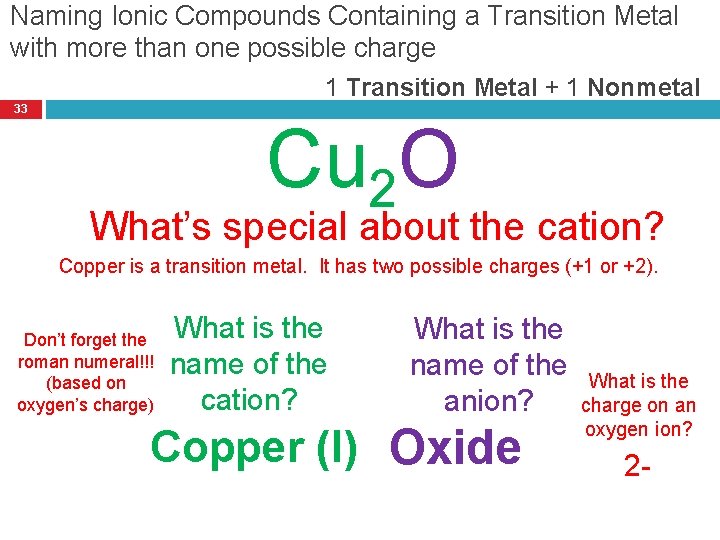
Naming Ionic Compounds Containing a Transition Metal with more than one possible charge 1 Transition Metal + 1 Nonmetal 33 Cu 2 O What’s special about the cation? Copper is a transition metal. It has two possible charges (+1 or +2). Don’t forget the roman numeral!!! (based on oxygen’s charge) What is the name of the cation? What is the name of the anion? Copper (I) Oxide What is the charge on an oxygen ion? 2 -

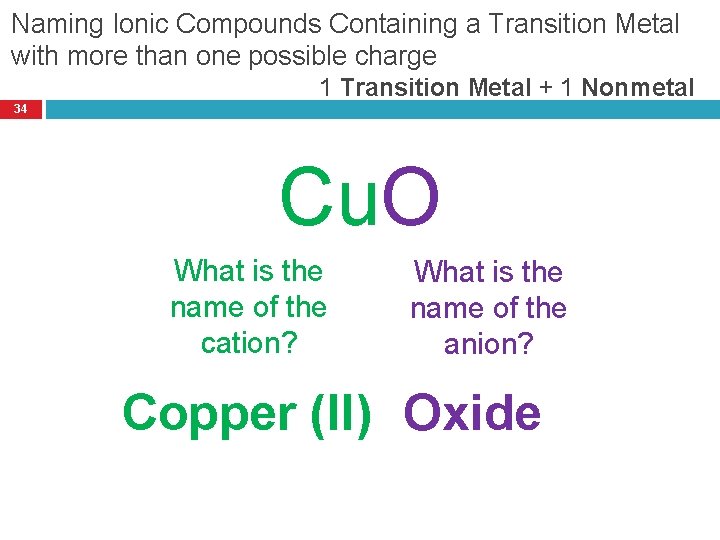
Naming Ionic Compounds Containing a Transition Metal with more than one possible charge 1 Transition Metal + 1 Nonmetal 34 Cu. O What is the name of the cation? What is the name of the anion? Copper (II) Oxide

Try It! 35 Name the following binary ionic compounds: 1. 2. 3. Na. F Fe. Cl 3 Fe. Cl 2 1. 2. 3. Sodium Fluoride Iron (III) Chloride Iron (II) Chloride

Writing Binary Ionic Formulas 36 If you know the name of a binary ionic compound, you can write the formula! Write the symbol of the cation and then the anion. Then, add the subscripts that are needed to balance the charges. The positive charge of the cation must balance the negative charge of the anion so that the net ionic charge of the compound is neutral.

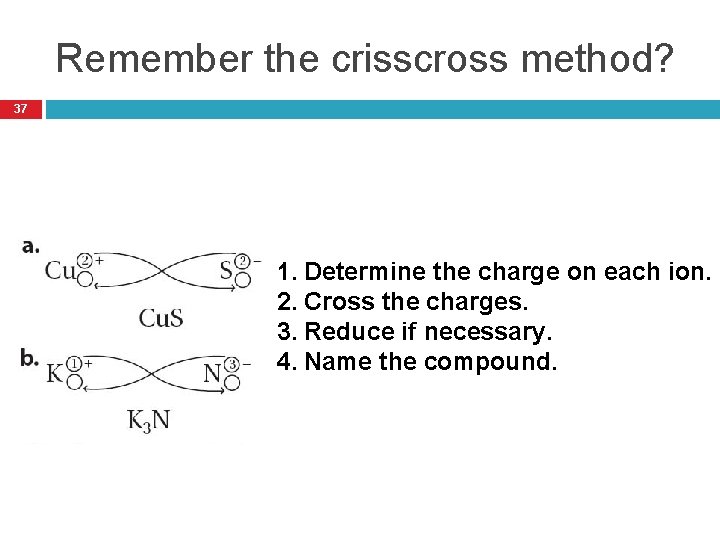
Remember the crisscross method? 37 1. Determine the charge on each ion. 2. Cross the charges. 3. Reduce if necessary. 4. Name the compound.

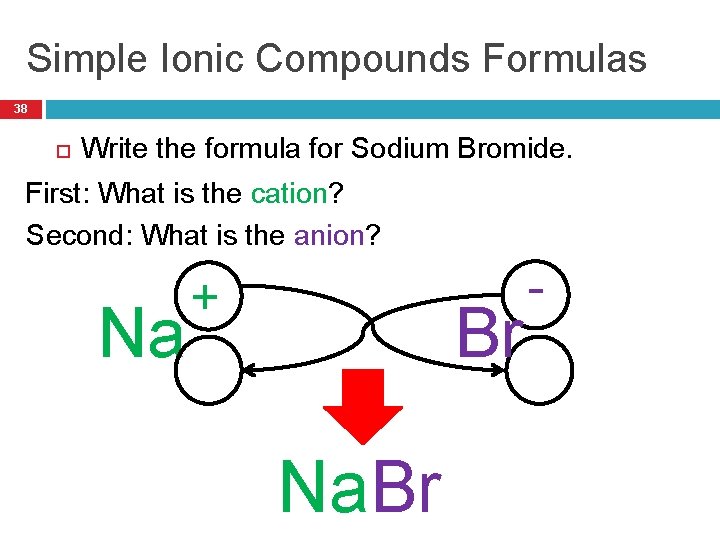
Simple Ionic Compounds Formulas 38 Write the formula for Sodium Bromide. First: What is the cation? Second: What is the anion? Na + Br Na. Br -

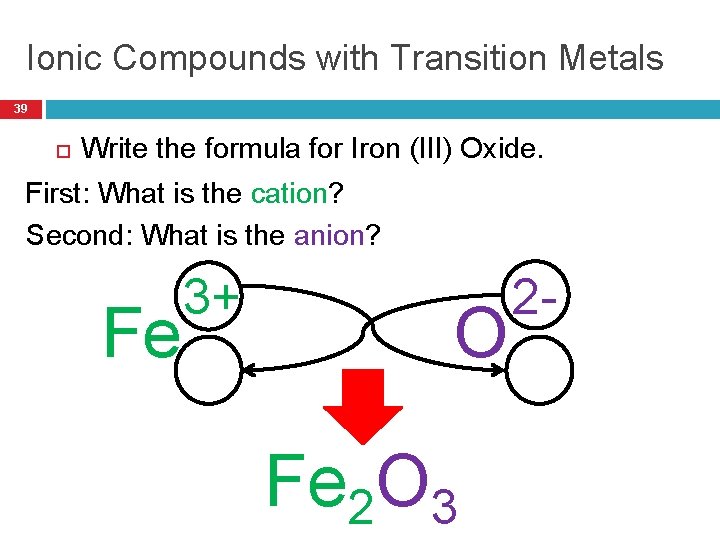
Ionic Compounds with Transition Metals 39 Write the formula for Iron (III) Oxide. First: What is the cation? Second: What is the anion? Fe 3+ O Fe 2 O 3 2 -

40 K 3 N Cu. S Na. I K 2 S Ca. I 2

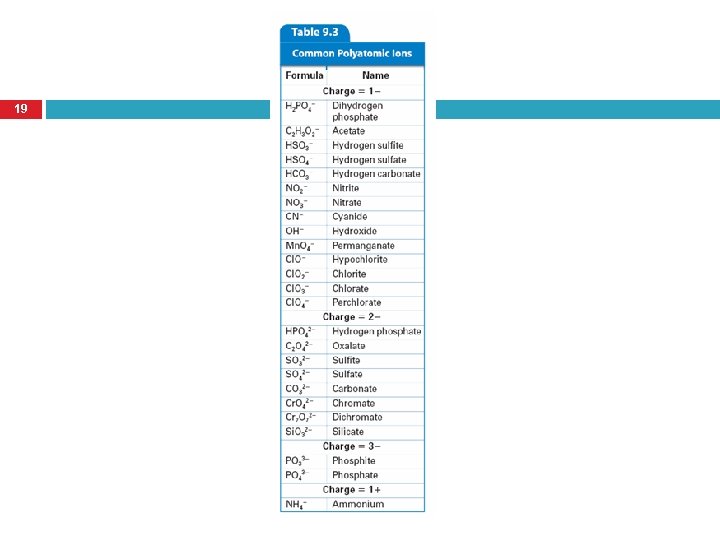
What is a polyatomic ion? 41 Tightly bound groups of atoms that behave as a unit and carry a charge

Polyatomic Ions behave as a UNIT. 42 You CANNOT change the charge or the number of atoms of each element in a polyatomic ion or you will change the identity of that ion!! – Cl. O 2 – Cl. O 3 – Cl. O 4 – Hypochlorite Chlorate Perchlorate

Naming Compounds with Polyatomic Ions 43 To name a compound containing a polyatomic ion, state the cation first, and then the anion. Just like naming binary ionic compounds! � Except you DO NOT change the anion name!

Naming Compounds with Polyatomic Ions Example 44 Ca. SO 4 What’s special about the anion? SO 4 is a polyatomic ion. What is the name of the cation? What is the name of the anion? Calcium Sulfate

Naming Compounds with Polyatomic Ions Example 45 Al. PO 4 What is the name of the cation? What is the name of the anion? Aluminum Phosphate

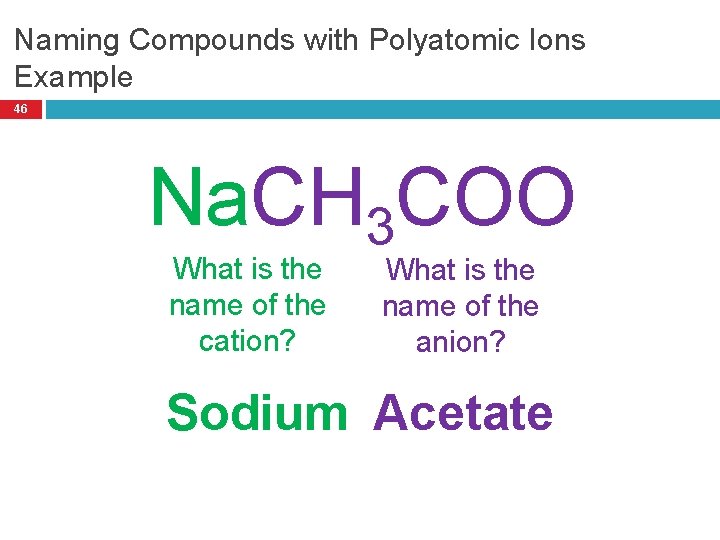
Naming Compounds with Polyatomic Ions Example 46 Na. CH 3 COO What is the name of the cation? What is the name of the anion? Sodium Acetate

Be aware – you can’t forget about your transition metals! 47 Cu. Si. O 3 What is the name of the cation? What is the name of the anion? Copper (II) Silicate Si. O 32 - is called silicate

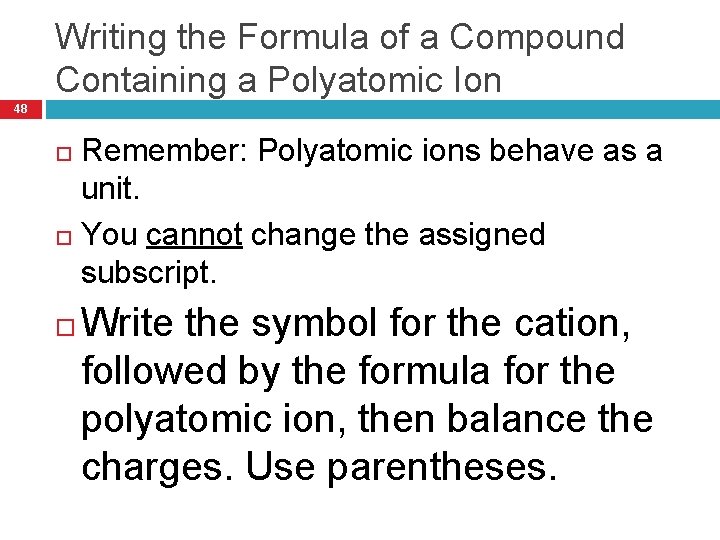
Writing the Formula of a Compound Containing a Polyatomic Ion 48 Remember: Polyatomic ions behave as a unit. You cannot change the assigned subscript. Write the symbol for the cation, followed by the formula for the polyatomic ion, then balance the charges. Use parentheses.

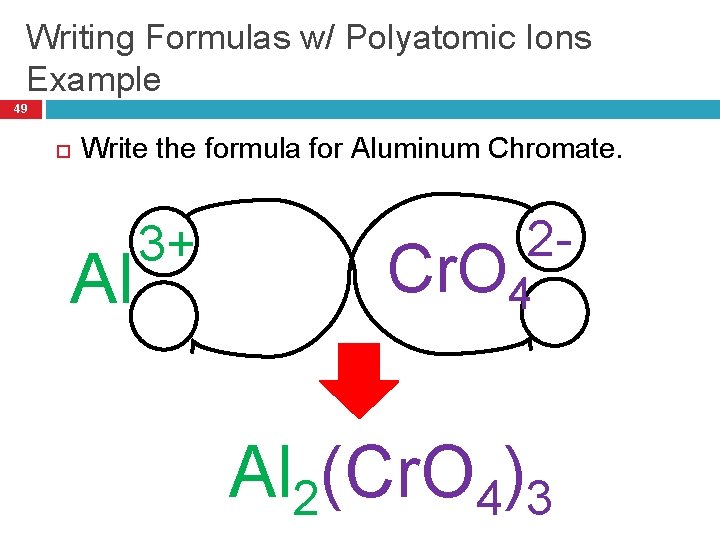
Writing Formulas w/ Polyatomic Ions Example 49 Write the formula for Aluminum Chromate. Al 3+ 2 - Cr. O 4 Al 2(Cr. O 4)3

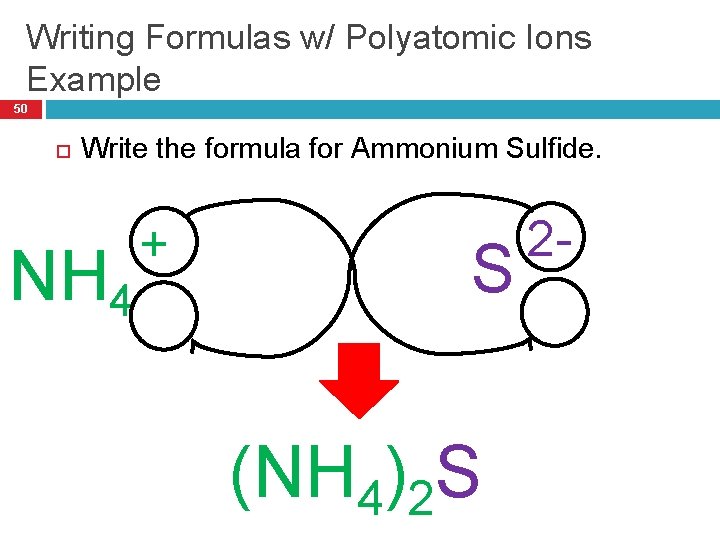
Writing Formulas w/ Polyatomic Ions Example 50 Write the formula for Ammonium Sulfide. NH 4 + S (NH 4)2 S 2 -

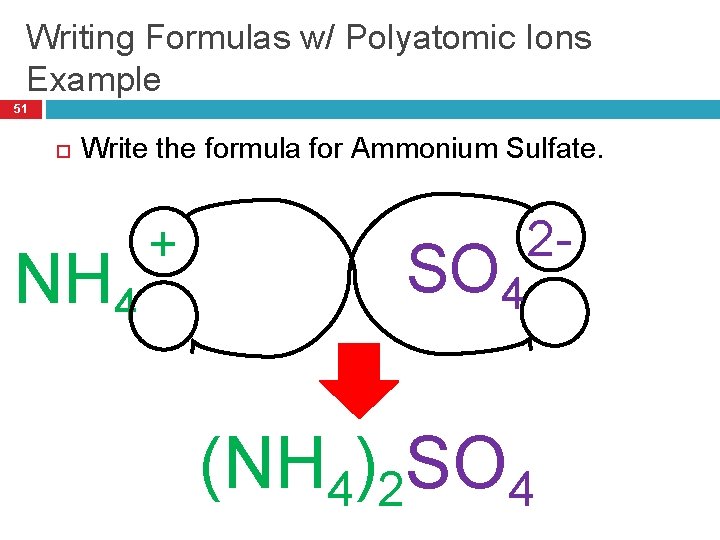
Writing Formulas w/ Polyatomic Ions Example 51 Write the formula for Ammonium Sulfate. NH 4 + SO 4 2 - (NH 4)2 SO 4

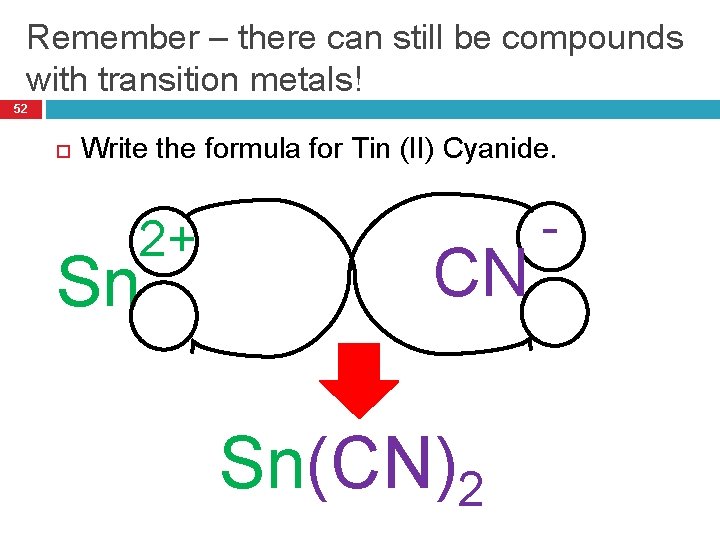
Remember – there can still be compounds with transition metals! 52 Write the formula for Tin (II) Cyanide. 2+ Sn CN Sn(CN)2 -

53 a. Mg(OH)2 b. K 2 SO 4

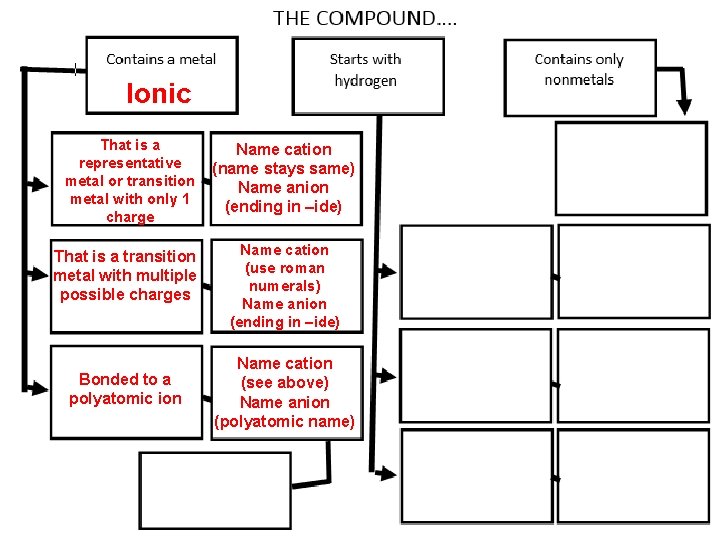
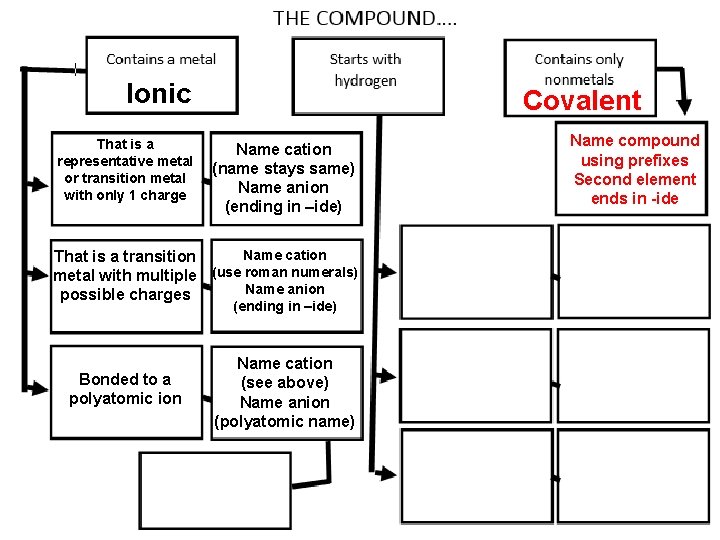
Ionic That is a representative metal or transition metal with only 1 charge That is a transition metal with multiple possible charges Bonded to a polyatomic ion 54 Name cation (name stays same) Name anion (ending in –ide) Name cation (use roman numerals) Name anion (ending in –ide) Name cation (see above) Name anion (polyatomic name)

Try It! 55 Write the formulas of the following binary ionic compounds: 1. 2. 3. 4. Tin (IV) sulfide Magnesium nitrate Calcium bromide Sodium hydroxide 1. 2. 3. 4. Sn. S 2 Mg(NO 3)2 Ca. Br 2 Na. OH

Quick Quiz Section 9. 2

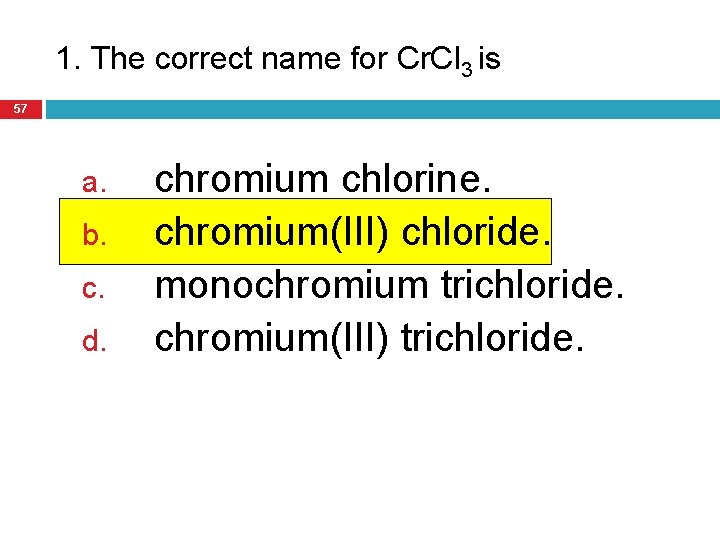
1. The correct name for Cr. Cl 3 is 57 a. b. c. d. chromium chlorine. chromium(III) chloride. monochromium trichloride. chromium(III) trichloride.

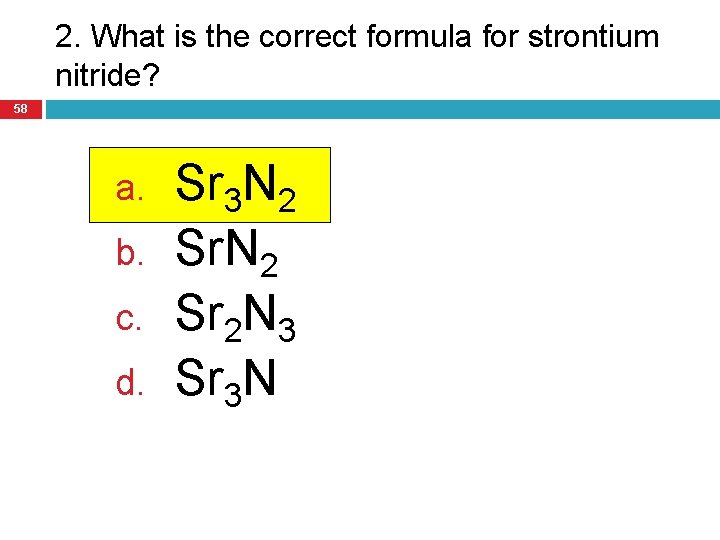
2. What is the correct formula for strontium nitride? 58 a. b. c. d. Sr 3 N 2 Sr 2 N 3 Sr 3 N

3. Which one of the following compounds is named correctly? 59 a. b. c. d. sodium chlorite, Na. Cl. O potassium nitrate, KNO 3 sodium acetate, Na 2 C 2 H 3 O 2 lithium sulfate, Li 2 SO 3

What’s Next? 60 Book Work �Page 258 (#8 -9) �Page 266 (#17 -19)

Page 258 #8 and 9 8. Page 266 #17 -19 Write the symbol for the ion of 17. Write the formulas of the following compounds: each element. Classify the ion as an anion or cation, and name the a. Beryllium Chloride ion. b. Cesium Sulfide a. Potassium c. Sodium Iodide b. Oxygen d. Strontium Oxide c. Tin (2 electrons lost) 18. Write the formulas of these d. Bromine polyatomic compounds: e. Beryllium a. Chromium (III) nitrate f. Cobalt (3 electrons lost) b. Sodium perchlorate 9. Write the symbol or formula c. Magnesium hydrogen carbonate (including charge) for each of the d. Calcium acetate following ions. 19. Identify any incorrect formulas a. Ammonium ion and explain your reasoning. b. Tin (II) ion a. Mg 2(SO 4)3 c. Chromate ion b. Rb 3 As d. Nitrate ion c. Be. Cl 3 61 d. Na. F

#8 – Write the symbol for the ion of each element. Classify the ion as an anion or cation, and name the ion. 62 a. Potassium K+, cation, Potassium b. Oxygen O 2 -, anion, Oxide a. Tin (2 electrons lost) Sn 2+, cation, Tin (II) b. Bromine Br-, anion, Bromide c. Beryllium Be 2+, cation, Beryllium d. Cobalt (3 electrons lost) Co 3+, cation, Cobalt (III)

#9 – Write the symbol or formula (including charge) for each of the following ions. 63 a. b. c. d. Ammonium ion NH 4 Tin (II) ion Sn 2+ 2 Chromate ion Cr. O 4 Nitrate ion NO 3 +

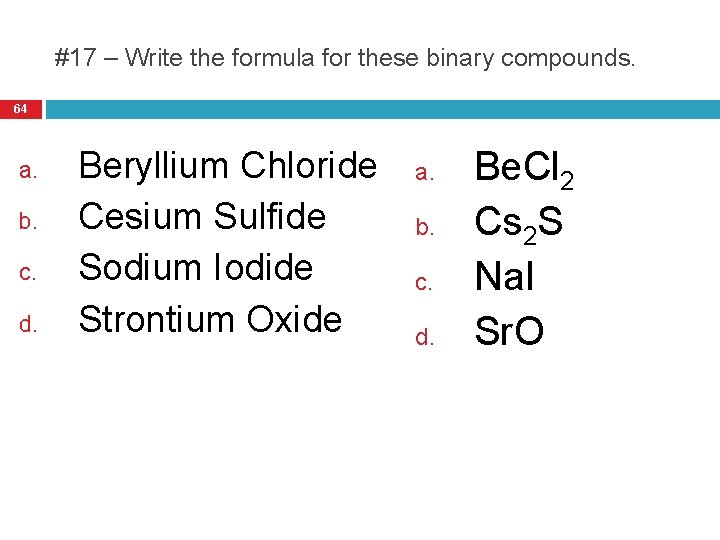
#17 – Write the formula for these binary compounds. 64 a. b. c. d. Beryllium Chloride Cesium Sulfide Sodium Iodide Strontium Oxide a. b. c. d. Be. Cl 2 Cs 2 S Na. I Sr. O

#18 – Write the formula these compounds containing polyatomic ions. 65 a. b. c. d. Chromium (III) nitrate Sodium Perchlorate Magnesium hydrogen carbonate Calcium acetate a. b. c. d. Cr(NO 3)3 Na. Cl. O 4 Mg(HCO 3)2 Ca(C 2 H 3 O 2)2 or Ca(CH 3 COO)2

#19 – Identify any incorrect formulas. Explain your answer. 66 a. b. c. d. Mg 2(SO 4)3 Rb 3 As Be. Cl 3 Na. F

Section 9. 3: Naming and Writing Formulas for Molecular Compounds Read pages 268 -270

Binary Molecular Compounds 68 A binary molecular compound is composed of two elements – both are nonmetals Binary molecular compounds do not contain ions – they are made of… molecules

69 Carbon and oxygen combine to form carbon monoxide (CO) and carbon dioxide (CO 2), but these two invisible gases are very different. Sitting in a room with small amounts of CO 2 in the air would not present any problems. If the same amount of CO were in the room, you could die of asphyxiation. A naming system that distinguishes between these two compounds is needed.

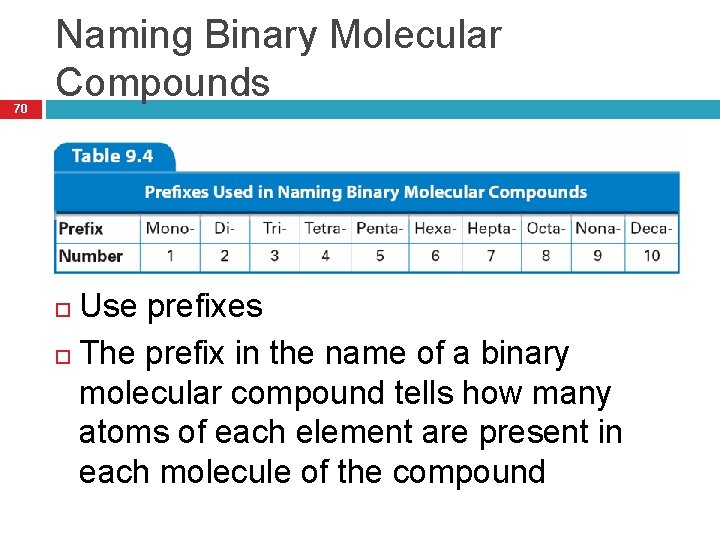
70 Naming Binary Molecular Compounds Use prefixes The prefix in the name of a binary molecular compound tells how many atoms of each element are present in each molecule of the compound

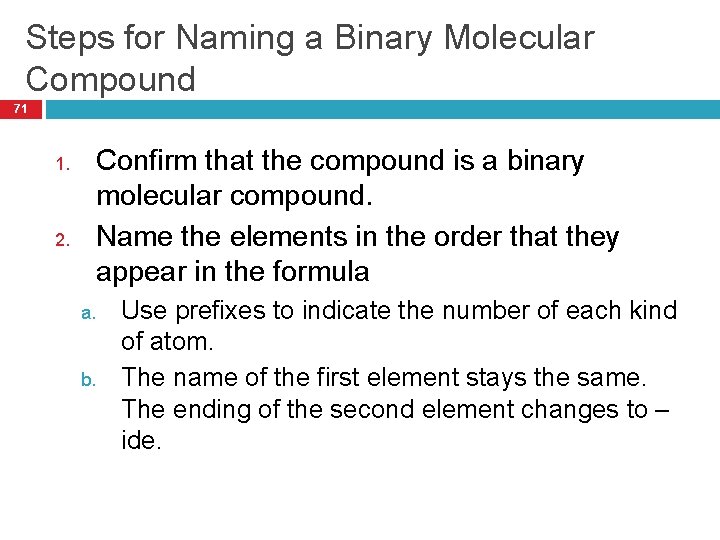
Steps for Naming a Binary Molecular Compound 71 1. 2. Confirm that the compound is a binary molecular compound. Name the elements in the order that they appear in the formula a. b. Use prefixes to indicate the number of each kind of atom. The name of the first element stays the same. The ending of the second element changes to – ide.

Quick Example – Add it to your notes! 72 S 2 Br 4 disulfur tetrabromide

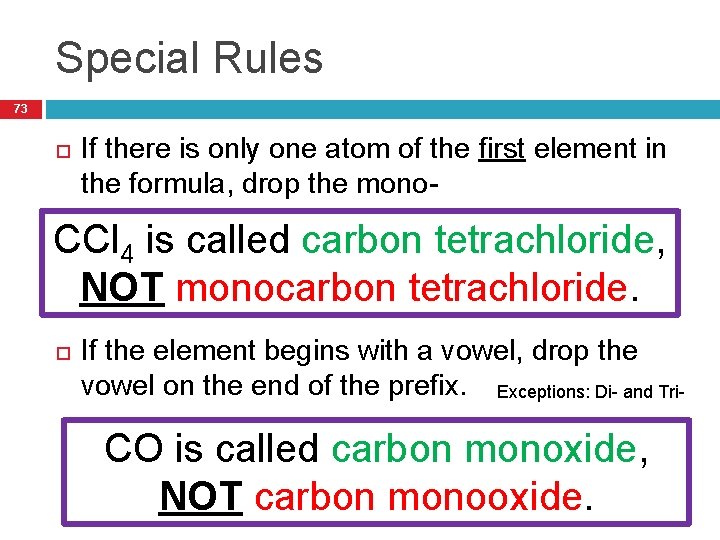
Special Rules 73 If there is only one atom of the first element in the formula, drop the mono- CCl 4 is called carbon tetrachloride, NOT monocarbon tetrachloride. If the element begins with a vowel, drop the vowel on the end of the prefix. Exceptions: Di- and Tri- CO is called carbon monoxide, NOT carbon monooxide.

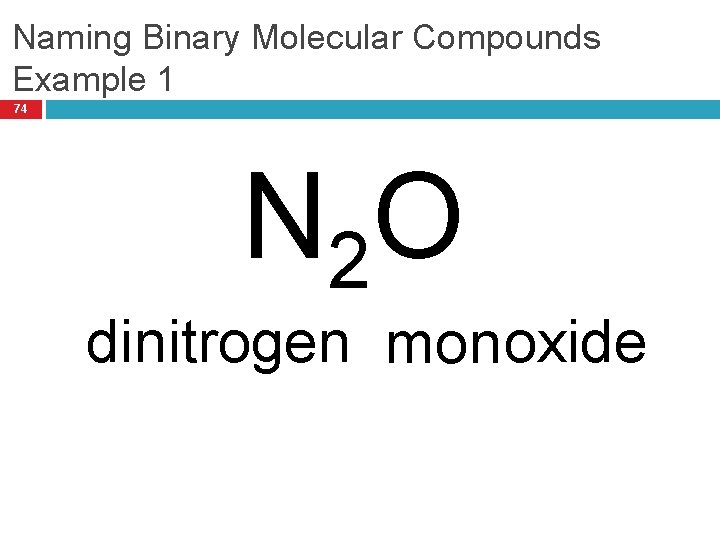
Naming Binary Molecular Compounds Example 1 74 N 2 O dinitrogen monoxide

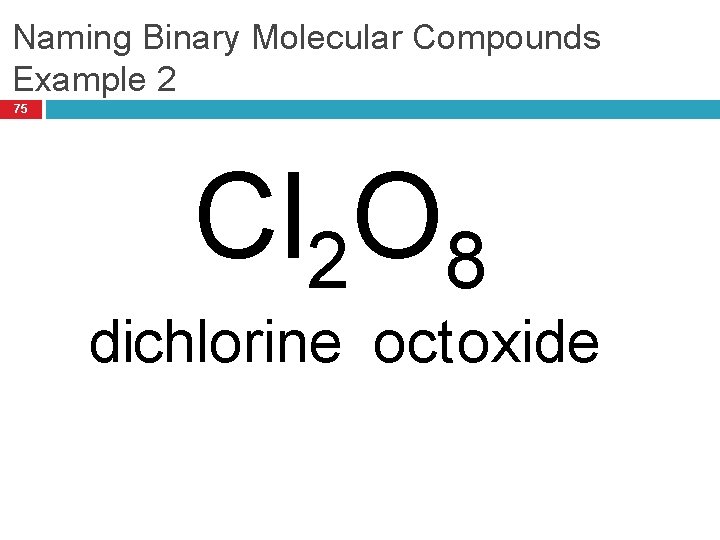
Naming Binary Molecular Compounds Example 2 75 Cl 2 O 8 dichlorine oct oxide

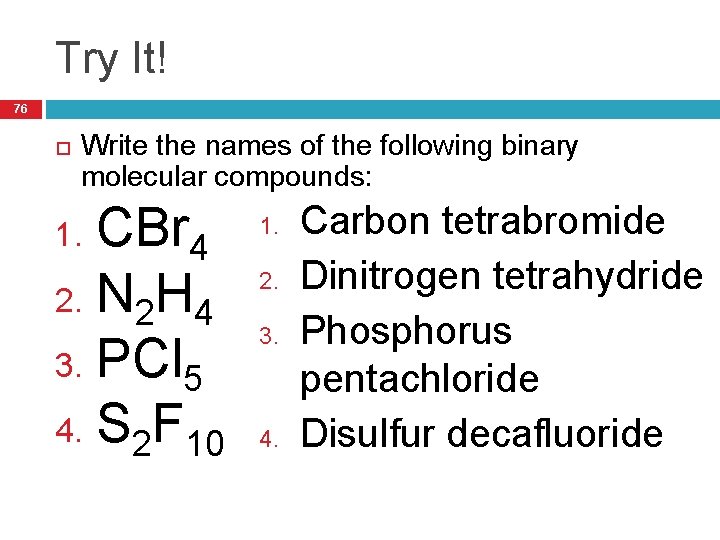
Try It! 76 Write the names of the following binary molecular compounds: CBr 4 2. N 2 H 4 3. PCl 5 4. S 2 F 10 1. 2. 3. 4. Carbon tetrabromide Dinitrogen tetrahydride Phosphorus pentachloride Disulfur decafluoride

77 Writing Formulas for Binary Molecular Compounds Use the prefixes in the name to tell you the subscript of each element in the formula. Then, write the correct symbols for the two elements with the appropriate subscripts.

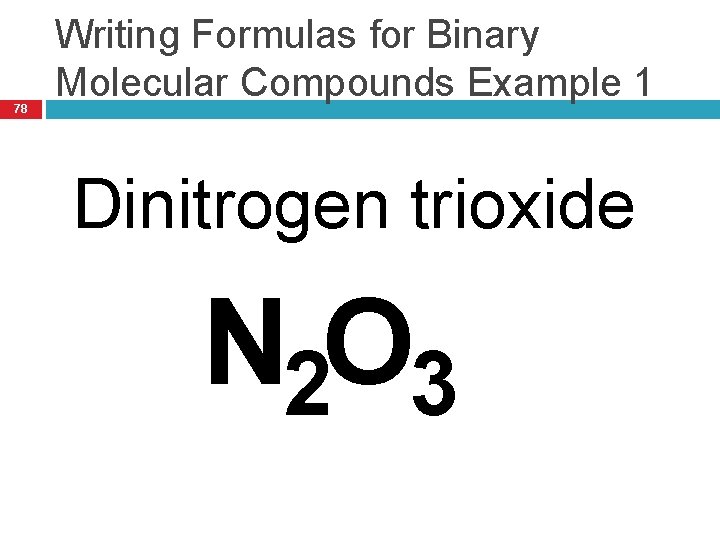
78 Writing Formulas for Binary Molecular Compounds Example 1 Dinitrogen trioxide N 2 O 3

79 Writing Formulas for Binary Molecular Compounds Example 2 Iodine heptafluoride I F 7

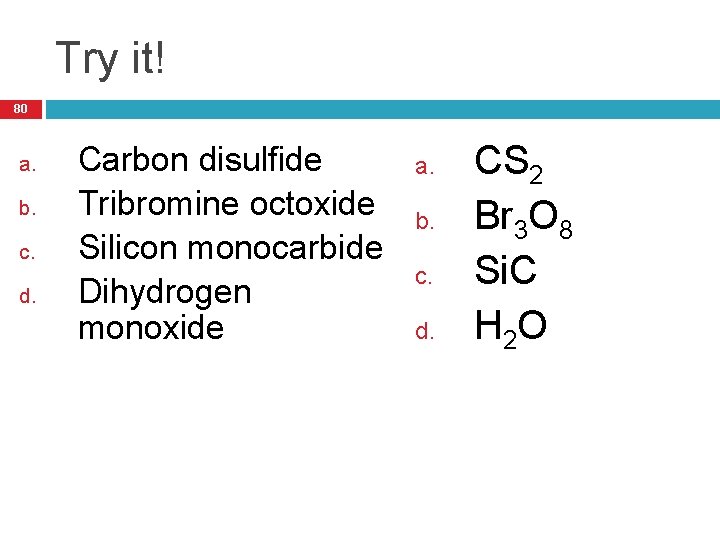
Try it! 80 a. b. c. d. Carbon disulfide Tribromine octoxide Silicon monocarbide Dihydrogen monoxide a. b. c. d. CS 2 Br 3 O 8 Si. C H 2 O

Ionic That is a representative metal or transition metal with only 1 charge Covalent Name cation (name stays same) Name anion (ending in –ide) Name cation That is a transition metal with multiple (use roman numerals) Name anion possible charges (ending in –ide) Bonded to a polyatomic ion 81 Name cation (see above) Name anion (polyatomic name) Name compound using prefixes Second element ends in -ide

Quick Quiz Section 9. 3

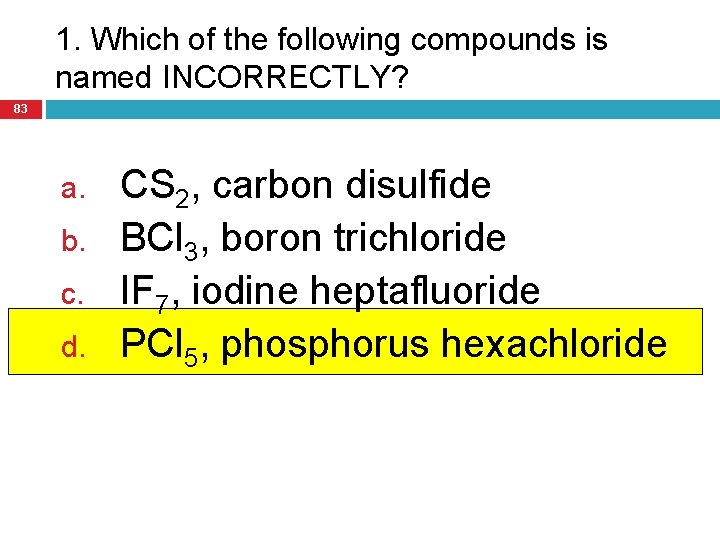
1. Which of the following compounds is named INCORRECTLY? 83 a. b. c. d. CS 2, carbon disulfide BCl 3, boron trichloride IF 7, iodine heptafluoride PCl 5, phosphorus hexachloride

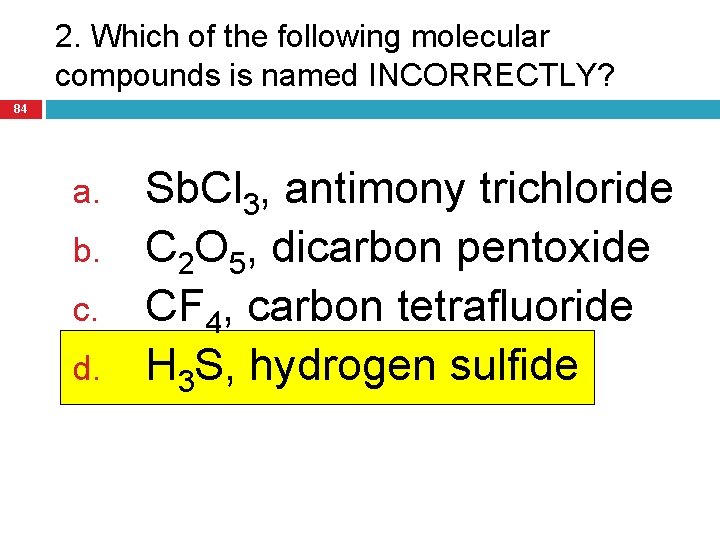
2. Which of the following molecular compounds is named INCORRECTLY? 84 a. b. c. d. Sb. Cl 3, antimony trichloride C 2 O 5, dicarbon pentoxide CF 4, carbon tetrafluoride H 3 S, hydrogen sulfide

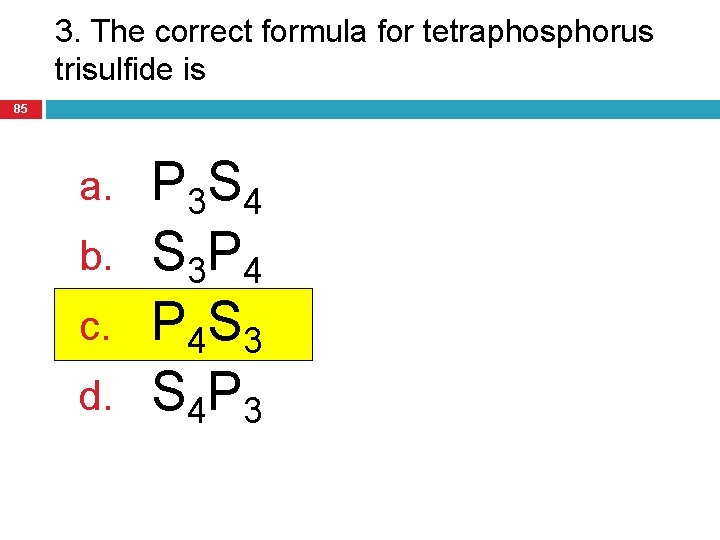
3. The correct formula for tetraphosphorus trisulfide is 85 a. b. c. d. P 3 S 4 S 3 P 4 P 4 S 3 S 4 P 3

Section 9. 4: Naming and Writing Formulas for Acids and Bases Read pages 271 -273

Naming Acids 87 Acid - a compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water Hydrogen is most commonly listed first in the compound � Formula: NOTE: H n. X Acids do not have to have hydrogen first, but usually do.

Rules for Naming Acids 88 Three rules Dependent on the anion (the non hydrogen part)

Rule #1 89 When the name of the anion ends in –ide, the acid name begins with hydro-. The stem of the anion has the suffix –ic and is followed by acid. Simply – if there is no oxygen in the acid formula, the formula for the name is hydro-root-ic acid. Example � HCl Cation – Hydrogen Anion – Chloride Name of Acid – Hydrochloric Acid

Rule #2 90 When the anion name ends in –ite, the acid name is the stem of the anion with the suffix –ous followed by the word acid. Formula for name: Root-ous acid Example � HCl. O 2 Cation – Hydrogen Anion – Chlorite Name of Acid – Chlorous acid

Rule #3 91 When the anion name ends in –ate, the acid name is the stem of the anion with the suffix –ic followed by the word acid. Formula for name: Root-ic acid Example � HCl. O 3 Cation – Hydrogen Anion – Chlorate Name of Acid – Chloric acid

Weird Roots 92 Sulfur and Phosphorus � Sulfuric, Sulfurous � Phosphorus Phosphoric, Phosphorous

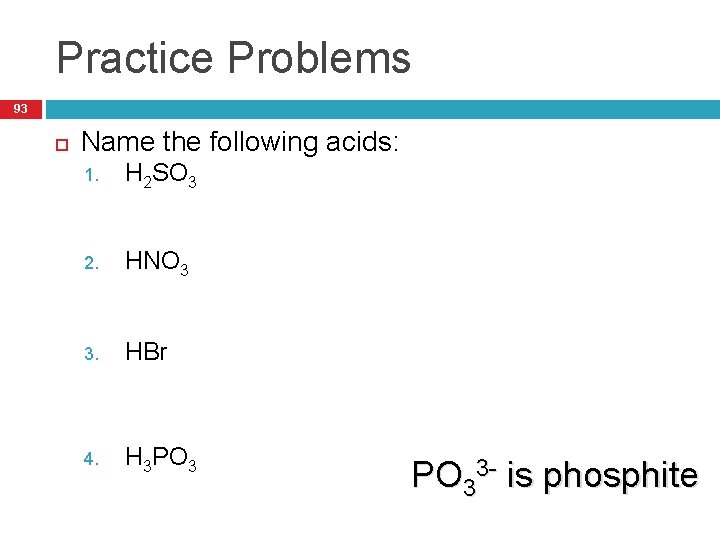
Practice Problems 93 Name the following acids: H 2 SO 3 1. 1. Sulfurous Acid HNO 3 2. 1. Nitric Acid HBr 3. 1. Hydrobromic Acid H 3 PO 3 4. 1. Phosphorous Acid PO 33 - is phosphite

Writing Formulas for Acids 94 Use the rules in reverse Example: � Write the formula for hydrocyanic acid. � Hydro-root-ic acid means the anion ends in –ide. The cation is Hydrogen (H+). The anion is Cyanide (CN-). The formula for the acid is HCN.

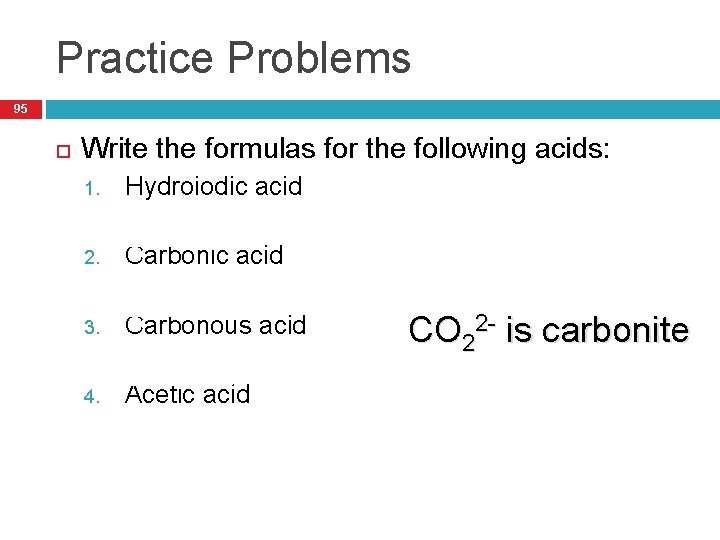
Practice Problems 95 Write the formulas for the following acids: Hydroiodic acid 1. 1. Carbonic acid 2. 1. H 2 CO 3 Carbonous acid 3. 1. 4. HI H 2 CO 2 Acetic acid HC 2 H 3 O 2 CO 22 - is carbonite

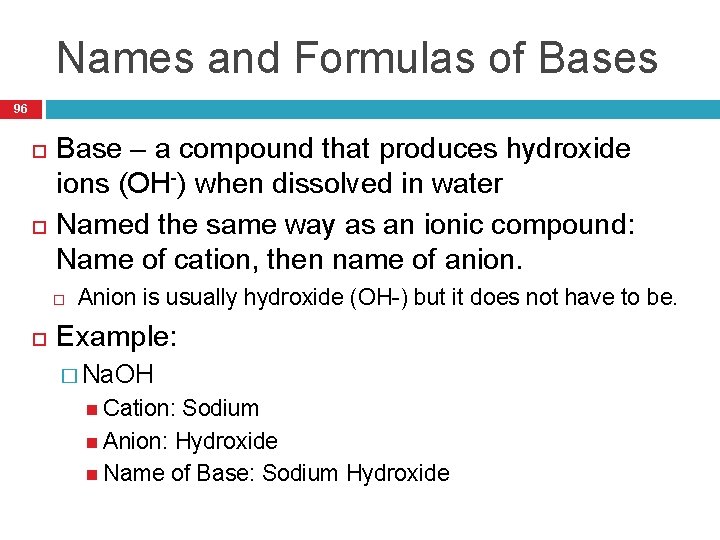
Names and Formulas of Bases 96 Base – a compound that produces hydroxide ions (OH-) when dissolved in water Named the same way as an ionic compound: Name of cation, then name of anion. Anion is usually hydroxide (OH-) but it does not have to be. Example: � Na. OH Cation: Sodium Anion: Hydroxide Name of Base: Sodium Hydroxide

Try it! 97 Provide the appropriate name or formula of the following bases: Potassium hydroxide 1. 1. KOH Be(OH)2 2. 1. Beryllium Hydroxide Rb. OH 3. 1. Rubidium Hydroxide Aluminum hydroxide 4. 1. Al(OH)3

A base to take note of: 98 NH 3 is ammonia. This is an example of a base that does NOT contain a hydroxide ion.

Weird Roots 99 Sulfur and Phosphorus � Sulfuric, Sulfurous � Phosphorus Phosphoric, Phosphorous

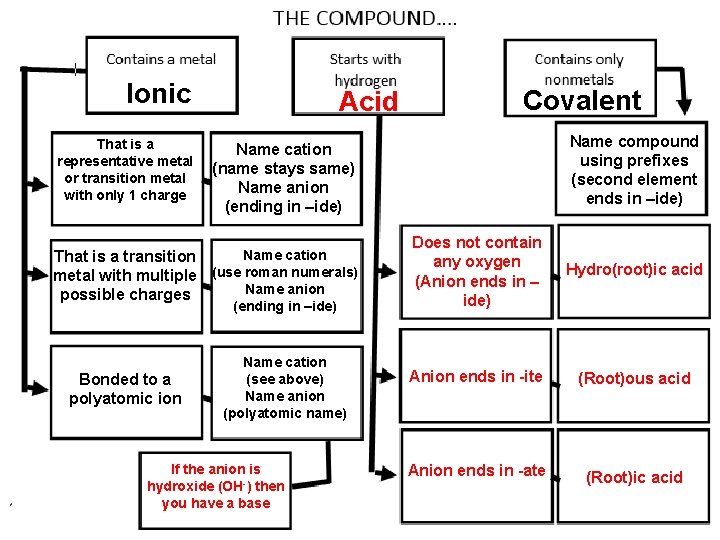
Ionic That is a representative metal or transition metal with only 1 charge Acid (ending in –ide) 100 Name compound using prefixes (second element ends in –ide) Name cation (name stays same) Name anion (ending in –ide) Name cation That is a transition metal with multiple (use roman numerals) Name anion possible charges Bonded to a polyatomic ion Covalent Name cation (see above) Name anion (polyatomic name) If the anion is hydroxide (OH-) then you have a base Does not contain any oxygen (Anion ends in – ide) Hydro(root)ic acid Anion ends in -ite (Root)ous acid Anion ends in -ate (Root)ic acid

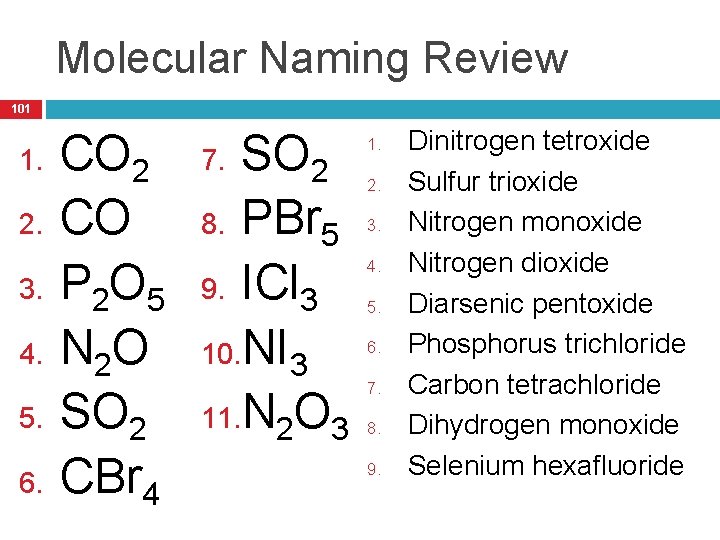
Molecular Naming Review 101 1. 2. 3. 4. 5. 6. CO 2 CO P 2 O 5 N 2 O SO 2 CBr 4 SO 2 8. PBr 5 9. ICl 3 10. NI 3 11. N 2 O 3 7. 1. 2. 3. 4. 5. 6. 7. 8. 9. Dinitrogen tetroxide Sulfur trioxide Nitrogen monoxide Nitrogen dioxide Diarsenic pentoxide Phosphorus trichloride Carbon tetrachloride Dihydrogen monoxide Selenium hexafluoride

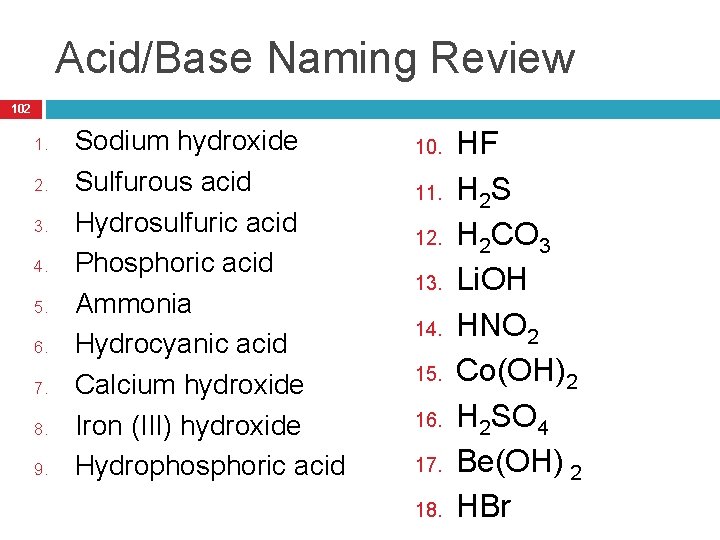
Acid/Base Naming Review 102 1. 2. 3. 4. 5. 6. 7. 8. 9. Sodium hydroxide Sulfurous acid Hydrosulfuric acid Phosphoric acid Ammonia Hydrocyanic acid Calcium hydroxide Iron (III) hydroxide Hydrophosphoric acid 10. 11. 12. 13. 14. 15. 16. 17. 18. HF H 2 S H 2 CO 3 Li. OH HNO 2 Co(OH)2 H 2 SO 4 Be(OH) 2 HBr

Quick Quiz Section 9. 4

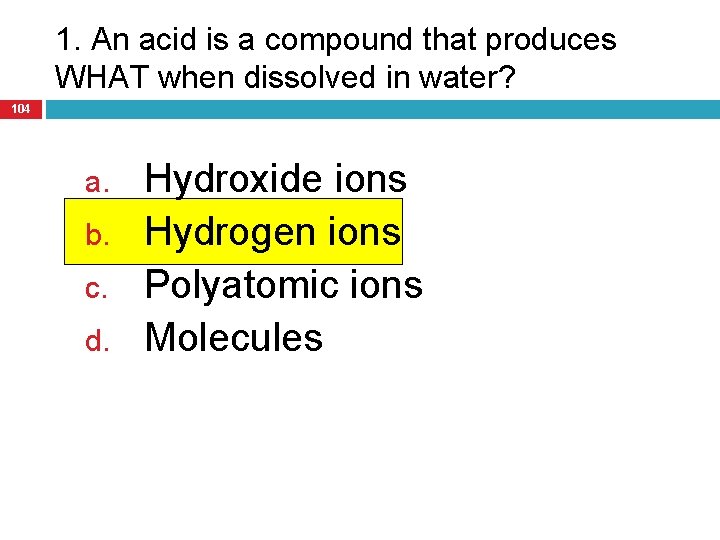
1. An acid is a compound that produces WHAT when dissolved in water? 104 a. b. c. d. Hydroxide ions Hydrogen ions Polyatomic ions Molecules

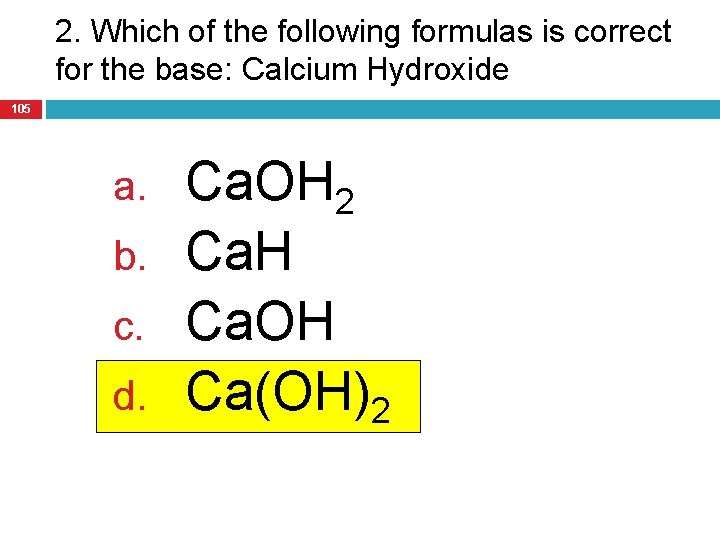
2. Which of the following formulas is correct for the base: Calcium Hydroxide 105 a. b. c. d. Ca. OH 2 Ca. H Ca. OH Ca(OH)2

What’s Next? 106 Practice Problems

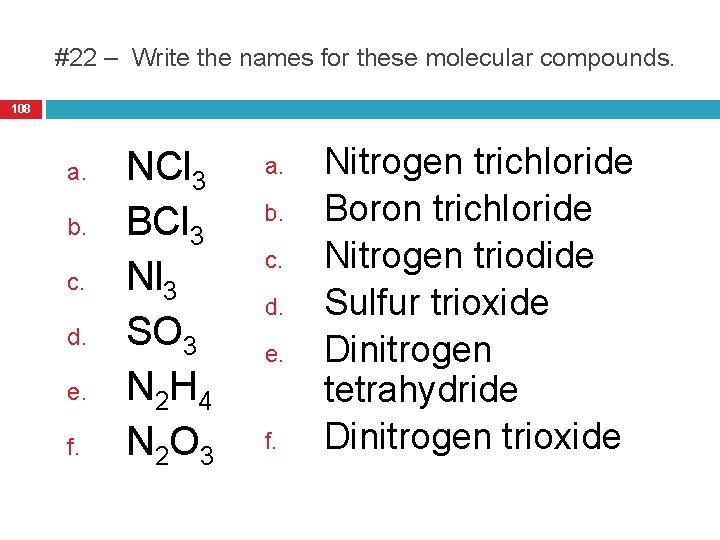
22. Write the names of the following 25. The name a student gives for the molecular compound Si. Cl 4 is compounds: monosilicon trichloride. Is this a. NCl 3 d. SO 3 name correct? Explain. b. BCl 3 e. N 2 H 4 29. Name the following acids: c. Nl 3 f. N 2 O 3 29. HNO 2 23. Write the formulas or names of 30. HMn. O 4 the following compounds: 31. HCN a. CS 2 32. H 2 S b. Carbon tetrabromide 30. Name the following bases: c. Cl 2 O 7 29. Li. OH d. Diphosphorus trioxide 30. Pb(OH)2 24. Write the molecular formulas of 31. Mg(OH)2 the following compounds: 32. Al(OH)3 a. Phosphorus pentachloride 31. Write formulas for each of the b. Iodine heptafluoride following acids and bases: c. Chlorine trifluoride 29. Carbonic acid d. Iodine dioxide 30. Sulfurous acid 31. Iron (III) hydroxide 32. Strontium hydroxide 107

#22 – Write the names for these molecular compounds. 108 a. b. c. d. e. f. NCl 3 BCl 3 Nl 3 SO 3 N 2 H 4 N 2 O 3 a. b. c. d. e. f. Nitrogen trichloride Boron trichloride Nitrogen triodide Sulfur trioxide Dinitrogen tetrahydride Dinitrogen trioxide

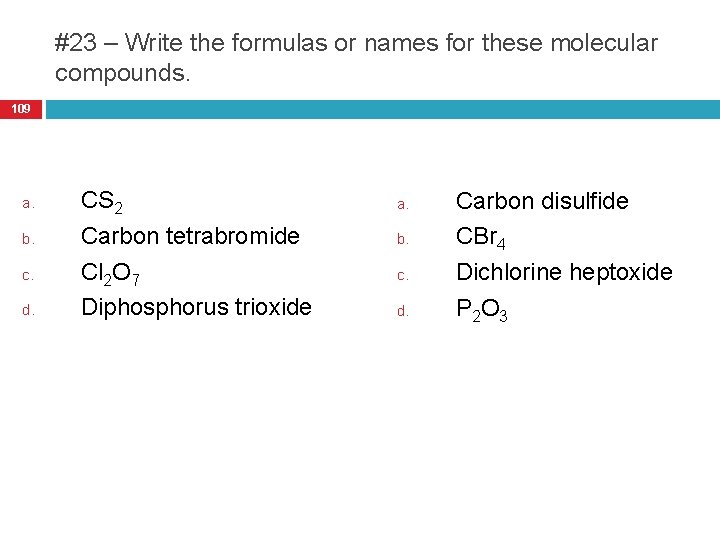
#23 – Write the formulas or names for these molecular compounds. 109 a. b. c. d. CS 2 Carbon tetrabromide Cl 2 O 7 Diphosphorus trioxide a. b. c. d. Carbon disulfide CBr 4 Dichlorine heptoxide P 2 O 3

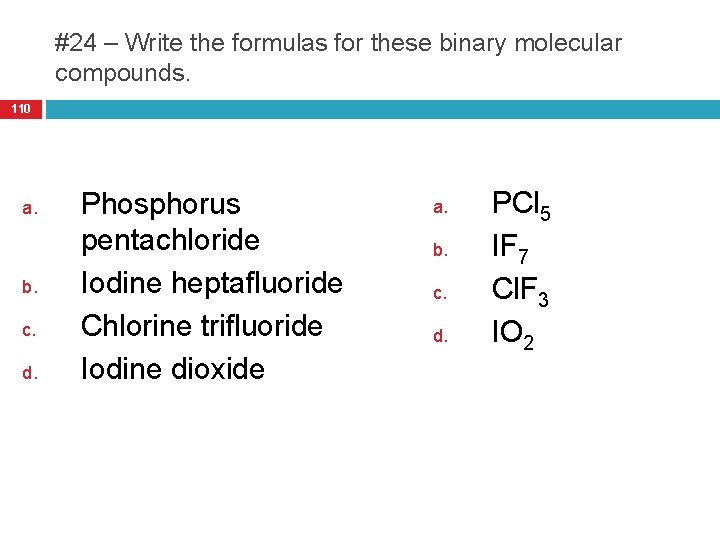
#24 – Write the formulas for these binary molecular compounds. 110 a. b. c. d. Phosphorus pentachloride Iodine heptafluoride Chlorine trifluoride Iodine dioxide a. b. c. d. PCl 5 IF 7 Cl. F 3 IO 2

#25 – The name a student gives for the molecular compound Si. Cl 4 is monosilicon trichloride. Is this name correct? Explain. 111 Silicon tetrachloride

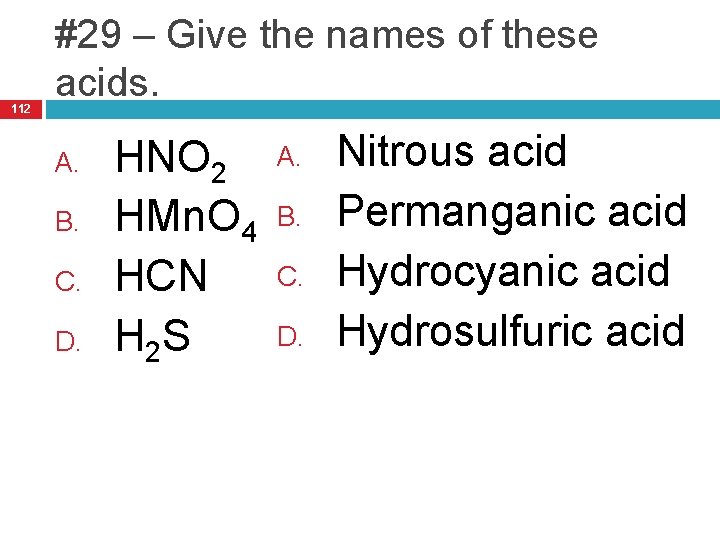
112 #29 – Give the names of these acids. A. B. C. D. HNO 2 HMn. O 4 HCN H 2 S A. B. C. D. Nitrous acid Permanganic acid Hydrocyanic acid Hydrosulfuric acid

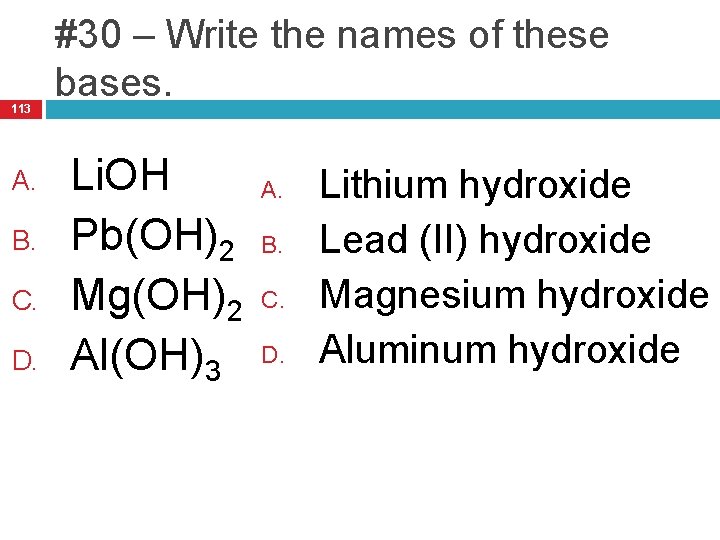
113 A. B. C. D. #30 – Write the names of these bases. Li. OH Pb(OH)2 Mg(OH)2 Al(OH)3 A. B. C. D. Lithium hydroxide Lead (II) hydroxide Magnesium hydroxide Aluminum hydroxide

114 A. B. C. D. #32 – Write the formulas of the following acids and bases: Carbonic acid Sulfurous acid Iron (III) hydroxide Strontium hydroxide A. B. C. D. H 2 CO 3 H 2 SO 3 Fe(OH)3 Sr(OH)2

Section 9. 5: The Laws Governing Formulas and Names Read pages 274 -279

The Laws Governing Formulas and Names 116 The rules for naming and writing formulas are possible because elements form compounds in predictable ways. Two Important Laws � The Law of Definite Proportions � The Law of Multiple Proportions

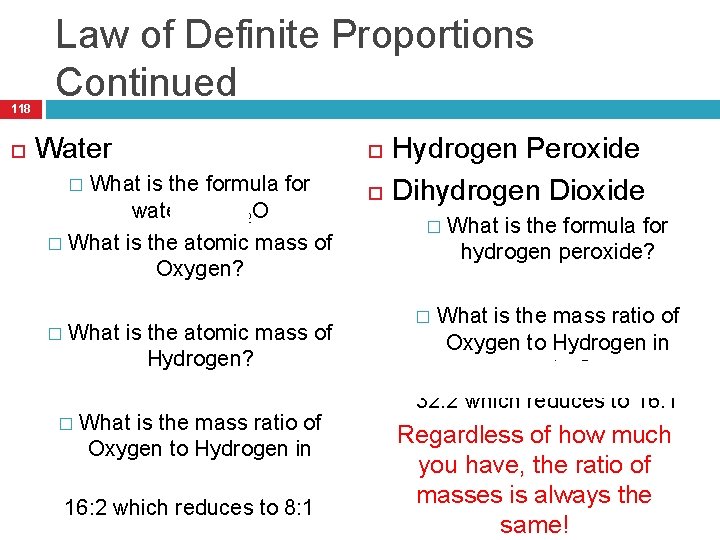
The Law of Definite Proportions 117 States that in samples of any chemical compound, the masses of the elements are always in the same proportions. So what does that mean?

118 Law of Definite Proportions Continued Water What is the formula for water? H 2 O � What is the atomic mass of Oxygen? 16. 00 � What is the atomic mass of Hydrogen? 1. 00 � What is the mass ratio of Oxygen to Hydrogen in water? 16: 2 which reduces to 8: 1 � Hydrogen Peroxide Dihydrogen Dioxide What is the formula for hydrogen peroxide? � H 2 O 2 � What is the mass ratio of Oxygen to Hydrogen in water? 32: 2 which reduces to 16: 1 � Regardless of how much you have, the ratio of masses is always the same!

Both water and hydrogen peroxide are composed of hydrogen and oxygen…. 119 But they have different properties.

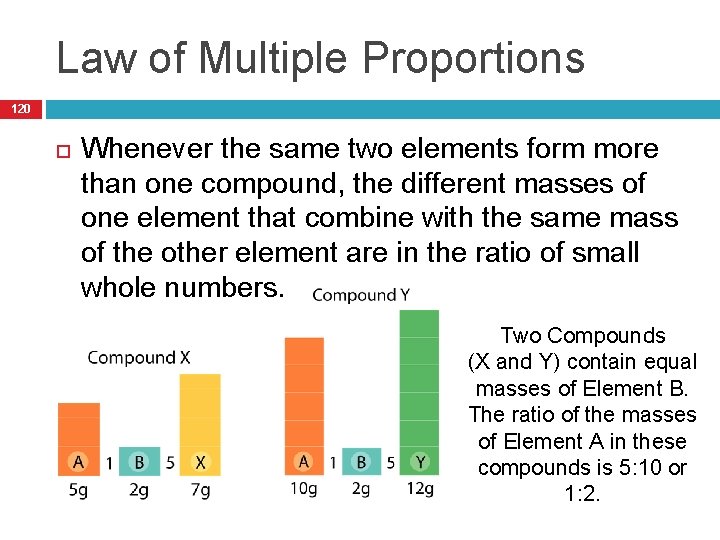
Law of Multiple Proportions 120 Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. Two Compounds (X and Y) contain equal masses of Element B. The ratio of the masses of Element A in these compounds is 5: 10 or 1: 2.

Main Idea: 121 In any chemical compound, the elements are always in the same proportion by MASS.

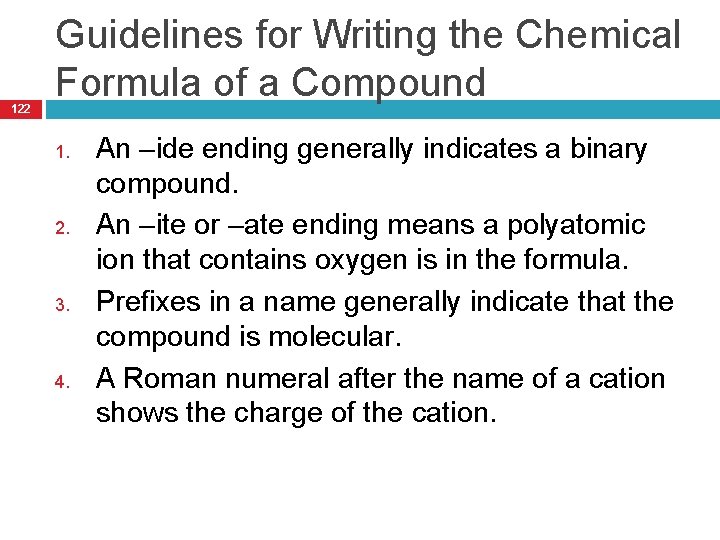
122 Guidelines for Writing the Chemical Formula of a Compound 1. 2. 3. 4. An –ide ending generally indicates a binary compound. An –ite or –ate ending means a polyatomic ion that contains oxygen is in the formula. Prefixes in a name generally indicate that the compound is molecular. A Roman numeral after the name of a cation shows the charge of the cation.

Naming Review 123 1. 2. 3. H in front of the formula? It’s an acid! OH in the formula? It’s a base! (Ionic name) Metal present? It’s ionic! 1. 4. Check to see if you have a transition metal with multiple charges. Only nonmetals? It’s covalent!

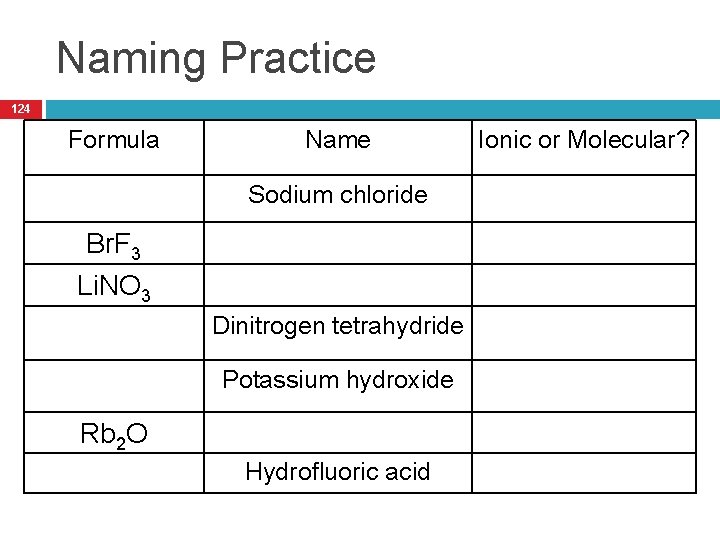
Naming Practice 124 Formula Name Sodium chloride Br. F 3 Li. NO 3 Dinitrogen tetrahydride Potassium hydroxide Rb 2 O Hydrofluoric acid Ionic or Molecular?

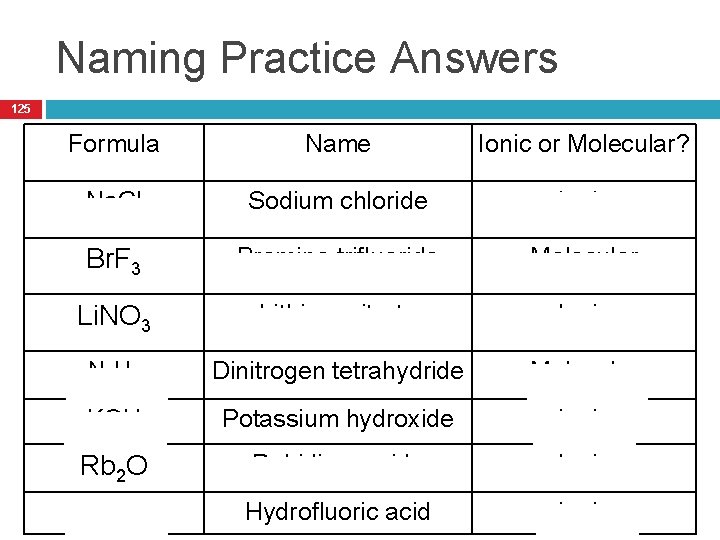
Naming Practice Answers 125 Formula Name Ionic or Molecular? Na. Cl Sodium chloride Ionic Br. F 3 Bromine trifluoride Molecular Li. NO 3 Lithium nitrate Ionic N 2 H 4 Dinitrogen tetrahydride Molecular KOH Potassium hydroxide Ionic Rb 2 O Rubidium oxide Ionic HF Hydrofluoric acid Ionic

What’s Next? 126 Book Work �Page 279 (#35, 39 -41)

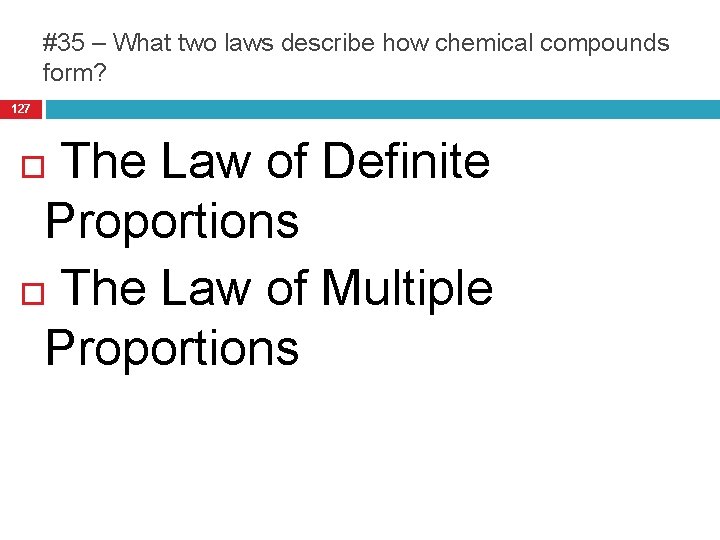
#35 – What two laws describe how chemical compounds form? 127 The Law of Definite Proportions The Law of Multiple Proportions

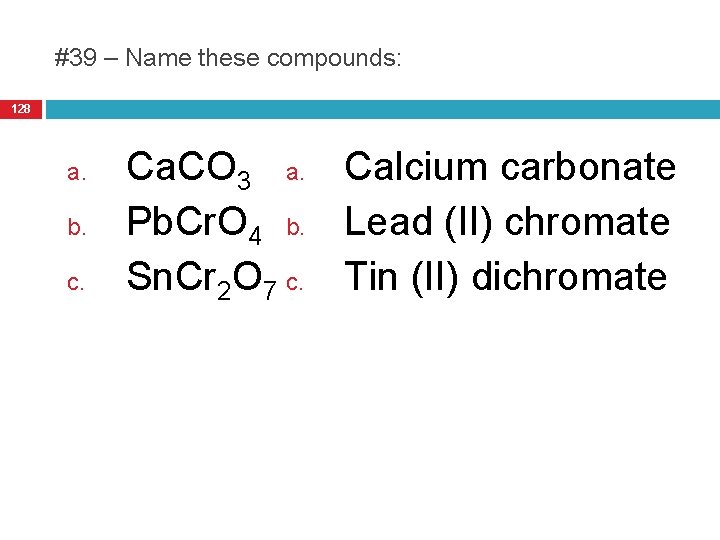
#39 – Name these compounds: 128 a. b. c. Ca. CO 3 a. Calcium carbonate Pb. Cr. O 4 b. Lead (II) chromate Sn. Cr 2 O 7 c. Tin (II) dichromate

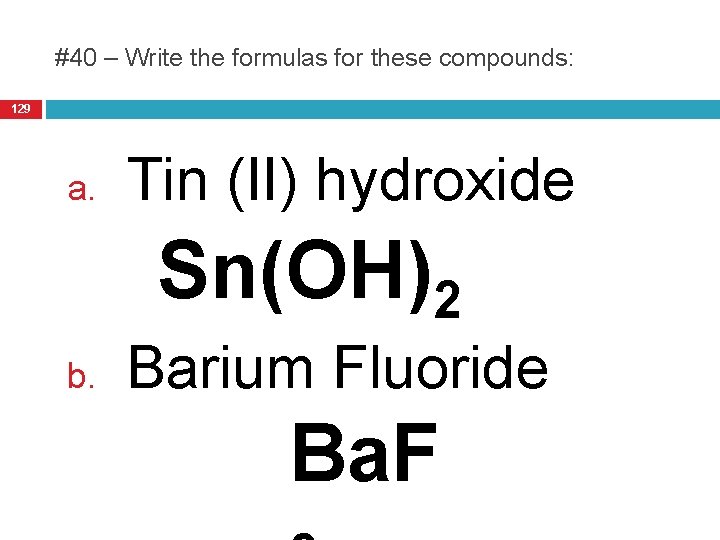
#40 – Write the formulas for these compounds: 129 a. Tin (II) hydroxide Sn(OH)2 b. Barium Fluoride Ba. F

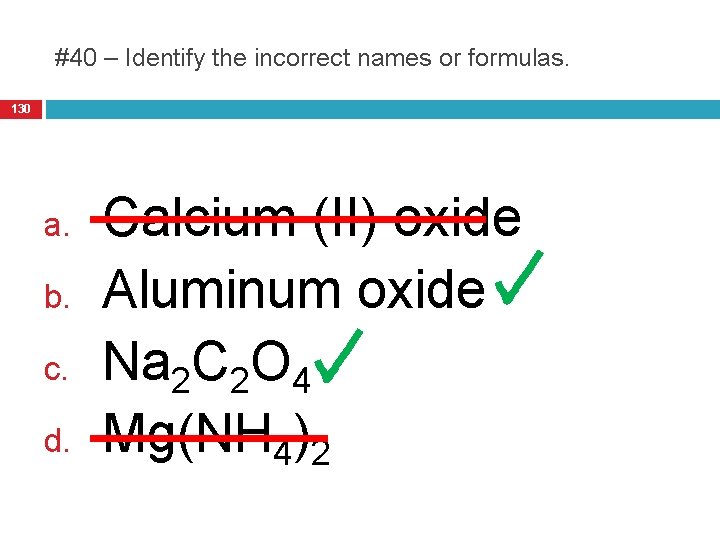
#40 – Identify the incorrect names or formulas. 130 a. b. c. d. Calcium (II) oxide Aluminum oxide Na 2 C 2 O 4 Mg(NH 4)2