Understanding Heat Transfer Conduction Convection and Radiation How































- Slides: 60

Understanding Heat Transfer, Conduction, Convection and Radiation

How ‘Heat’ Moves Review of past terms: • Define “Energy”: The ability to do work or cause change. • What is the basic unit of measure for energy? Joules. 2

How ‘Heat’ Moves • Define “Heat”: Heat is the movement of thermal energy from a substance at a higher temperature to another substance at a lower temperature. 3

Heat Transfer • Heat always moves from a warmer place to a cooler place. • Hot objects in a cooler room will cool to room temperature. • Cold objects in a warmer room will heat up to room temperature.

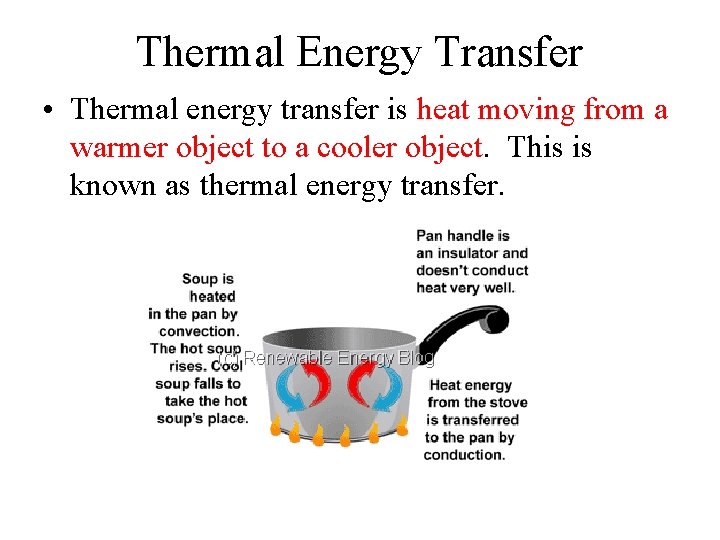
Thermal Energy Transfer • Thermal energy transfer is heat moving from a warmer object to a cooler object. This is known as thermal energy transfer.

The Nature of Heat moves in only one direction: • Under normal conditions and in nature, heat energy will ALWAYS flow the warmer object to the cooler object. • Heat energy will flow from one substance to another until the two substances have the same temperature. 6

Question • If a cup of coffee and a red popsickle were left on the table in this room what would happen to them? Why? • The cup of coffee will cool until it reaches room temperature. The popsickle will melt and then the liquid will warm to room temperature.

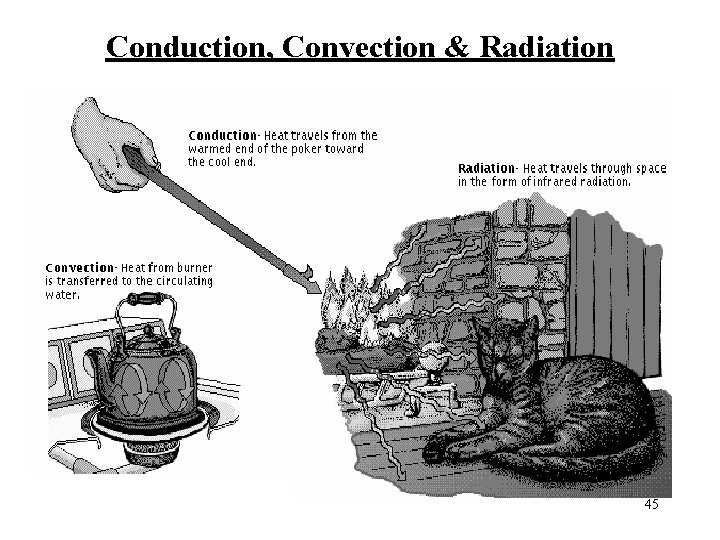
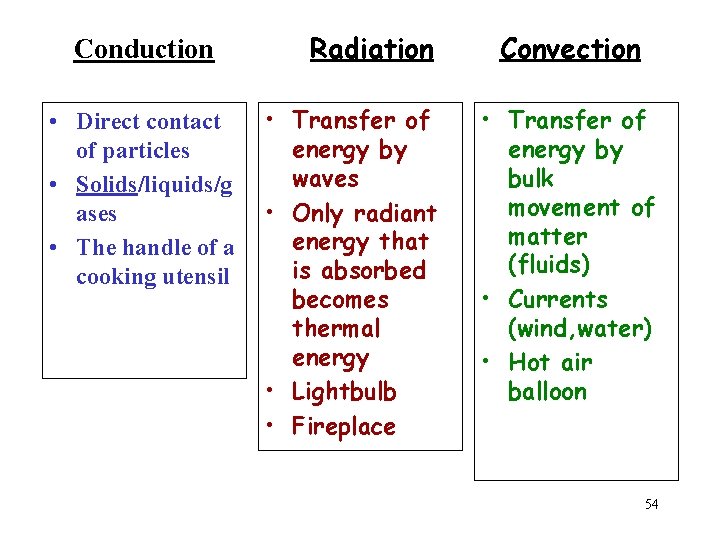
Heat Transfer Methods • Heat transfers in three ways: – Conduction – Convection – Radiation

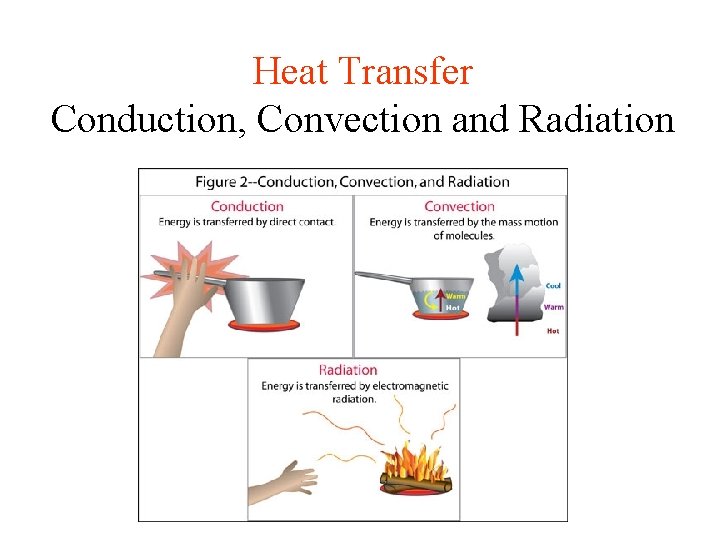
Heat Transfer Conduction, Convection and Radiation

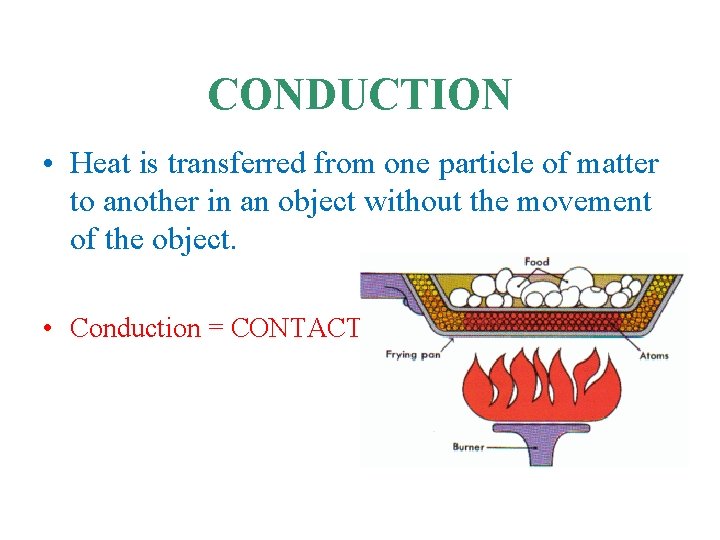
CONDUCTION • Heat is transferred from one particle of matter to another in an object without the movement of the object. • Conduction = CONTACT

Have you ever… • Touched a metal spoon sitting in a pan of boiling water only to be surprised by HOW hot it is? ? Think back to what you know about metals and nonmetals. What conducts heat better, metal or nonmetal? Why?

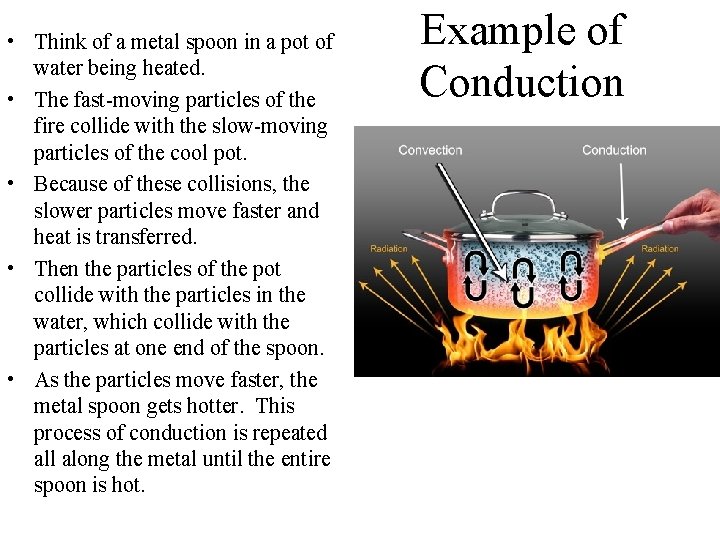
• Think of a metal spoon in a pot of water being heated. • The fast-moving particles of the fire collide with the slow-moving particles of the cool pot. • Because of these collisions, the slower particles move faster and heat is transferred. • Then the particles of the pot collide with the particles in the water, which collide with the particles at one end of the spoon. • As the particles move faster, the metal spoon gets hotter. This process of conduction is repeated all along the metal until the entire spoon is hot. Example of Conduction

EXAMPLE OF CONDUCTION • A piece of cheese melts as heat is transferred from the meat to the cheese (Contact)

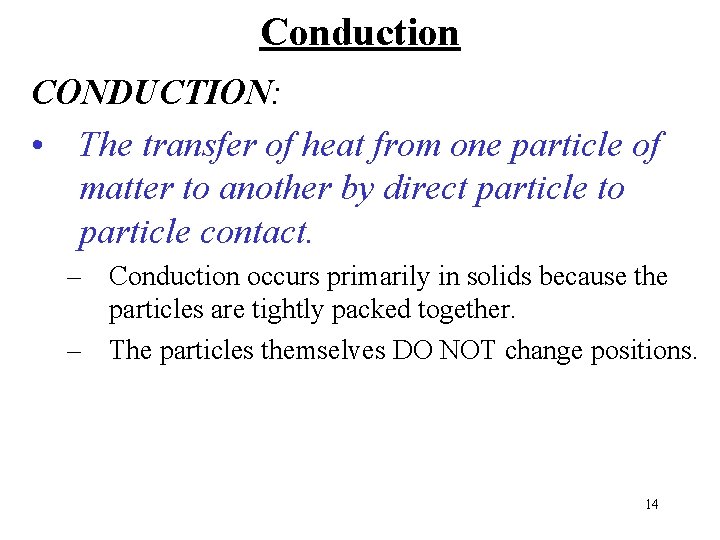
Conduction CONDUCTION: • The transfer of heat from one particle of matter to another by direct particle to particle contact. – Conduction occurs primarily in solids because the particles are tightly packed together. – The particles themselves DO NOT change positions. 14

Conduction Example: A metal spoon in a pot of water being heated on an electric stove. a. First, the electrical energy is converted to thermal energy by the stove. b. The rapidly vibrating particles of the hot electric coil collide with the particles of the cool pot. c. Heat energy is transferred, causing the particles in the pot to vibrate faster. 15

Conduction d. The rapidly vibrating particles of the pot now collide with the particles of the water at the bottom of the pot. e. The water particles absorb energy and vibrate and flow more rapidly and its temperature increases. f. Now, the energetic (hot) particles of water collide with the particles of the submerged end of the spoon. g. As the particles of the spoon absorb energy and vibrate more rapidly. The temperature of the spoon increases. 16

Conduction h. As the particles at this end of the spoon absorb energy and vibrate faster they collide with other particles in the spoon. As they collide, energy is transferred to the other particles (similar to momentum) and they begin to vibrate more rapidly. i. This process of conduction is repeated all along the metal spoon until the entire metal spoon becomes hot. 17

Conduction Brainstorming: What are other examples of conduction? Application: Describe the process of conduction when you place a hot spoon into a bowl of ice cream. 18

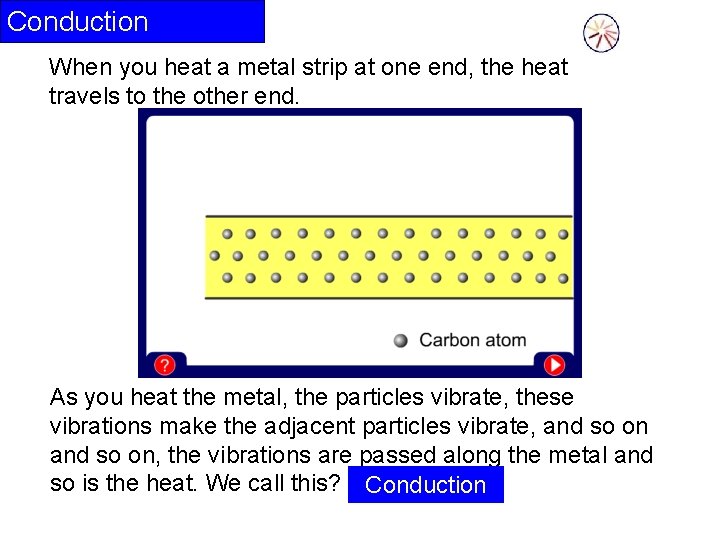
Conduction When you heat a metal strip at one end, the heat travels to the other end. As you heat the metal, the particles vibrate, these vibrations make the adjacent particles vibrate, and so on, the vibrations are passed along the metal and so is the heat. We call this? Conduction

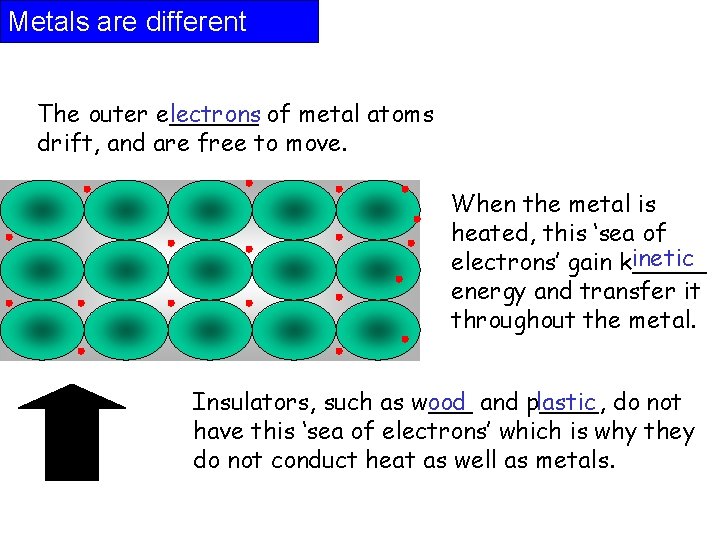
Metals are different The outer e______ lectrons of metal atoms drift, and are free to move. When the metal is heated, this ‘sea of inetic electrons’ gain k_____ energy and transfer it throughout the metal. Insulators, such as w___ ood and p____, lastic do not have this ‘sea of electrons’ which is why they do not conduct heat as well as metals.

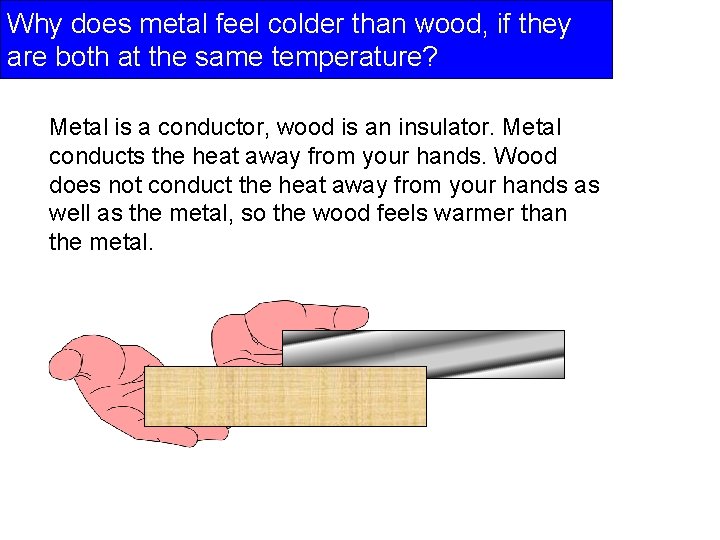
Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. Metal conducts the heat away from your hands. Wood does not conduct the heat away from your hands as well as the metal, so the wood feels warmer than the metal.

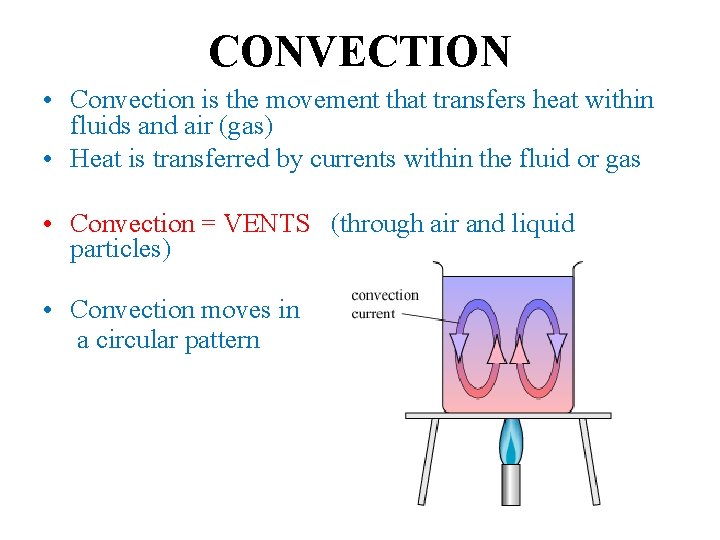
CONVECTION • Convection is the movement that transfers heat within fluids and air (gas) • Heat is transferred by currents within the fluid or gas • Convection = VENTS (through air and liquid particles) • Convection moves in a circular pattern

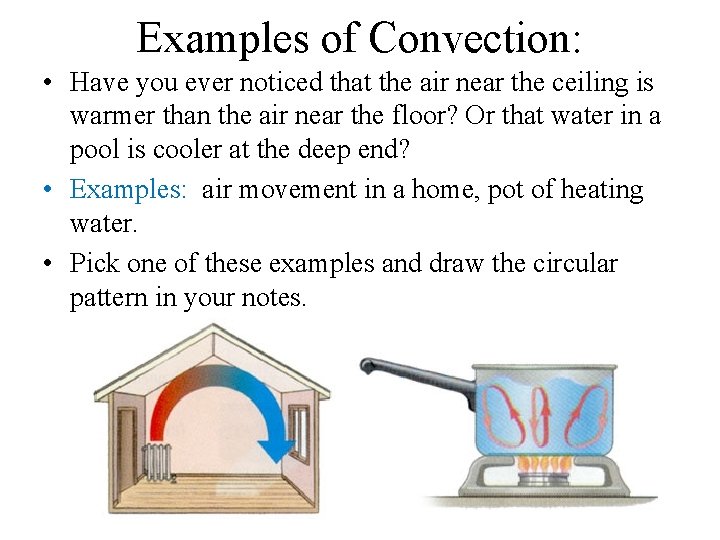
Examples of Convection: • Have you ever noticed that the air near the ceiling is warmer than the air near the floor? Or that water in a pool is cooler at the deep end? • Examples: air movement in a home, pot of heating water. • Pick one of these examples and draw the circular pattern in your notes.

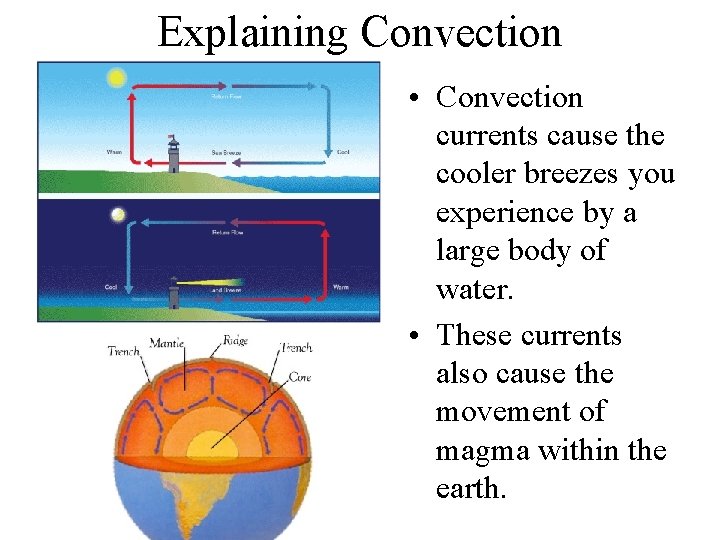
Explaining Convection • Convection currents cause the cooler breezes you experience by a large body of water. • These currents also cause the movement of magma within the earth.

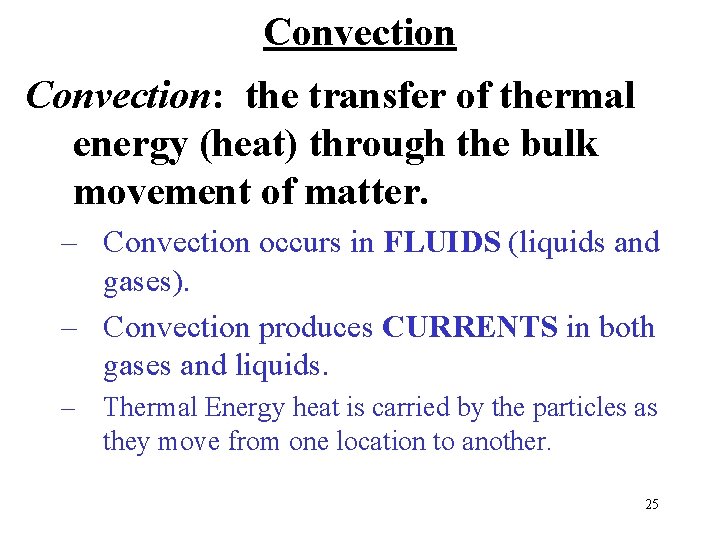
Convection: the transfer of thermal energy (heat) through the bulk movement of matter. – Convection occurs in FLUIDS (liquids and gases). – Convection produces CURRENTS in both gases and liquids. – Thermal Energy heat is carried by the particles as they move from one location to another. 25

Convection Example: Heating water: a. When the water at the bottom of the pot (nearest the burner) is heated, the particles absorb energy by conduction as they touch the hot pot. b. The water particles vibrate more rapidly. c. The particles also move farther apart and the hot water becomes less dense than the surrounding cool water. d. This causes the heated (hot) water to rise. 26

Convection e. The surrounding denser cooler water is forced downward near the burner by the rising hot water. f. This process continues to repeat. g. This FLOW creates a circular motion known as a convection current. Application: How do convection currents form in a room when the heater is turned on? 27

Convection • The warm air from the heater vent will rise. Why? , – The warm air is less dense than the surrounding cooler air. • The cool air is pushed down by the rising warm air. What is the best location for a heat vent in a room and why? Near the ceiling or the floor? Floor: Because the warm air will rise to the ceiling. How about the return vent? 28

Convection currents occur in the environment as well. They produce: – Global winds that contribute to Earth’s weather. – Ocean and lake currents 29

Convection Brainstorming: On a hot summer day the breeze near the beach blows toward the water. However, later in the day the breeze reverses direction and blows toward land will get increasingly stronger. Why? 30

Convection Answer: In the morning the water may be warmer than the sand causing the air over the water to rise. In the afternoon, the sand has become much hotter than the water and the air above it rises. The air over the water rushes in to fill its void causing a wind. 31

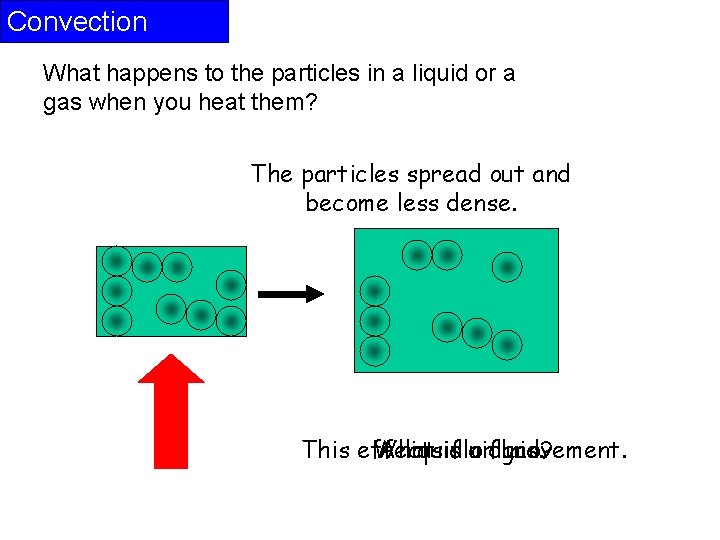
Convection What happens to the particles in a liquid or a gas when you heat them? The particles spread out and become less dense. This effects What A liquid isfluid aorfluid? gas. movement.

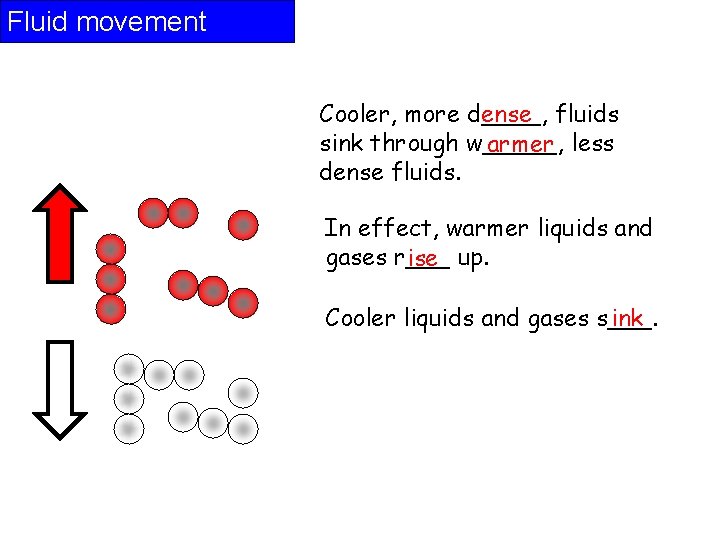
Fluid movement Cooler, more d____, ense fluids sink through w_____, armer less dense fluids. In effect, warmer liquids and gases r___ ise up. Cooler liquids and gases s___. ink

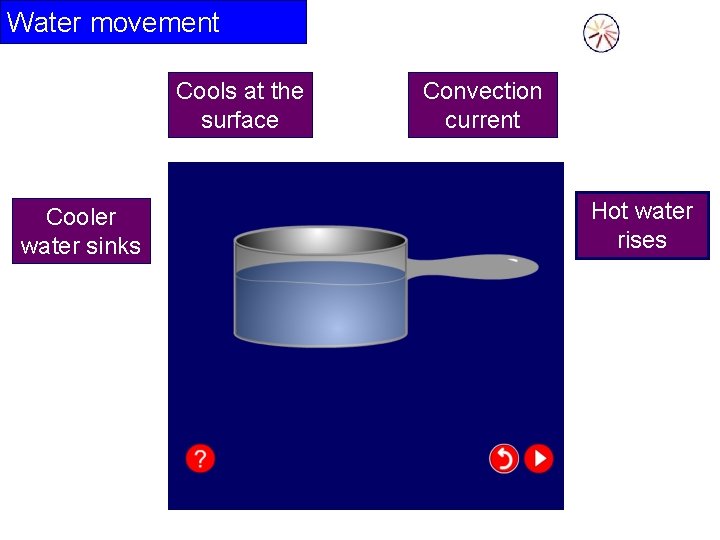
Water movement Cools at the surface Cooler water sinks Convection current Hot water rises

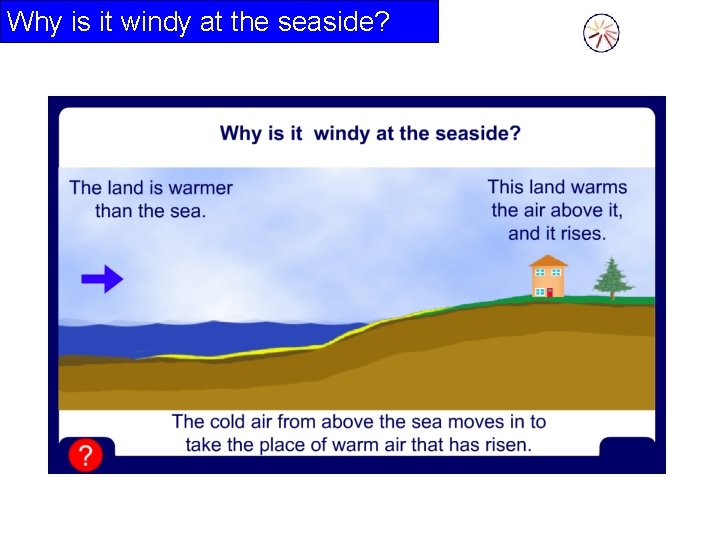
Why is it windy at the seaside?

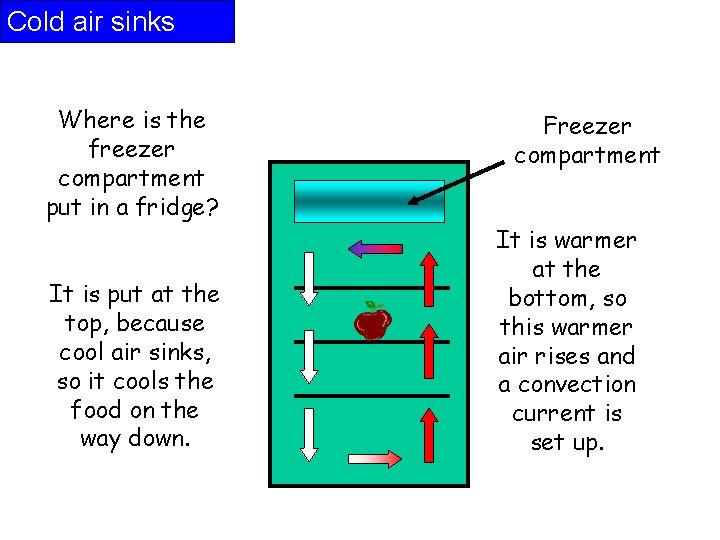
Cold air sinks Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up.

RADIATION • Radiation is the transfer of energy by electromagnetic waves • Radiation does NOT require matter to transfer thermal energy • Radiation = Radiates (heat escaping the sun)

Radiation May Come From Other Sources Have you ever sat too close to a campfire while cooking marshmallows? You’re enjoying the warmth …. . only to notice that your skin is really warm?

Examples of RADIATION 1. Fire 2. Heat Lamps 3. Sun

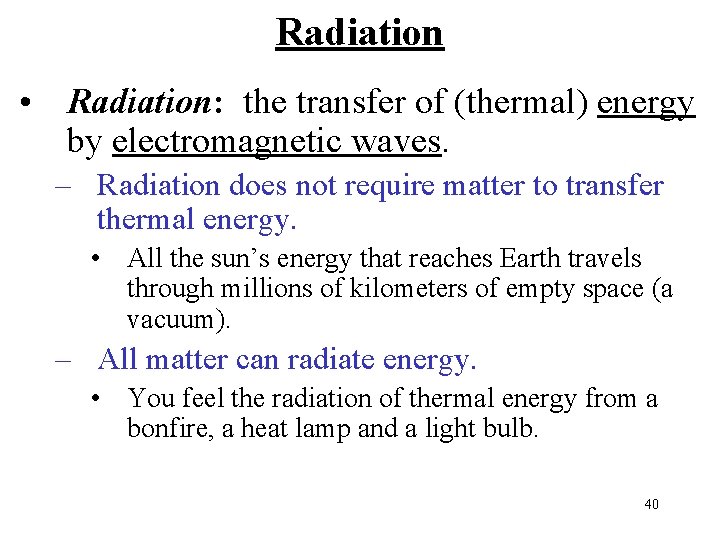
Radiation • Radiation: the transfer of (thermal) energy by electromagnetic waves. – Radiation does not require matter to transfer thermal energy. • All the sun’s energy that reaches Earth travels through millions of kilometers of empty space (a vacuum). – All matter can radiate energy. • You feel the radiation of thermal energy from a bonfire, a heat lamp and a light bulb. 40

Radiation • Other examples of the transfer of heat by Radiation: a. Charcoal grill. b. Hot tin roof. c. Burner on a stove top. d. ? e. ? 41

Radiation Key Point: For radiation to be felt as heat it must first be absorbed by a material. Example: Why do blue jeans feel hotter in the sun than a yellow shirt, even though they are both exposed to the same amount of sunlight? – The blue jean fabric absorbs more radiant energy from the sun than the yellow shirt because of its dark color. 42

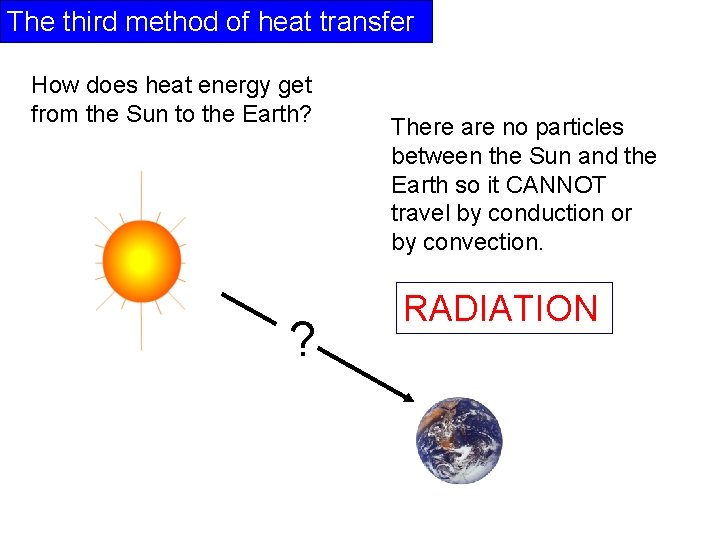
The third method of heat transfer How does heat energy get from the Sun to the Earth? ? There are no particles between the Sun and the Earth so it CANNOT travel by conduction or by convection. RADIATION

Radiation travels in straight lines True/False Radiation can travel through a vacuum True/False Radiation requires particles to travel True/False Radiation travels at the speed of light True/False

Conduction, Convection & Radiation 45

Energy from the Sun 46

Convection, Conduction & Radiation 47

Emission experiment Four containers were filled with warm water. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black shiny metal container would be the warmest after ten The _____ radiation back minutes because its shiny surface reflects heat _______ dull black container into the container so less is lost. The ____ emitting heat would be the coolest because it is the best at _______ radiation.

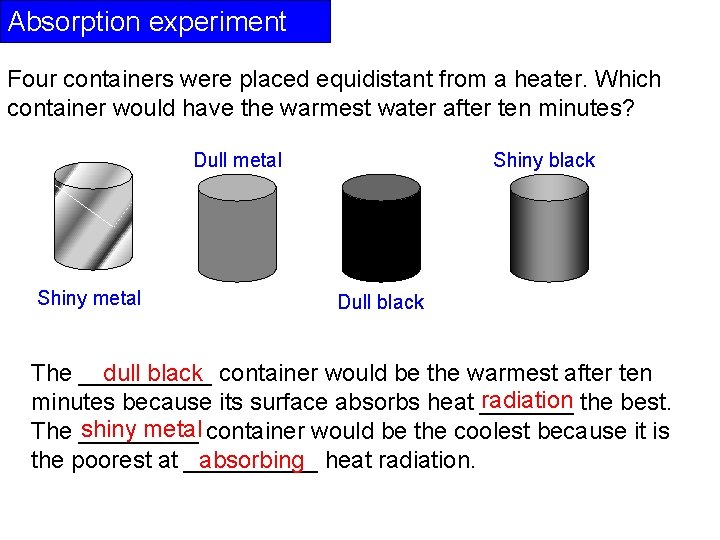
Absorption experiment Four containers were placed equidistant from a heater. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black dull black container would be the warmest after ten The _____ radiation the best. minutes because its surface absorbs heat _______ shiny metal container would be the coolest because it is The _____ the poorest at _____ absorbing heat radiation.

Convection questions Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air. Why are boilers placed beneath hot water tanks in people’s homes? Hot water rises. So when the boiler heats the water, and the hot water rises, the water tank is filled with hot water.

Radiation questions Why are houses painted white in hot countries? White reflects heat radiation and keeps the house cooler. Why are shiny foil blankets wrapped around marathon runners at the end of a race? The shiny metal reflects the heat radiation from the runner back in, this stops the runner getting cold.

The Nature of Heat What happens when you put ice in a warm soft drink? – The heat energy moves from the soft drink into the ice by conduction (particle to particle contact) causing the ice to melt. 52

Review Describe three kinds of heat transfer. a. Conduction – transfer of heat energy from one particle to another by direct contact. (Primarily in solids) b. Convection – transfer of heat energy in fluids-gases and liquids) through the bulk movement of matter from one place to another. (Produces currents) c. Radiation – transfer of energy through electromagnetic waves. (Matter is not required!) (Radiant & infrared radiation from the sun) 53

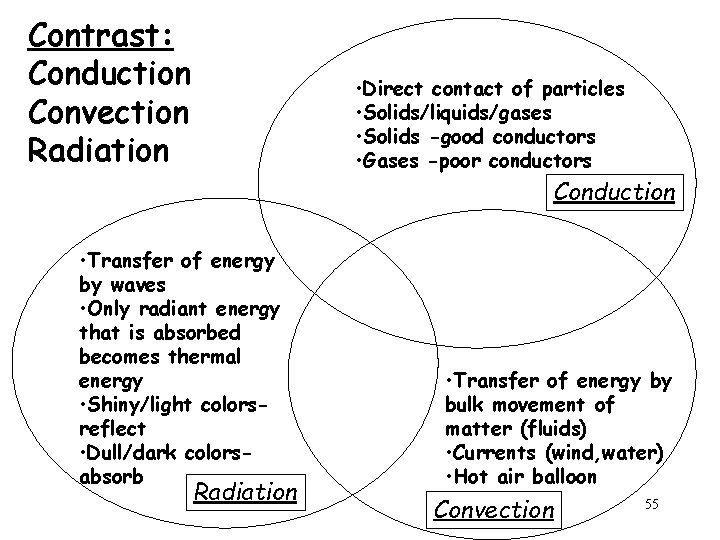
Conduction • Direct contact of particles • Solids/liquids/g ases • The handle of a cooking utensil Radiation • Transfer of energy by waves • Only radiant energy that is absorbed becomes thermal energy • Lightbulb • Fireplace Convection • Transfer of energy by bulk movement of matter (fluids) • Currents (wind, water) • Hot air balloon 54

Contrast: Conduction Convection Radiation • Direct contact of particles • Solids/liquids/gases • Solids -good conductors • Gases -poor conductors Conduction • Transfer of energy by waves • Only radiant energy that is absorbed becomes thermal energy • Shiny/light colorsreflect • Dull/dark colorsabsorb Radiation • Transfer of energy by bulk movement of matter (fluids) • Currents (wind, water) • Hot air balloon Convection 55

1. Which of the following is not a method of heat transfer? A. Radiation B. Insulation C. Conduction D. Convection

2. In which of the following are the particles closest together? A. Solid B. Liquid C. Gas D. Fluid

3. How does heat energy reach the Earth from the Sun? A. Radiation B. Conduction C. Convection D. Insulation

4. Which is the best surface for reflecting heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black

5. Which is the best surface for absorbing heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black