Chemistry A Molecular Approach 2 nd Ed Nivaldo
































- Slides: 191

Chemistry: A Molecular Approach, 2 nd Ed. Nivaldo Tro Chapter 11 Liquids, Solids, and Intermolecular Forces Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Copyright 2011 Pearson Education, Inc.

Climbing Geckos • Geckos can adhere to almost any surface • Recent studies indicate that this amazing ability • is related to intermolecular attractive forces Geckos have millions of tiny hairs on their feet that branch out and flatten out on the end ü setae = hairs, spatulae = flat ends • This structure allows the gecko to have unusually close contact to the surface – allowing the intermolecular attractive forces to act strongly Tro: Chemistry: A Molecular Approach, 2/e 2 Copyright 2011 Pearson Education, Inc.

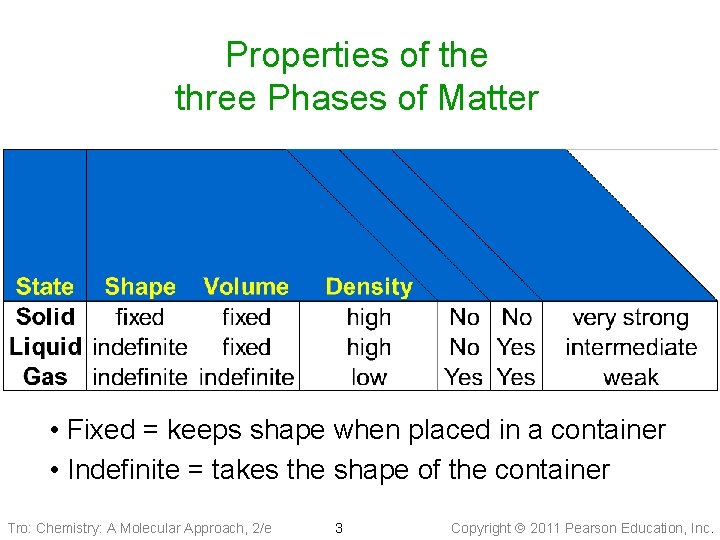
Properties of the three Phases of Matter • Fixed = keeps shape when placed in a container • Indefinite = takes the shape of the container Tro: Chemistry: A Molecular Approach, 2/e 3 Copyright 2011 Pearson Education, Inc.

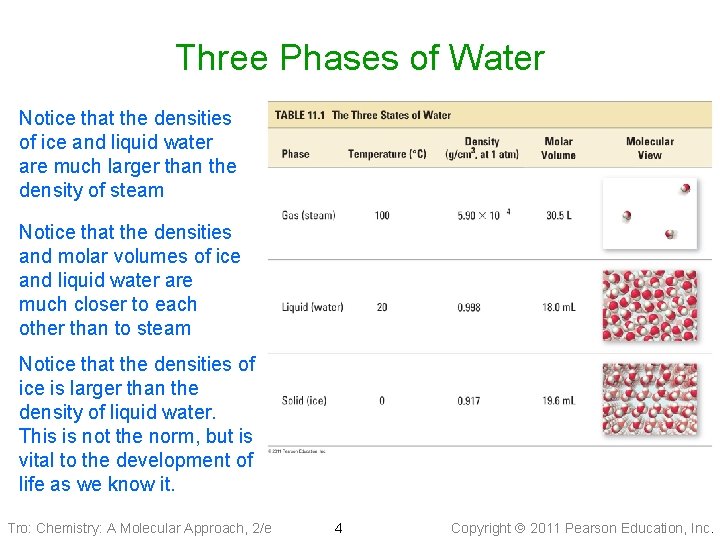
Three Phases of Water Notice that the densities of ice and liquid water are much larger than the density of steam Notice that the densities and molar volumes of ice and liquid water are much closer to each other than to steam Notice that the densities of ice is larger than the density of liquid water. This is not the norm, but is vital to the development of life as we know it. Tro: Chemistry: A Molecular Approach, 2/e 4 Copyright 2011 Pearson Education, Inc.

Degrees of Freedom • Particles may have one or several types of freedom of motion ü and various degrees of each type • Translational freedom is the ability to move • • from one position in space to another Rotational freedom is the ability to reorient the particles direction in space Vibrational freedom is the ability to oscillate about a particular point in space Tro: Chemistry: A Molecular Approach, 2/e 5 Copyright 2011 Pearson Education, Inc.

Solids • The particles in a solid are packed close together and are fixed in position ü though they may vibrate • The close packing of the particles • results in solids being incompressible The inability of the particles to move around results in solids retaining their shape and volume when placed in a new container, and prevents the solid from flowing Tro: Chemistry: A Molecular Approach, 2/e 6 Copyright 2011 Pearson Education, Inc.

Solids • Some solids have their particles arranged in an orderly geometric pattern – we call these crystalline solids ü salt and diamonds • Other solids have particles that do not show a regular geometric pattern over a long range – we call these amorphous solids ü plastic and glass Tro: Chemistry: A Molecular Approach, 2/e 7 Copyright 2011 Pearson Education, Inc.

Liquids • The particles in a liquid are • • closely packed, but they have some ability to move around The close packing results in liquids being incompressible But the ability of the particles to move allows liquids to take the shape of their container and to flow – however, they don’t have enough freedom to escape or expand to fill the container Tro: Chemistry: A Molecular Approach, 2/e 8 Copyright 2011 Pearson Education, Inc.

Gases • In the gas state, the particles have • complete freedom of motion and are not held together The particles are constantly flying around, bumping into each other and the container • There is a large amount of space between the particles ü compared to the size of the particles ü therefore the molar volume of the gas state of a material is much larger than the molar volume of the solid or liquid states Tro: Chemistry: A Molecular Approach, 2/e 9 Copyright 2011 Pearson Education, Inc.

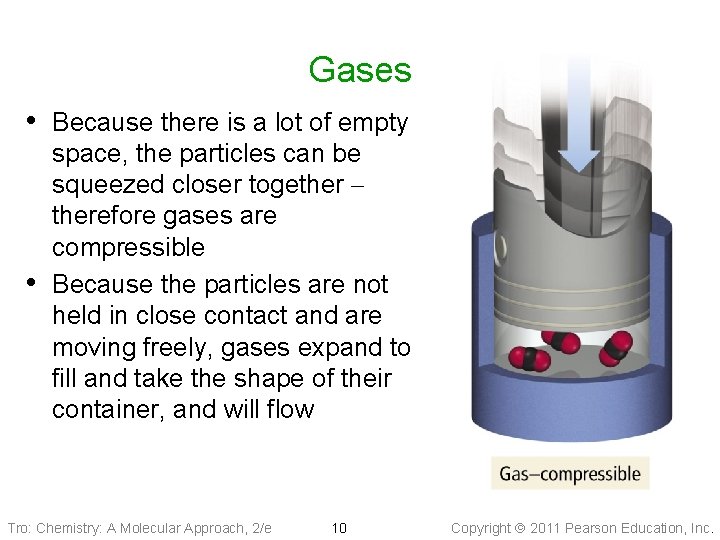
Gases • Because there is a lot of empty • space, the particles can be squeezed closer together – therefore gases are compressible Because the particles are not held in close contact and are moving freely, gases expand to fill and take the shape of their container, and will flow Tro: Chemistry: A Molecular Approach, 2/e 10 Copyright 2011 Pearson Education, Inc.

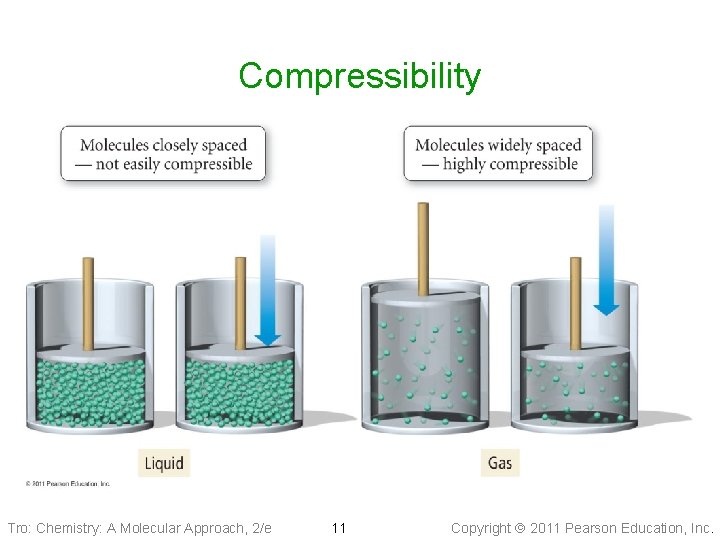
Compressibility Tro: Chemistry: A Molecular Approach, 2/e 11 Copyright 2011 Pearson Education, Inc.

Kinetic – Molecular Theory • What state a material is in depends largely on two major factors 1. the amount of kinetic energy the particles possess 2. the strength of attraction between the particles • These two factors are in competition with each other Tro: Chemistry: A Molecular Approach, 2/e 12 Copyright 2011 Pearson Education, Inc.

States and Degrees of Freedom • The molecules in a gas have complete freedom of motion ü their kinetic energy overcomes the attractive forces between the molecules • The molecules in a solid are locked in place, they cannot move around ü though they do vibrate, they don’t have enough kinetic energy to overcome the attractive forces • The molecules in a liquid have limited freedom – they can move around a little within the structure of the liquid ü they have enough kinetic energy to overcome some of the attractive forces, but not enough to escape each other Tro: Chemistry: A Molecular Approach, 2/e 13 Copyright 2011 Pearson Education, Inc.

Kinetic Energy • Increasing kinetic energy increases the • • motion energy of the particles The more motion energy the molecules have, the more freedom they can have The average kinetic energy is directly proportional to the temperature ü KEavg = 1. 5 k. T Tro: Chemistry: A Molecular Approach, 2/e 14 Copyright 2011 Pearson Education, Inc.

Attractive Forces • The particles are attracted to each other by • • • electrostatic forces The strength of the attractive forces varies, some are small and some are large The strength of the attractive forces depends on the kind(s) of particles The stronger the attractive forces between the particles, the more they resist moving ü though no material completely lacks particle motion Tro: Chemistry: A Molecular Approach, 2/e 15 Copyright 2011 Pearson Education, Inc.

Kinetic–Molecular Theory of Gases • When the kinetic energy is so large it overcomes • • the attractions between particles, the material will be a gas In an ideal gas, the particles have complete freedom of motion – especially translational This allows gas particles to expand to fill their container ü gases flow • It also leads to there being large spaces between the particles ü therefore low density and compressibility Tro: Chemistry: A Molecular Approach, 2/e 16 Copyright 2011 Pearson Education, Inc.

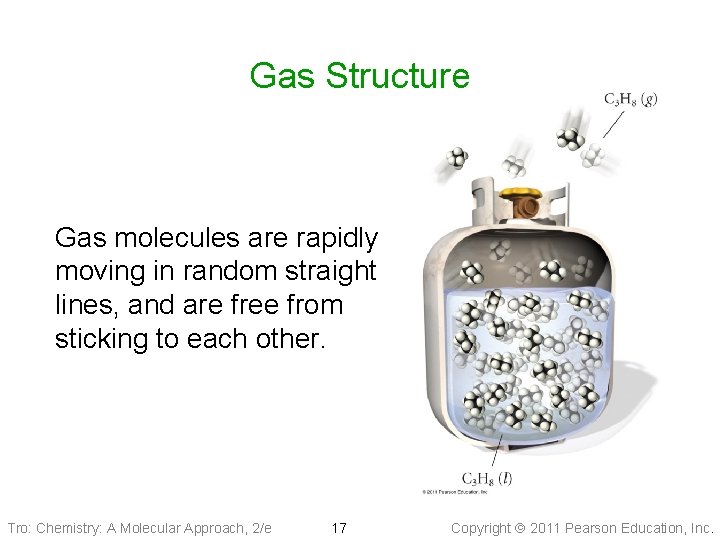
Gas Structure Gas molecules are rapidly moving in random straight lines, and are free from sticking to each other. Tro: Chemistry: A Molecular Approach, 2/e 17 Copyright 2011 Pearson Education, Inc.

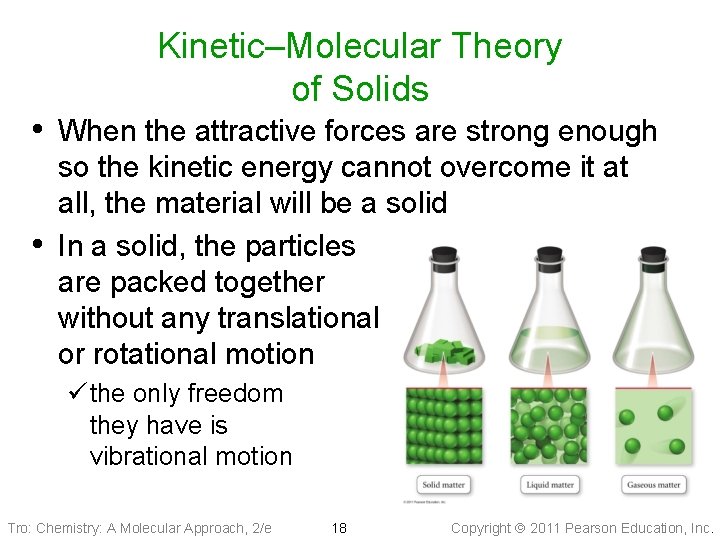
Kinetic–Molecular Theory of Solids • When the attractive forces are strong enough • so the kinetic energy cannot overcome it at all, the material will be a solid In a solid, the particles are packed together without any translational or rotational motion ü the only freedom they have is vibrational motion Tro: Chemistry: A Molecular Approach, 2/e 18 Copyright 2011 Pearson Education, Inc.

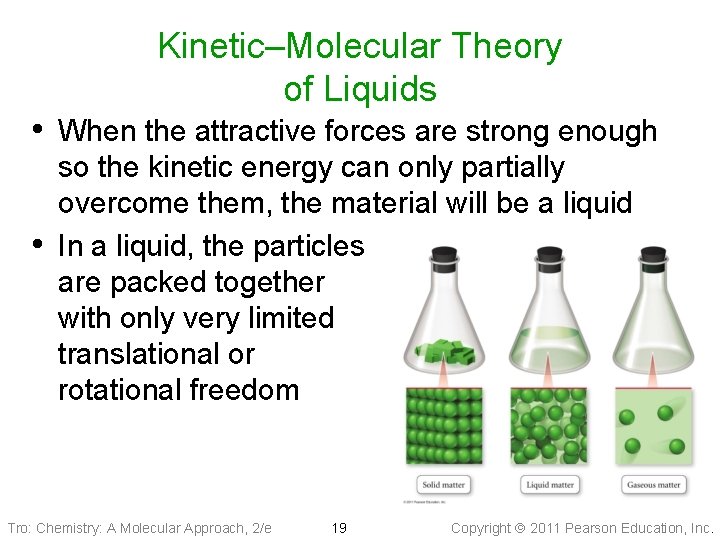
Kinetic–Molecular Theory of Liquids • When the attractive forces are strong enough • so the kinetic energy can only partially overcome them, the material will be a liquid In a liquid, the particles are packed together with only very limited translational or rotational freedom Tro: Chemistry: A Molecular Approach, 2/e 19 Copyright 2011 Pearson Education, Inc.

Explaining the Properties of Liquids • Liquids have higher densities than gases and are • • • incompressible because the particles are in contact They have an indefinite shape because the limited translational freedom of the particles allows them to move around enough to get to the container walls It also allows them to flow But they have a definite volume because the limit on their freedom keeps the particles from escaping each other Tro: Chemistry: A Molecular Approach, 2/e 20 Copyright 2011 Pearson Education, Inc.

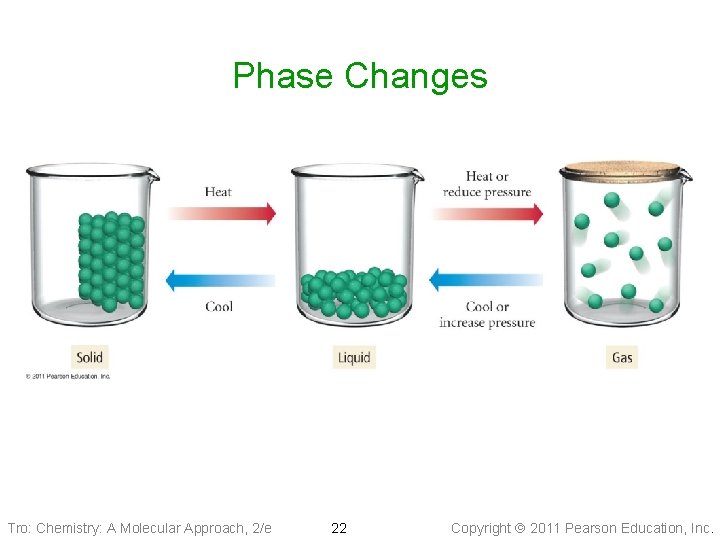
Phase Changes • Because the attractive forces between the molecules are • • fixed, changing the material’s state requires changing the amount of kinetic energy the particles have, or limiting their freedom Solids melt when heated because the particles gain enough kinetic energy to partially overcome the attactive forces Liquids boil when heated because the particles gain enough kinetic energy to completely overcome the attractive forces ü the stronger the attractive forces, the higher you will need to raise the temperature • Gases can be condensed by decreasing the temperature and/or increasing the pressure ü pressure can be increased by decreasing the gas volume ü reducing the volume reduces the amount of translational freedom the particles have Tro: Chemistry: A Molecular Approach, 2/e 21 Copyright 2011 Pearson Education, Inc.

Phase Changes Tro: Chemistry: A Molecular Approach, 2/e 22 Copyright 2011 Pearson Education, Inc.

Intermolecular Attractions • The strength of the attractions between the • • particles of a substance determines its state At room temperature, moderate to strong attractive forces result in materials being solids or liquids The stronger the attractive forces are, the higher will be the boiling point of the liquid and the melting point of the solid ü other factors also influence the melting point Tro: Chemistry: A Molecular Approach, 2/e 23 Copyright 2011 Pearson Education, Inc.

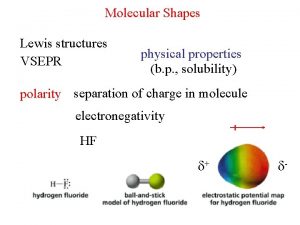
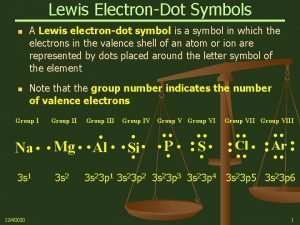
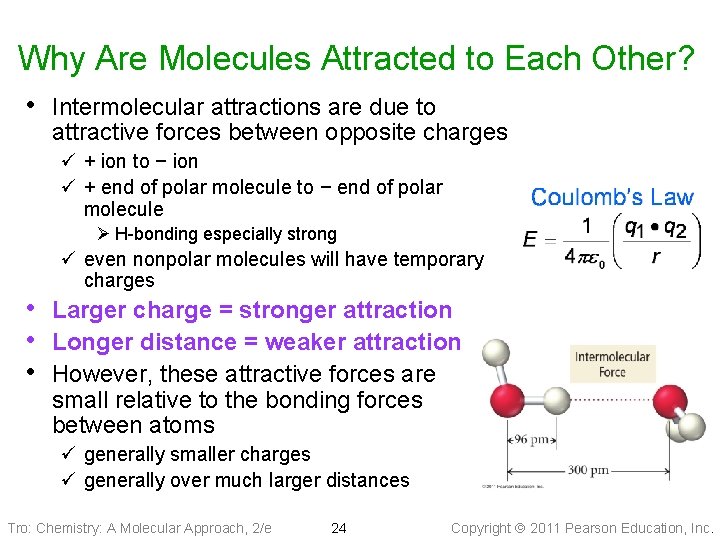
Why Are Molecules Attracted to Each Other? • Intermolecular attractions are due to attractive forces between opposite charges ü + ion to − ion ü + end of polar molecule to − end of polar molecule Ø H-bonding especially strong ü even nonpolar molecules will have temporary charges • Larger charge = stronger attraction • Longer distance = weaker attraction • However, these attractive forces are small relative to the bonding forces between atoms ü generally smaller charges ü generally over much larger distances Tro: Chemistry: A Molecular Approach, 2/e 24 Copyright 2011 Pearson Education, Inc.

Trends in the Strength of Intermolecular Attraction • The stronger the attractions between the atoms or • molecules, the more energy it will take to separate them Boiling a liquid requires we add enough energy to overcome all the attractions between the particles ü However, not breaking the covalent bonds • The higher the normal boiling point of the liquid, the stronger the intermolecular attractive forces Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc.

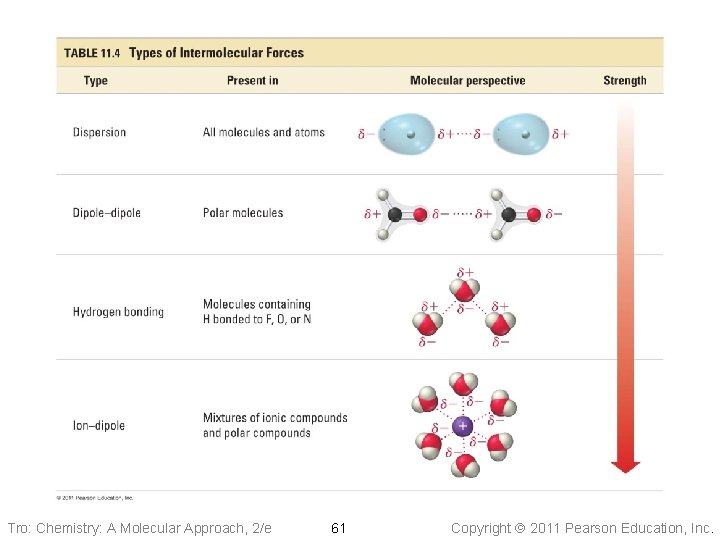
Kinds of Attractive Forces • Temporary polarity in the molecules due to • • unequal electron distribution leads to attractions called dispersion forces Permanent polarity in the molecules due to their structure leads to attractive forces called dipole–dipole attractions An especially strong dipole–dipole attraction results when H is attached to an extremely electronegative atom. These are called hydrogen bonds. Tro: Chemistry: A Molecular Approach, 2/e 26 Copyright 2011 Pearson Education, Inc.

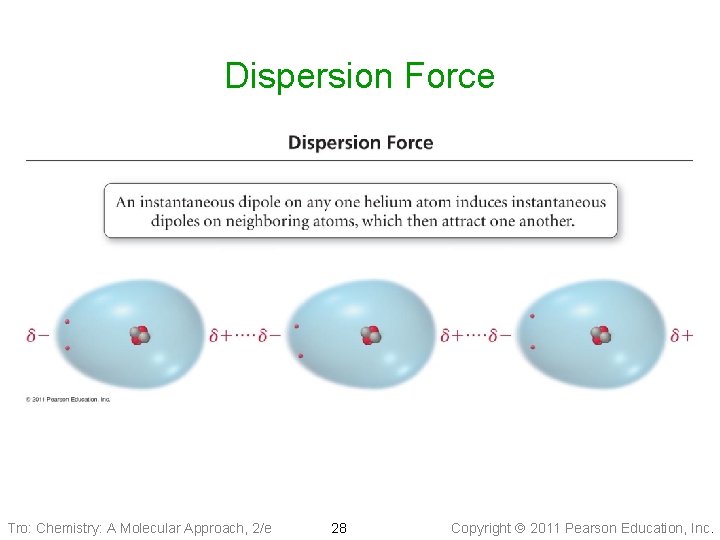
Dispersion Forces • Fluctuations in the electron distribution in atoms and molecules result in a temporary dipole ü region with excess electron density has partial (─) charge ü region with depleted electron density has partial (+) charge • The attractive forces caused by these temporary dipoles are called dispersion forces ü aka London Forces • All molecules and atoms will have them • As a temporary dipole is established in one molecule, it induces a dipole in all the surrounding molecules Tro: Chemistry: A Molecular Approach, 2/e 27 Copyright 2011 Pearson Education, Inc.

Dispersion Force Tro: Chemistry: A Molecular Approach, 2/e 28 Copyright 2011 Pearson Education, Inc.

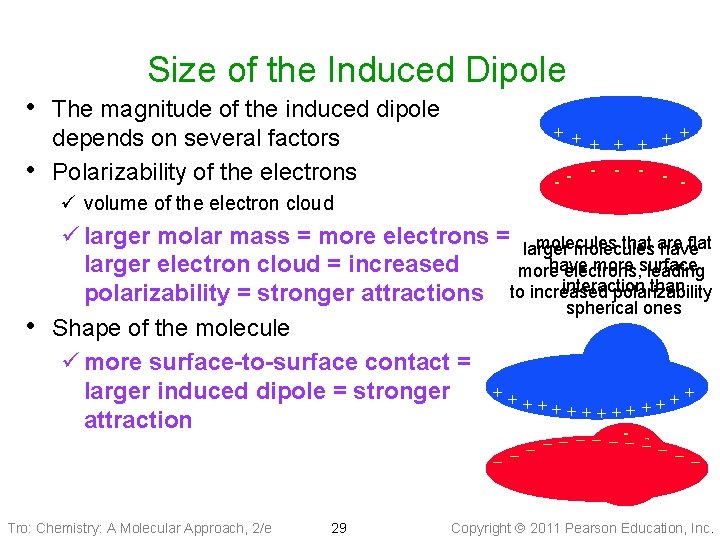
Size of the Induced Dipole • The magnitude of the induced dipole • depends on several factors Polarizability of the electrons + + + + - -- ü volume of the electron cloud ü larger molar mass = more electrons = larger molecules that are flat molecules have more surface larger electron cloud = increased more electrons, leading interaction than polarizability = stronger attractions to increased spherical ones • Shape of the molecule ü more surface-to-surface contact = + + ++ + larger induced dipole = stronger + + + + ++ + + attraction − −- − −− − Tro: Chemistry: A Molecular Approach, 2/e 29 Copyright 2011 Pearson Education, Inc.

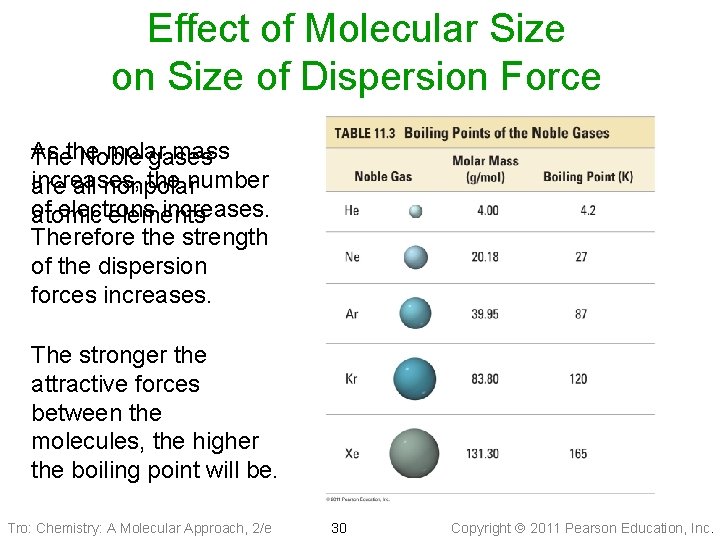
Effect of Molecular Size on Size of Dispersion Force As molar mass Thethe Noble gases increases, the number are all nonpolar of electrons increases. atomic elements Therefore the strength of the dispersion forces increases. The stronger the attractive forces between the molecules, the higher the boiling point will be. Tro: Chemistry: A Molecular Approach, 2/e 30 Copyright 2011 Pearson Education, Inc.

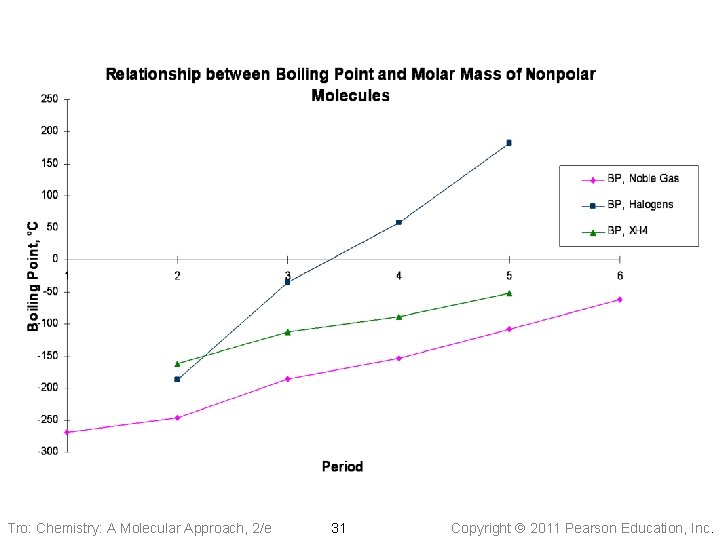
Tro: Chemistry: A Molecular Approach, 2/e 31 Copyright 2011 Pearson Education, Inc.

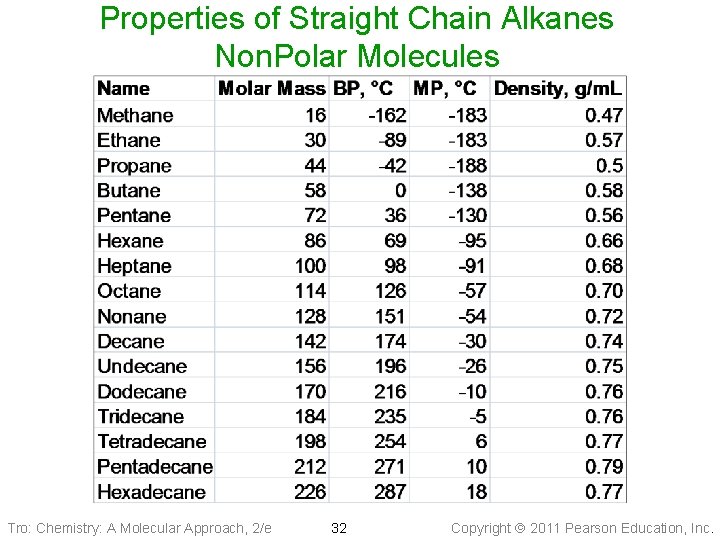
Properties of Straight Chain Alkanes Non. Polar Molecules Tro: Chemistry: A Molecular Approach, 2/e 32 Copyright 2011 Pearson Education, Inc.

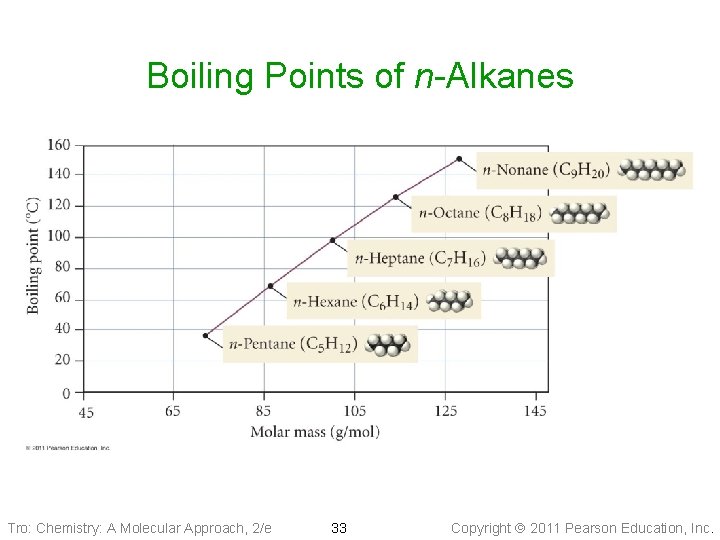
Boiling Points of n-Alkanes Tro: Chemistry: A Molecular Approach, 2/e 33 Copyright 2011 Pearson Education, Inc.

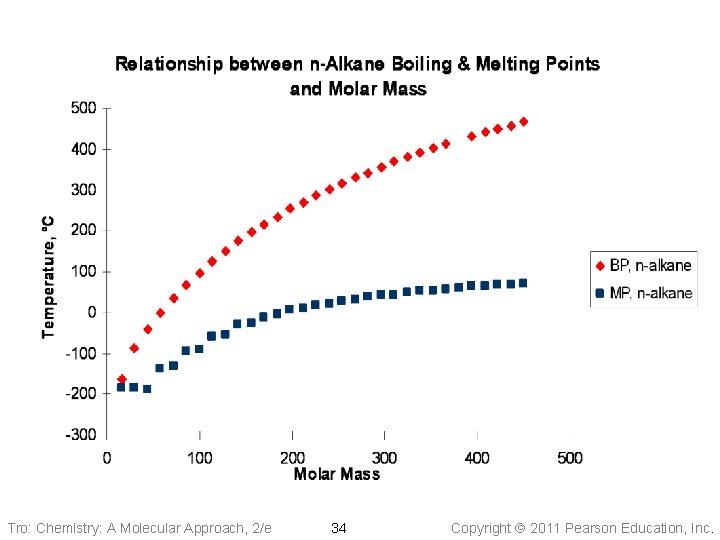
Tro: Chemistry: A Molecular Approach, 2/e 34 Copyright 2011 Pearson Education, Inc.

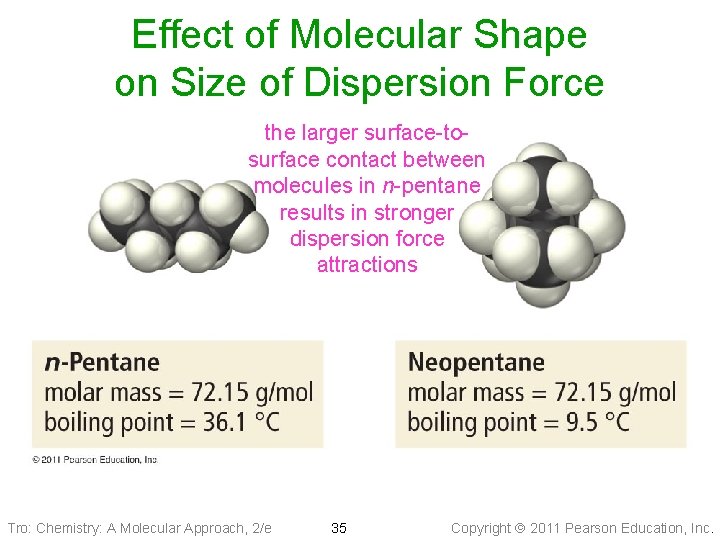
Effect of Molecular Shape on Size of Dispersion Force the larger surface-tosurface contact between molecules in n-pentane results in stronger dispersion force attractions Tro: Chemistry: A Molecular Approach, 2/e 35 Copyright 2011 Pearson Education, Inc.

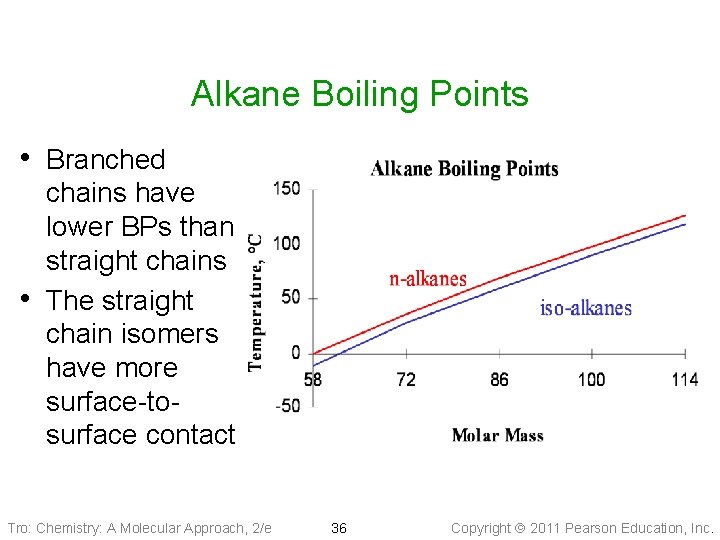
Alkane Boiling Points • Branched • chains have lower BPs than straight chains The straight chain isomers have more surface-tosurface contact Tro: Chemistry: A Molecular Approach, 2/e 36 Copyright 2011 Pearson Education, Inc.

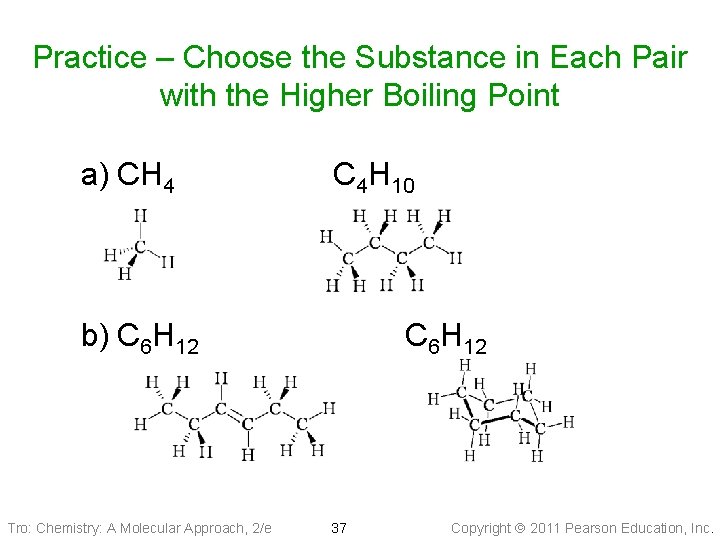
Practice – Choose the Substance in Each Pair with the Higher Boiling Point a) CH 4 C 4 H 10 b) C 6 H 12 Tro: Chemistry: A Molecular Approach, 2/e C 6 H 12 37 Copyright 2011 Pearson Education, Inc.

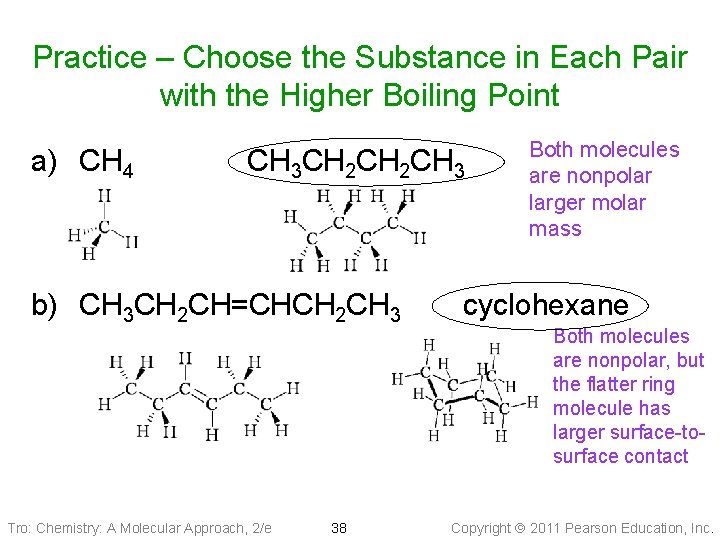
Practice – Choose the Substance in Each Pair with the Higher Boiling Point a) CH 4 CH 3 CH 2 CH 3 b) CH 3 CH 2 CH=CHCH 2 CH 3 Tro: Chemistry: A Molecular Approach, 2/e 38 Both molecules are nonpolar larger molar mass cyclohexane Both molecules are nonpolar, but the flatter ring molecule has larger surface-tosurface contact Copyright 2011 Pearson Education, Inc.

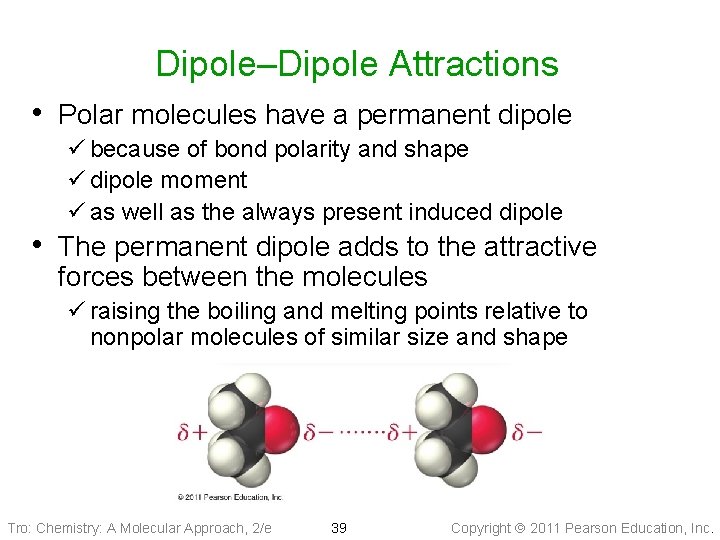
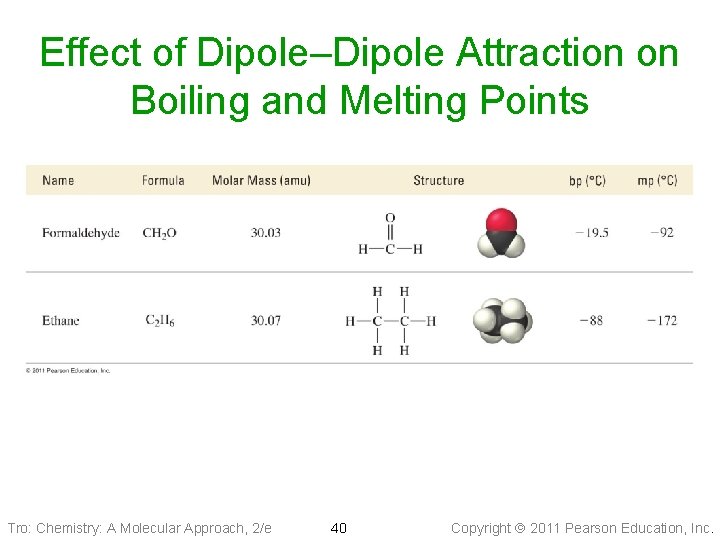
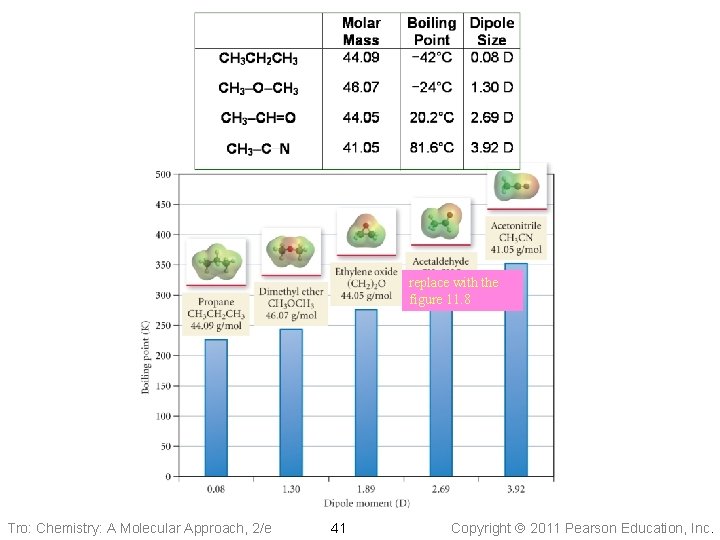
Dipole–Dipole Attractions • Polar molecules have a permanent dipole ü because of bond polarity and shape ü dipole moment ü as well as the always present induced dipole • The permanent dipole adds to the attractive forces between the molecules ü raising the boiling and melting points relative to nonpolar molecules of similar size and shape Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc.

Effect of Dipole–Dipole Attraction on Boiling and Melting Points Tro: Chemistry: A Molecular Approach, 2/e 40 Copyright 2011 Pearson Education, Inc.

replace with the figure 11. 8 Tro: Chemistry: A Molecular Approach, 2/e 41 Copyright 2011 Pearson Education, Inc.

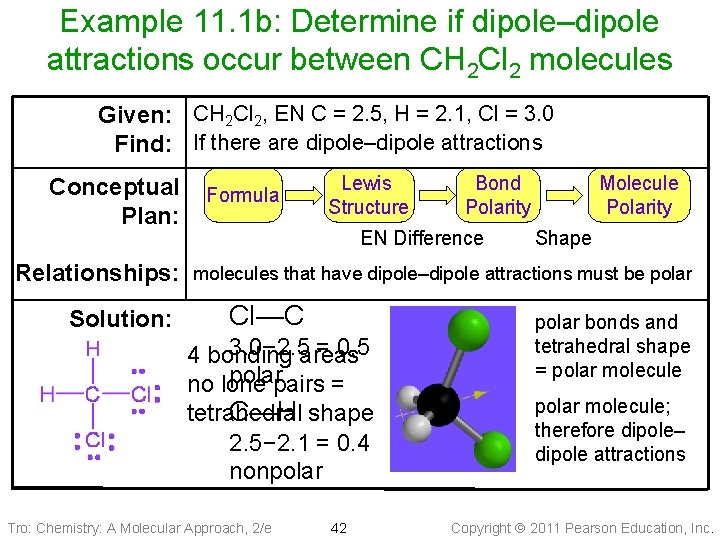
Example 11. 1 b: Determine if dipole–dipole attractions occur between CH 2 Cl 2 molecules Given: CH 2 Cl 2, EN C = 2. 5, H = 2. 1, Cl = 3. 0 Find: If there are dipole–dipole attractions Conceptual Plan: Relationships: Solution: Formula Lewis Structure Bond Polarity EN Difference Molecule Polarity Shape molecules that have dipole–dipole attractions must be polar Cl—C 3. 0− 2. 5 areas = 0. 5 4 bonding polarpairs = no lone tetrahedral C—H shape 2. 5− 2. 1 = 0. 4 nonpolar Tro: Chemistry: A Molecular Approach, 2/e 42 polar bonds and tetrahedral shape = polar molecule; therefore dipole– dipole attractions Copyright 2011 Pearson Education, Inc.

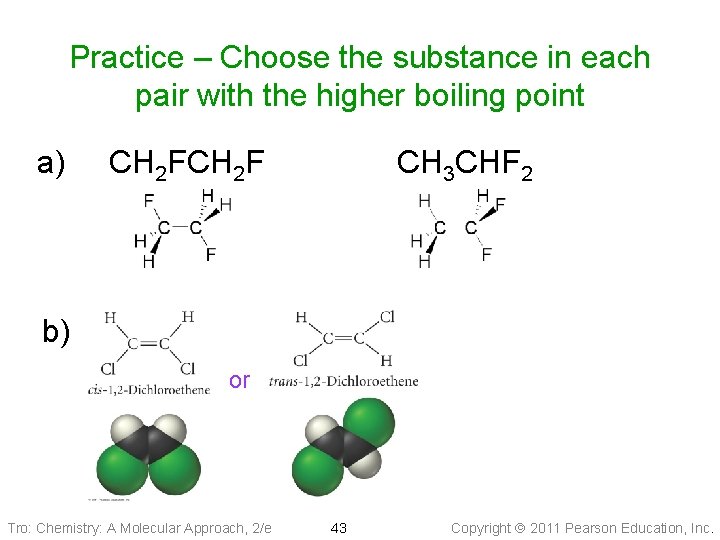
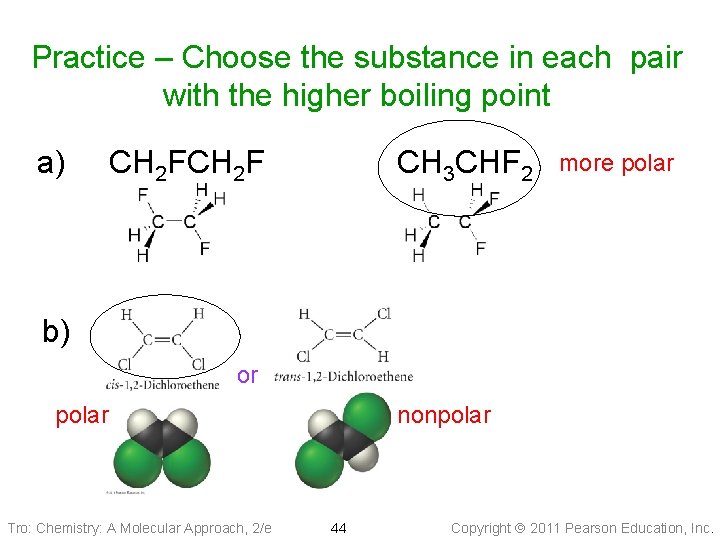
Practice – Choose the substance in each pair with the higher boiling point a) CH 2 F CH 3 CHF 2 b) or Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc.

Practice – Choose the substance in each pair with the higher boiling point a) CH 2 F CH 3 CHF 2 more polar b) or polar Tro: Chemistry: A Molecular Approach, 2/e nonpolar 44 Copyright 2011 Pearson Education, Inc.

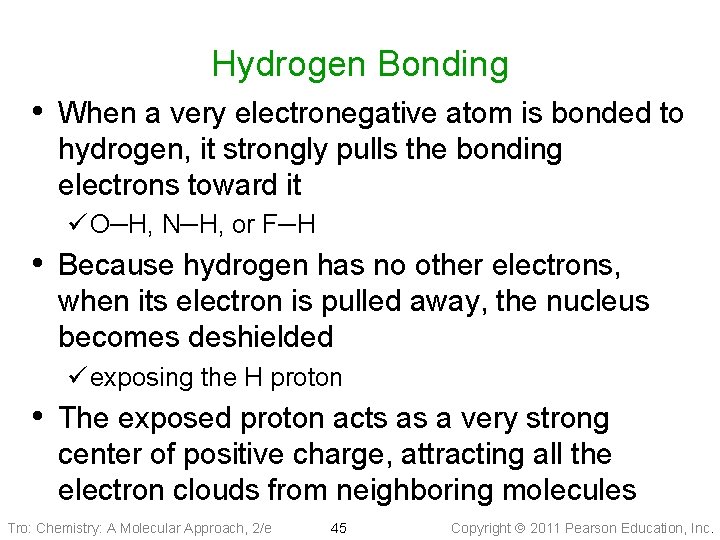
Hydrogen Bonding • When a very electronegative atom is bonded to hydrogen, it strongly pulls the bonding electrons toward it ü O─H, N─H, or F─H • Because hydrogen has no other electrons, when its electron is pulled away, the nucleus becomes deshielded ü exposing the H proton • The exposed proton acts as a very strong center of positive charge, attracting all the electron clouds from neighboring molecules Tro: Chemistry: A Molecular Approach, 2/e 45 Copyright 2011 Pearson Education, Inc.

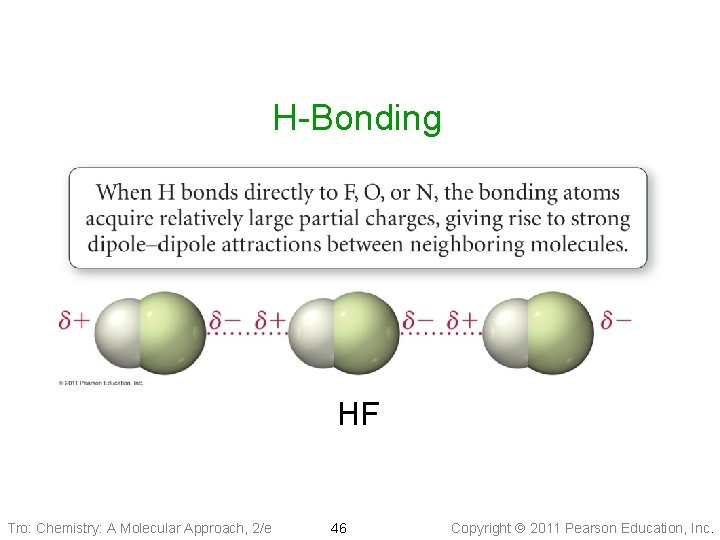
H-Bonding HF Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc.

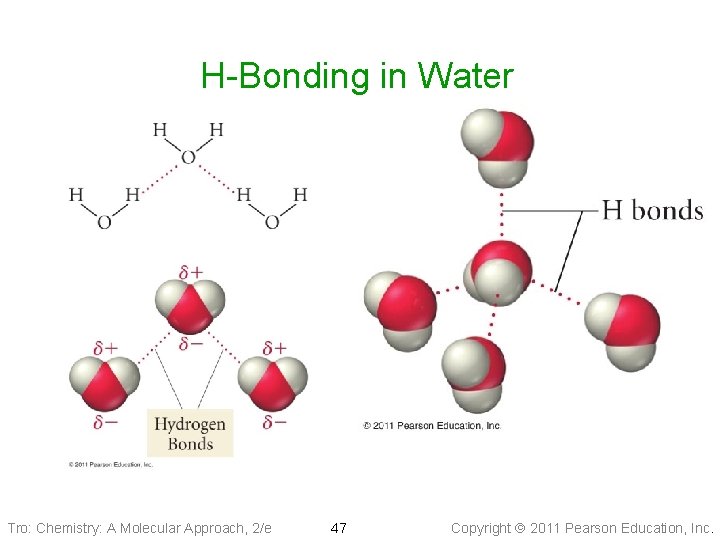
H-Bonding in Water Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc.

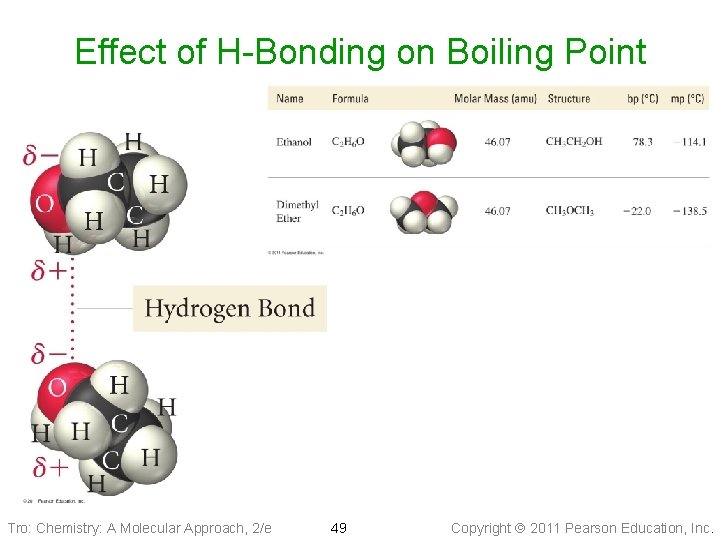
H-Bonds • Hydrogen bonds are very strong intermolecular attractive forces ü stronger than dipole–dipole or dispersion forces • Substances that can hydrogen bond will have • higher boiling points and melting points than similar substances that cannot But hydrogen bonds are not nearly as strong as chemical bonds ü 2 to 5% the strength of covalent bonds Tro: Chemistry: A Molecular Approach, 2/e 48 Copyright 2011 Pearson Education, Inc.

Effect of H-Bonding on Boiling Point Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc.

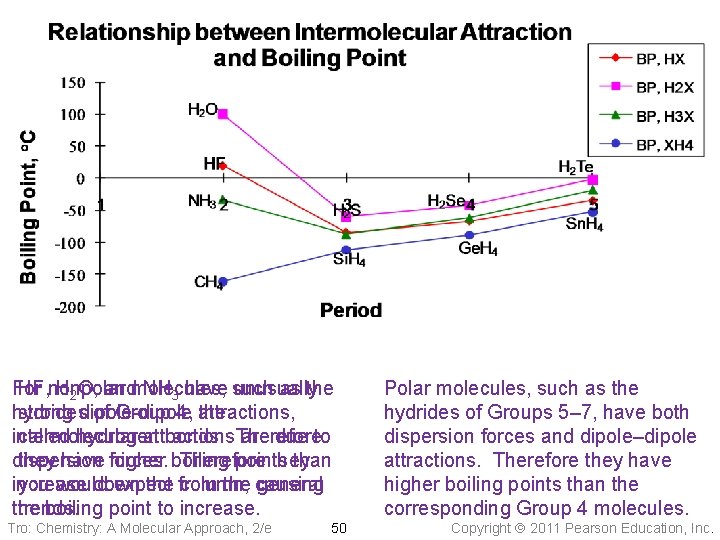
For HF, nonpolar H 2 O, andmolecules, NH 3 have such unusually as the hydrides strong dipole-dipole of Group 4, attractions, the intermolecular called hydrogen attractions bonds. Therefore are due to dispersion they have forces. higher boiling Therefore points they than increase you would down expect the from column, the causing general the trends. boiling point to increase. Tro: Chemistry: A Molecular Approach, 2/e 50 Polar molecules, such as the hydrides of Groups 5– 7, have both dispersion forces and dipole–dipole attractions. Therefore they have higher boiling points than the corresponding Group 4 molecules. Copyright 2011 Pearson Education, Inc.

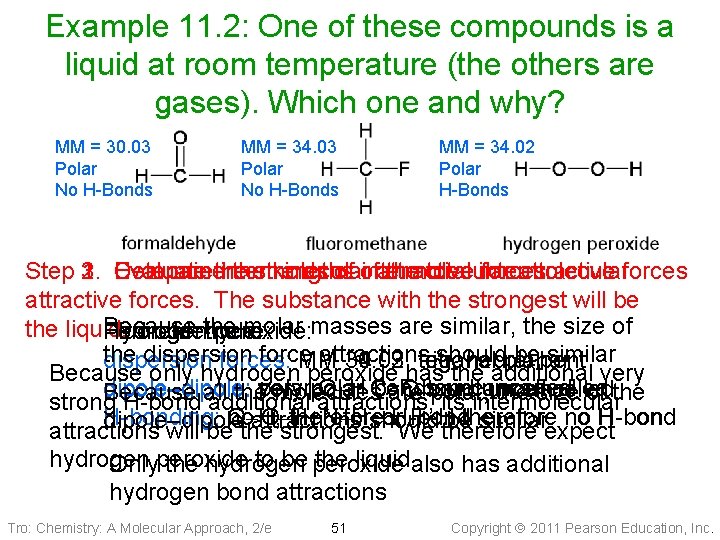
Example 11. 2: One of these compounds is a liquid at room temperature (the others are gases). Which one and why? MM = 30. 03 Polar No H-Bonds MM = 34. 02 Polar H-Bonds Step 2. 3. Compare 1. Evaluate the Determine intermolecular thestrengths kinds of of intermolecular attractive the total intermolecular forces attractive forces. The substance with the strongest will be Because the molar masses are similar, the size of the liquid. Hydrogen Formaldehyde: Fluoromethane: peroxide: the dispersion force attractions shouldplanar bebent similar dispersion forces: MM 34. 02, 30. 03, tetrahedral 34. 03, trigonal Because only hydrogen peroxide has the additional very dipole–dipole: very polar O–H C–F bonds uncancelled C=O bond uncancelled Because thepolar molecules arebond polar, the size of the strong H-bond all additional attractions, its intermolecular no H-bonding: O–H, noattractions O–H, therefore N–H, should or H-bond F–Hbetherefore dipole–dipole similarexpect attractions will be the strongest. We therefore hydrogen to be peroxide the liquid. also has additional Onlyperoxide the hydrogen bond attractions Tro: Chemistry: A Molecular Approach, 2/e 51 Copyright 2011 Pearson Education, Inc.

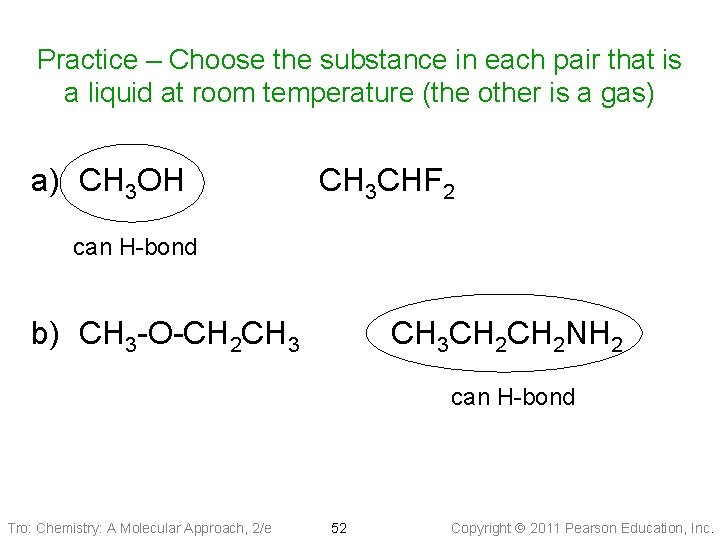
Practice – Choose the substance in each pair that is a liquid at room temperature (the other is a gas) a) CH 3 OH CH 3 CHF 2 can H-bond b) CH 3 -O-CH 2 CH 3 CH 2 NH 2 can H-bond Tro: Chemistry: A Molecular Approach, 2/e 52 Copyright 2011 Pearson Education, Inc.

Attractive Forces and Solubility • Solubility depends, in part, on the attractive forces of the solute and solvent molecules ü like dissolves like ü miscible liquids will always dissolve in each other • Polar substances dissolve in polar solvents ü hydrophilic groups = OH, CHO, C=O, COOH, NH 2, Cl • Nonpolar molecules dissolve in nonpolar solvents ü hydrophobic groups = C-H, C-C • Many molecules have both hydrophilic and hydrophobic parts – solubility in water becomes a competition between the attraction of the polar groups for the water and the attraction of the nonpolar groups for their own kind Tro: Chemistry: A Molecular Approach, 2/e 53 Copyright 2011 Pearson Education, Inc.

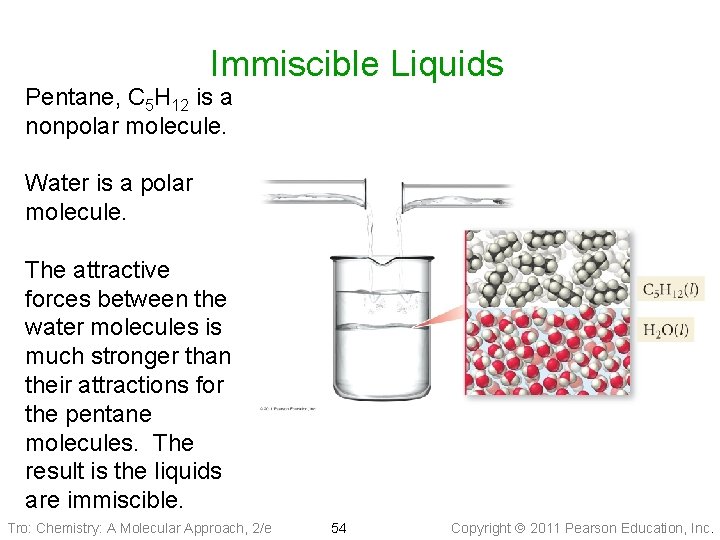
Immiscible Liquids Pentane, C 5 H 12 is a nonpolar molecule. Water is a polar molecule. The attractive forces between the water molecules is much stronger than their attractions for the pentane molecules. The result is the liquids are immiscible. Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc.

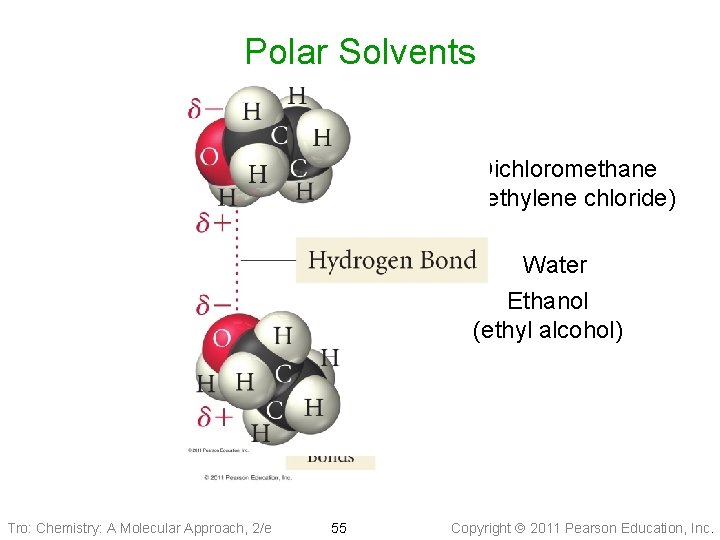
Polar Solvents Dichloromethane (methylene chloride) Water Ethanol (ethyl alcohol) Tro: Chemistry: A Molecular Approach, 2/e 55 Copyright 2011 Pearson Education, Inc.

Nonpolar Solvents Tro: Chemistry: A Molecular Approach, 2/e 56 Copyright 2011 Pearson Education, Inc.

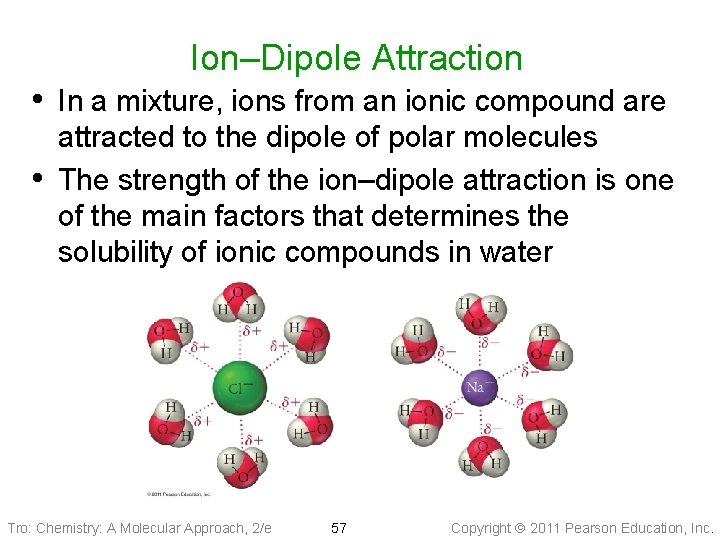
Ion–Dipole Attraction • In a mixture, ions from an ionic compound are • attracted to the dipole of polar molecules The strength of the ion–dipole attraction is one of the main factors that determines the solubility of ionic compounds in water Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc.

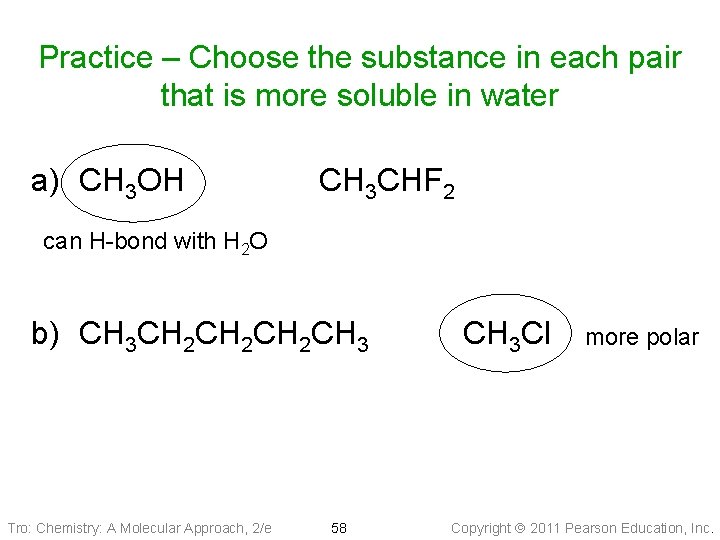
Practice – Choose the substance in each pair that is more soluble in water a) CH 3 OH CH 3 CHF 2 can H-bond with H 2 O b) CH 3 CH 2 CH 2 CH 3 Tro: Chemistry: A Molecular Approach, 2/e 58 CH 3 Cl more polar Copyright 2011 Pearson Education, Inc.

Summary • Dispersion forces are the weakest of the • • • intermolecular attractions Dispersion forces are present in all molecules and atoms The magnitude of the dispersion forces increases with molar mass Polar molecules also have dipole–dipole attractive forces Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc.

Summary (cont’d) • Hydrogen bonds are the strongest of the intermolecular attractive forces ü a pure substance can have • Hydrogen bonds will be present when a molecule has H directly bonded to either O , N, or F atoms ü only example of H bonded to F is HF • Ion–dipole attractions are present in mixtures of • • ionic compounds with polar molecules. Ion–dipole attractions are the strongest intermolecular attraction Ion–dipole attractions are especially important in aqueous solutions of ionic compounds Tro: Chemistry: A Molecular Approach, 2/e 60 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc.

Liquids Properties & Structure Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Surface Tension • Surface tension is a property of liquids that • results from the tendency of liquids to minimize their surface area To minimize their surface area, liquids form drops that are spherical ü as long as there is no gravity Tro: Chemistry: A Molecular Approach, 2/e 63 Copyright 2011 Pearson Education, Inc.

Surface Tension • The layer of molecules on the surface behave differently than the interior ü because the cohesive forces on the surface molecules have a net pull into the liquid interior • The surface layer acts like an elastic skin ü allowing you to “float” a paper clip even though steel is denser than water Tro: Chemistry: A Molecular Approach, 2/e 64 Copyright 2011 Pearson Education, Inc.

Surface Tension • Because they have fewer neighbors to attract them, the surface molecules are less stable than those in the interior ü have a higher potential energy • The surface tension of a liquid is the energy required to increase the surface area a given amount ü surface tension of H 2 O = 72. 8 m. J/m 2 Ø at room temperature ü surface tension of C 6 H 6 = 28 m. J/m 2 Tro: Chemistry: A Molecular Approach, 2/e 65 Copyright 2011 Pearson Education, Inc.

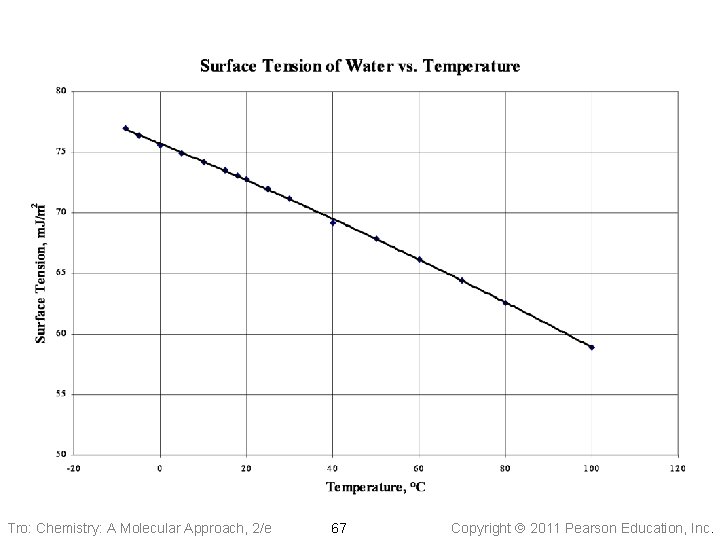
Factors Affecting Surface Tension • The stronger the intermolecular attractive • forces, the higher the surface tension will be Raising the temperature of a liquid reduces its surface tension ü raising the temperature of the liquid increases the average kinetic energy of the molecules ü the increased molecular motion makes it easier to stretch the surface Tro: Chemistry: A Molecular Approach, 2/e 66 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 67 Copyright 2011 Pearson Education, Inc.

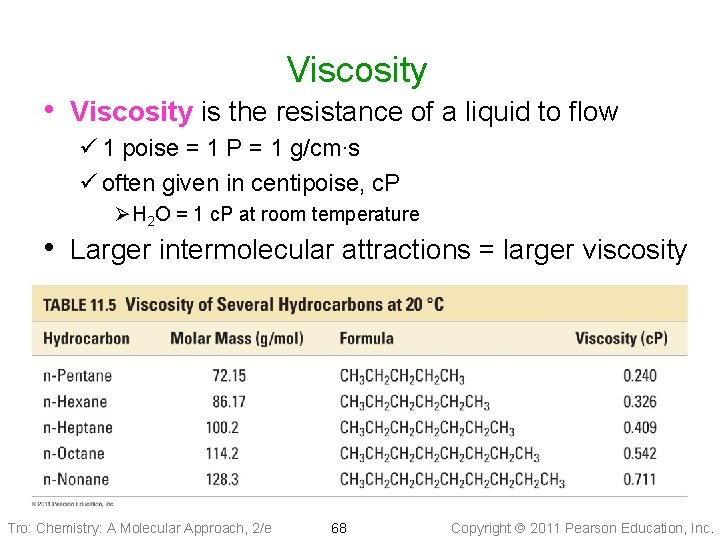
Viscosity • Viscosity is the resistance of a liquid to flow ü 1 poise = 1 P = 1 g/cm∙s ü often given in centipoise, c. P Ø H 2 O = 1 c. P at room temperature • Larger intermolecular attractions = larger viscosity Tro: Chemistry: A Molecular Approach, 2/e 68 Copyright 2011 Pearson Education, Inc.

Factors Affecting Viscosity • The stronger the intermolecular attractive forces, the • higher the liquid’s viscosity will be The more spherical the molecular shape, the lower the viscosity will be ü molecules roll more easily ü less surface-to-surface contact lowers attractions • Raising the temperature of a liquid reduces its viscosity ü raising the temperature of the liquid increases the average kinetic energy of the molecules ü the increased molecular motion makes it easier to overcome the intermolecular attractions and flow Tro: Chemistry: A Molecular Approach, 2/e 69 Copyright 2011 Pearson Education, Inc.

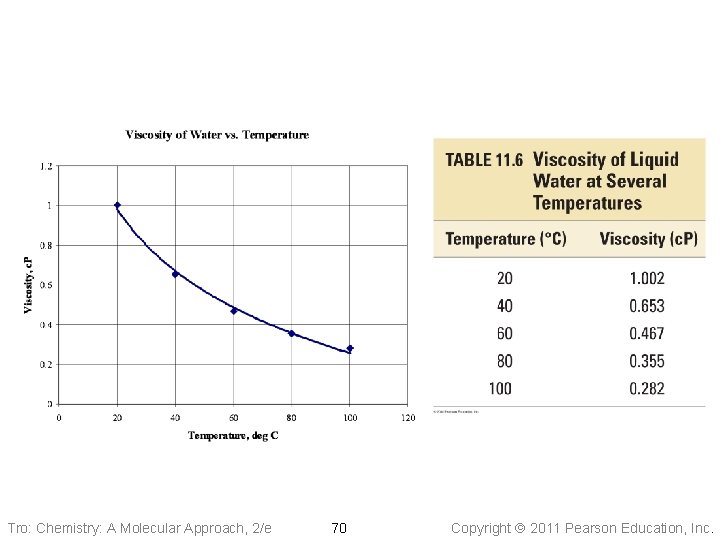
Insert Table 11. 6 Tro: Chemistry: A Molecular Approach, 2/e 70 Copyright 2011 Pearson Education, Inc.

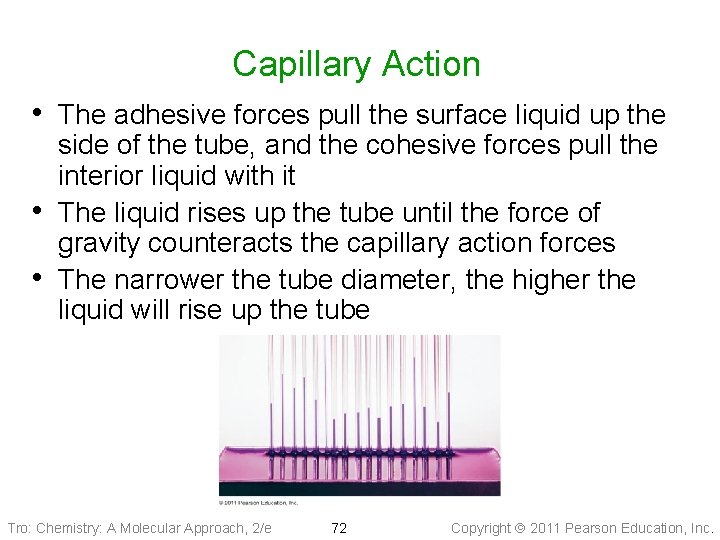
Capillary Action • Capillary action is the ability of a liquid to flow up a thin tube against the influence of gravity ü the narrower the tube, the higher the liquid rises • Capillary action is the result of two forces working in conjunction, the cohesive and adhesive forces ü cohesive forces hold the liquid molecules together ü adhesive forces attract the outer liquid molecules to the tube’s surface Tro: Chemistry: A Molecular Approach, 2/e 71 Copyright 2011 Pearson Education, Inc.

Capillary Action • The adhesive forces pull the surface liquid up the • • side of the tube, and the cohesive forces pull the interior liquid with it The liquid rises up the tube until the force of gravity counteracts the capillary action forces The narrower the tube diameter, the higher the liquid will rise up the tube Tro: Chemistry: A Molecular Approach, 2/e 72 Copyright 2011 Pearson Education, Inc.

Meniscus • The curving of the liquid surface in a • • thin tube is due to the competition between adhesive and cohesive forces The meniscus of water is concave in a glass tube because its adhesion to the glass is stronger than its cohesion for itself The meniscus of mercury is convex in a glass tube because its cohesion for itself is stronger than its adhesion for the glass ü metallic bonds are stronger than intermolecular attractions Tro: Chemistry: A Molecular Approach, 2/e 73 Copyright 2011 Pearson Education, Inc.

The Molecular Dance • Molecules in the liquid are constantly in motion ü vibrational, and limited rotational and translational • The average kinetic energy is proportional • to the temperature However, some molecules have more kinetic energy than the average, and others have less Tro: Chemistry: A Molecular Approach, 2/e 74 Copyright 2011 Pearson Education, Inc.

Vaporization • If these high energy molecules are at the surface, they may have enough energy to overcome the attractive forces ü therefore – the larger the surface area, the faster the rate of evaporation • This will allow them to escape the liquid and become a vapor Tro: Chemistry: A Molecular Approach, 2/e 75 Copyright 2011 Pearson Education, Inc.

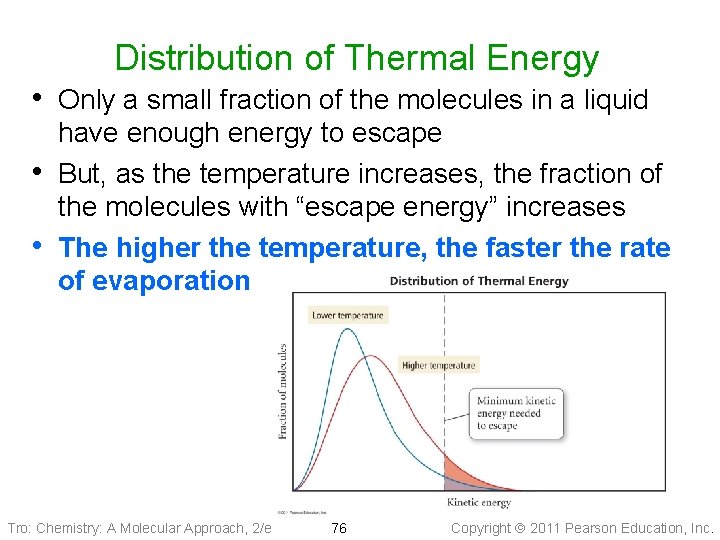
Distribution of Thermal Energy • Only a small fraction of the molecules in a liquid • • have enough energy to escape But, as the temperature increases, the fraction of the molecules with “escape energy” increases The higher the temperature, the faster the rate of evaporation Tro: Chemistry: A Molecular Approach, 2/e 76 Copyright 2011 Pearson Education, Inc.

Condensation • Some molecules of the vapor will lose energy • • through molecular collisions The result will be that some of the molecules will get captured back into the liquid when they collide with it Also some may stick and gather together to form droplets of liquid ü particularly on surrounding surfaces • We call this process condensation Tro: Chemistry: A Molecular Approach, 2/e 77 Copyright 2011 Pearson Education, Inc.

Evaporation vs. Condensation • Vaporization and condensation are opposite processes • In an open container, the vapor molecules generally • • • spread out faster than they can condense The net result is that the rate of vaporization is greater than the rate of condensation, and there is a net loss of liquid However, in a closed container, the vapor is not allowed to spread out indefinitely The net result in a closed container is that at some time the rates of vaporization and condensation will be equal Tro: Chemistry: A Molecular Approach, 2/e 78 Copyright 2011 Pearson Education, Inc.

Effect of Intermolecular Attraction on Evaporation and Condensation • The weaker the attractive forces between molecules, • • • the less energy they will need to vaporize Also, weaker attractive forces means that more energy will need to be removed from the vapor molecules before they can condense The net result will be more molecules in the vapor phase, and a liquid that evaporates faster – the weaker the attractive forces, the faster the rate of evaporation Liquids that evaporate easily are said to be volatile ü e. g. , gasoline, fingernail polish remover ü liquids that do not evaporate easily are called nonvolatile Ø e. g. , motor oil Tro: Chemistry: A Molecular Approach, 2/e 79 Copyright 2011 Pearson Education, Inc.

Energetics of Vaporization • When the high energy molecules are lost from • the liquid, it lowers the average kinetic energy If energy is not drawn back into the liquid, its temperature will decrease – therefore, vaporization is an endothermic process ü and condensation is an exothermic process • Vaporization requires input of energy to overcome the attractions between molecules Tro: Chemistry: A Molecular Approach, 2/e 80 Copyright 2011 Pearson Education, Inc.

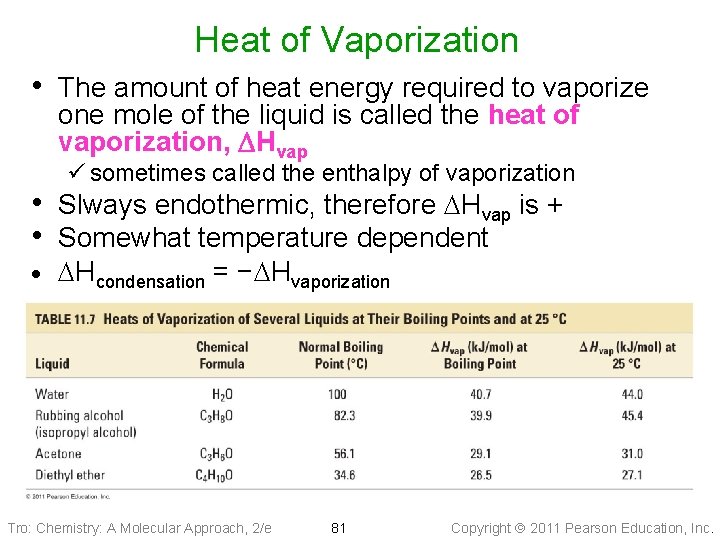
Heat of Vaporization • The amount of heat energy required to vaporize one mole of the liquid is called the heat of vaporization, DHvap ü sometimes called the enthalpy of vaporization • Slways endothermic, therefore DHvap is + • Somewhat temperature dependent · DHcondensation = −DHvaporization Tro: Chemistry: A Molecular Approach, 2/e 81 Copyright 2011 Pearson Education, Inc.

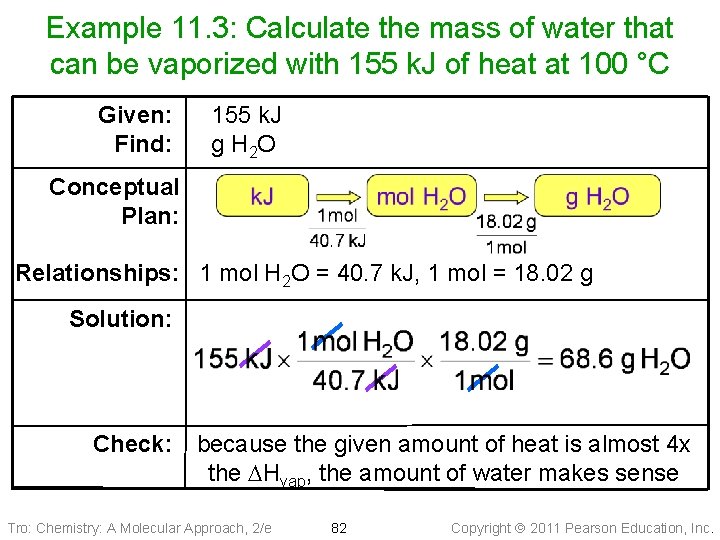
Example 11. 3: Calculate the mass of water that can be vaporized with 155 k. J of heat at 100 °C Given: Find: 155 k. J g H 2 O Conceptual Plan: Relationships: 1 mol H 2 O = 40. 7 k. J, 1 mol = 18. 02 g Solution: Check: because the given amount of heat is almost 4 x the DHvap, the amount of water makes sense Tro: Chemistry: A Molecular Approach, 2/e 82 Copyright 2011 Pearson Education, Inc.

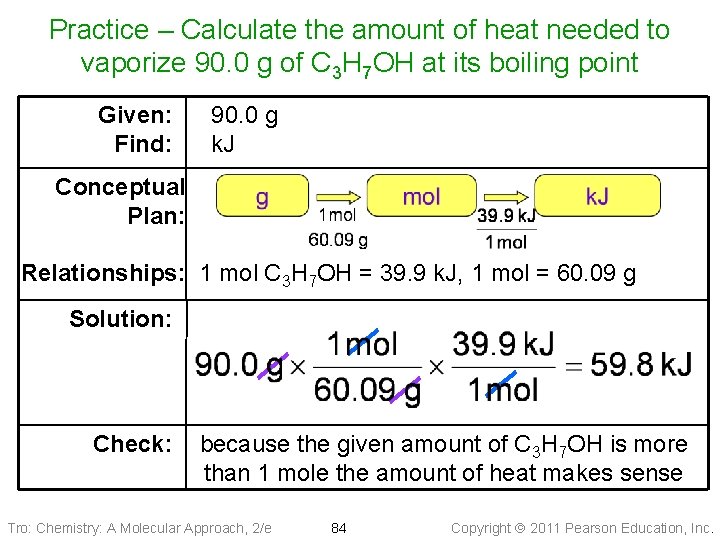
Practice – Calculate the amount of heat needed to vaporize 90. 0 g of C 3 H 7 OH at its boiling point (DHvap = 39. 9 k. J/mol) Tro: Chemistry: A Molecular Approach, 2/e 83 Copyright 2011 Pearson Education, Inc.

Practice – Calculate the amount of heat needed to vaporize 90. 0 g of C 3 H 7 OH at its boiling point Given: Find: 90. 0 g k. J Conceptual Plan: Relationships: 1 mol C 3 H 7 OH = 39. 9 k. J, 1 mol = 60. 09 g Solution: Check: because the given amount of C 3 H 7 OH is more than 1 mole the amount of heat makes sense Tro: Chemistry: A Molecular Approach, 2/e 84 Copyright 2011 Pearson Education, Inc.

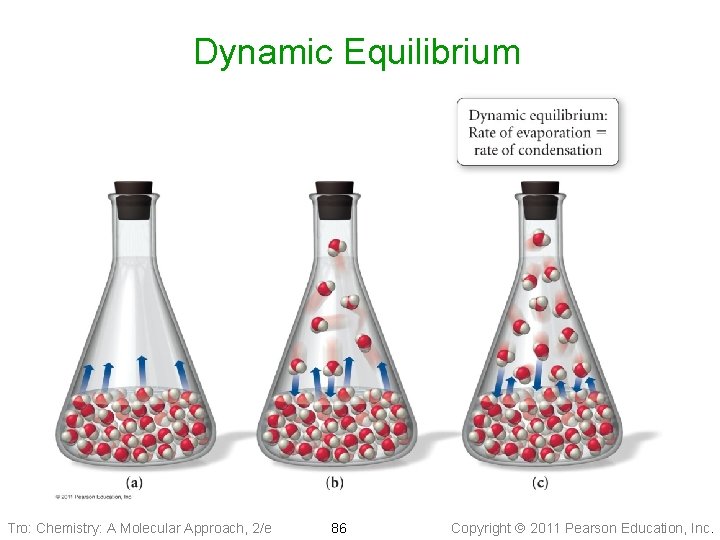
Dynamic Equilibrium • In a closed container, once the rates of • • vaporization and condensation are equal, the total amount of vapor and liquid will not change Evaporation and condensation are still occurring, but because they are opposite processes, there is no net gain or loss of either vapor or liquid When two opposite processes reach the same rate so that there is no gain or loss of material, we call it a dynamic equilibrium ü this does not mean there are equal amounts of vapor and liquid – it means that they are changing by equal amounts Tro: Chemistry: A Molecular Approach, 2/e 85 Copyright 2011 Pearson Education, Inc.

Dynamic Equilibrium Tro: Chemistry: A Molecular Approach, 2/e 86 Copyright 2011 Pearson Education, Inc.

Vapor Pressure • The pressure exerted by the vapor when it is in dynamic equilibrium with its liquid is called the vapor pressure ü remember using Dalton’s Law of Partial Pressures to account for the pressure of the water vapor when collecting gases by water displacement? • The weaker the attractive forces between the • molecules, the more molecules will be in the vapor Therefore, the weaker the attractive forces, the higher the vapor pressure ü the higher the vapor pressure, the more volatile the liquid Tro: Chemistry: A Molecular Approach, 2/e 87 Copyright 2011 Pearson Education, Inc.

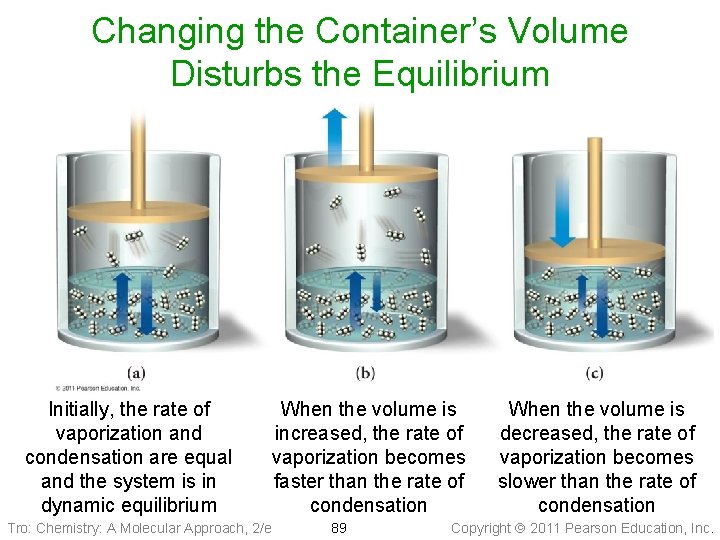
Vapor–Liquid Dynamic Equilibrium • If the volume of the chamber is increased, it will decrease the pressure of the vapor inside the chamber ü at that point, there are fewer vapor molecules in a given volume, causing the rate of condensation to slow • Therefore, for a period of time, the rate of vaporization • will be faster than the rate of condensation, and the amount of vapor will increase Eventually enough vapor accumulates so that the rate of the condensation increases to the point where it is once again as fast as evaporation ü equilibrium is reestablished • At this point, the vapor pressure will be the same as it was before Tro: Chemistry: A Molecular Approach, 2/e 88 Copyright 2011 Pearson Education, Inc.

Changing the Container’s Volume Disturbs the Equilibrium Initially, the rate of vaporization and condensation are equal and the system is in dynamic equilibrium Tro: Chemistry: A Molecular Approach, 2/e When the volume is increased, the rate of vaporization becomes faster than the rate of condensation 89 When the volume is decreased, the rate of vaporization becomes slower than the rate of condensation Copyright 2011 Pearson Education, Inc.

Dynamic Equilibrium • A system in dynamic equilibrium can respond • to changes in the conditions When conditions change, the system shifts its position to relieve or reduce the effects of the change Tro: Chemistry: A Molecular Approach, 2/e 90 Copyright 2011 Pearson Education, Inc.

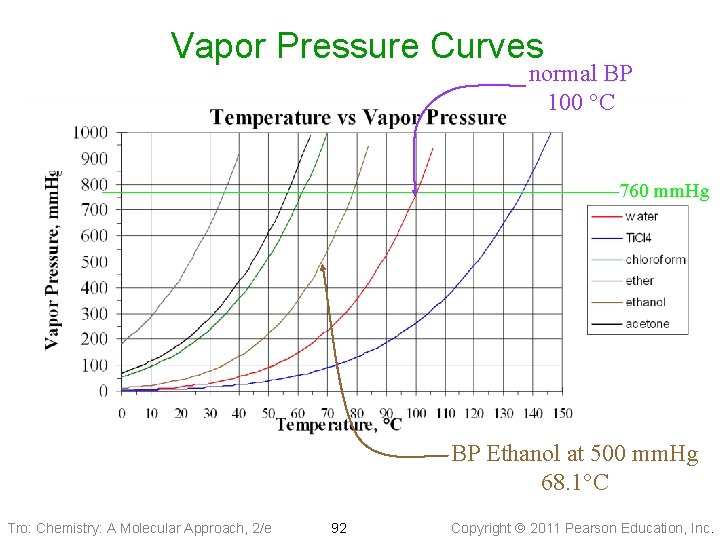
Vapor Pressure vs. Temperature • Increasing the temperature increases the • • number of molecules able to escape the liquid The net result is that as the temperature increases, the vapor pressure increases Small changes in temperature can make big changes in vapor pressure ü the rate of growth depends on strength of the intermolecular forces Tro: Chemistry: A Molecular Approach, 2/e 91 Copyright 2011 Pearson Education, Inc.

Vapor Pressure Curves normal BP 100 °C 760 mm. Hg BP Ethanol at 500 mm. Hg 68. 1°C Tro: Chemistry: A Molecular Approach, 2/e 92 Copyright 2011 Pearson Education, Inc.

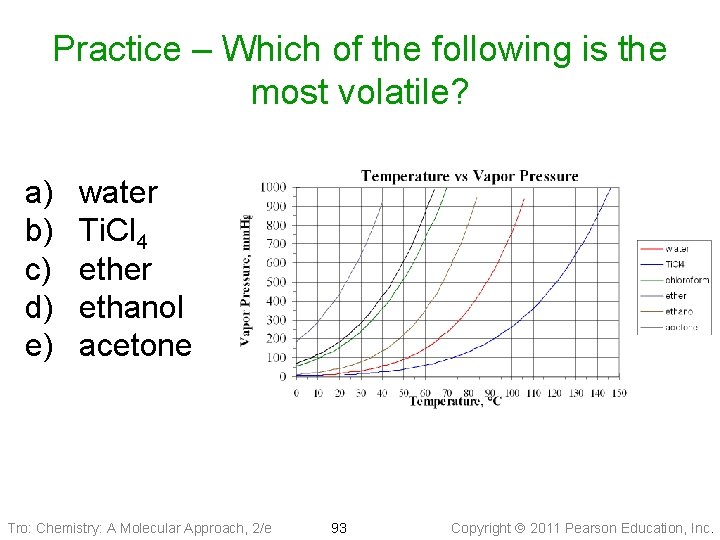
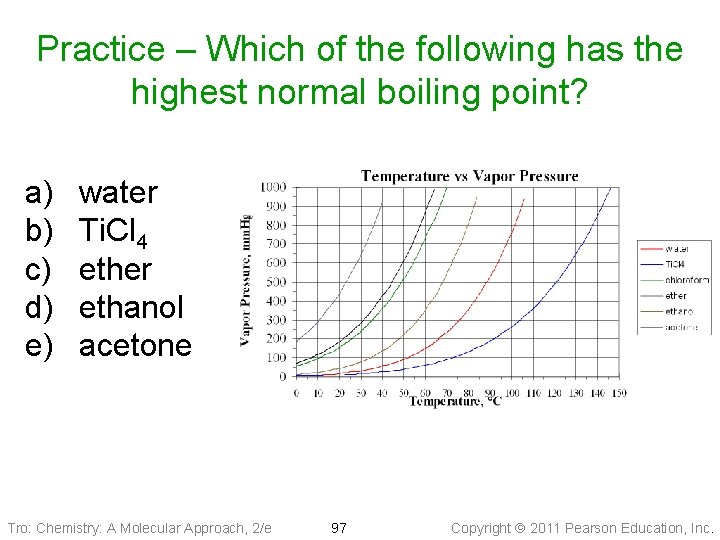
Practice – Which of the following is the most volatile? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular Approach, 2/e 93 Copyright 2011 Pearson Education, Inc.

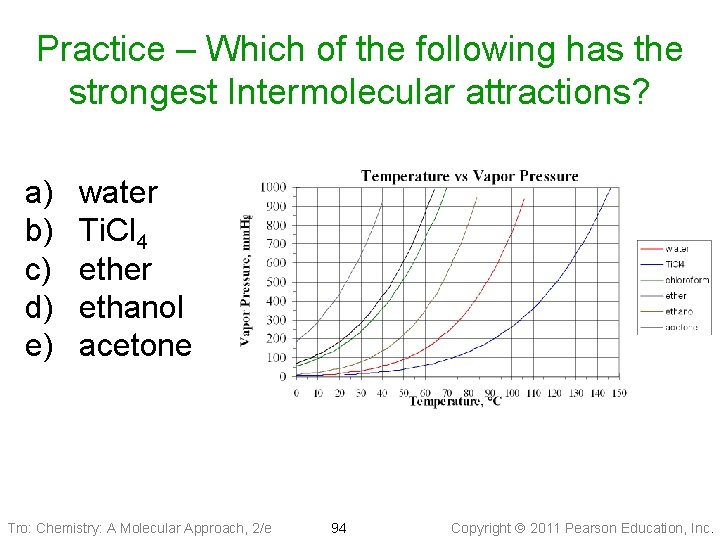
Practice – Which of the following has the strongest Intermolecular attractions? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular Approach, 2/e 94 Copyright 2011 Pearson Education, Inc.

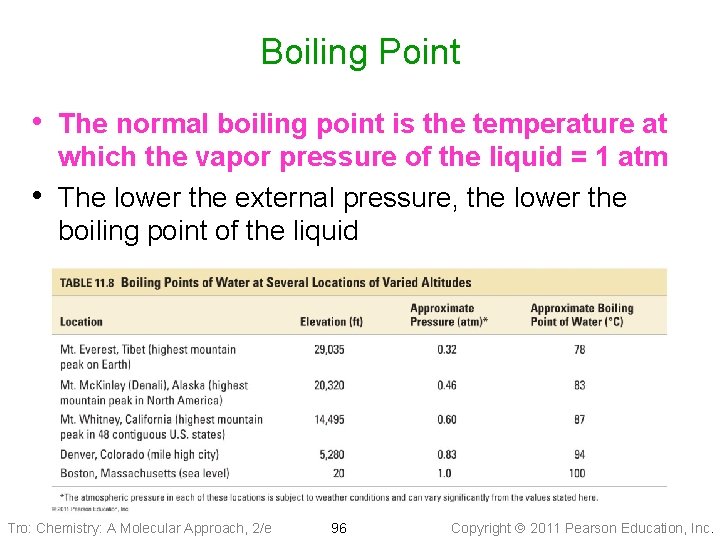
Boiling Point • When the temperature of a liquid reaches a point where its vapor pressure is the same as the external pressure, vapor bubbles can form anywhere in the liquid ü not just on the surface • This phenomenon is what is called boiling and the temperature at which the vapor pressure = external pressure is the boiling point Tro: Chemistry: A Molecular Approach, 2/e 95 Copyright 2011 Pearson Education, Inc.

Boiling Point • The normal boiling point is the temperature at • which the vapor pressure of the liquid = 1 atm The lower the external pressure, the lower the boiling point of the liquid Tro: Chemistry: A Molecular Approach, 2/e 96 Copyright 2011 Pearson Education, Inc.

Practice – Which of the following has the highest normal boiling point? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular Approach, 2/e 97 Copyright 2011 Pearson Education, Inc.

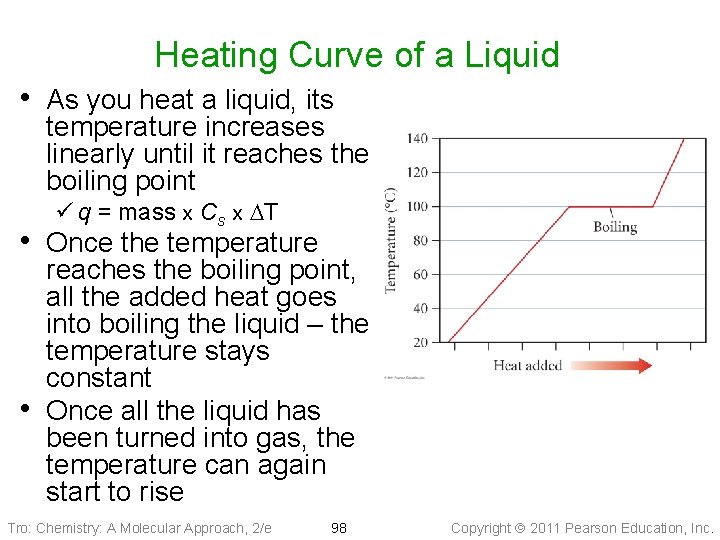
Heating Curve of a Liquid • As you heat a liquid, its temperature increases linearly until it reaches the boiling point ü q = mass x Cs x DT • Once the temperature • reaches the boiling point, all the added heat goes into boiling the liquid – the temperature stays constant Once all the liquid has been turned into gas, the temperature can again start to rise Tro: Chemistry: A Molecular Approach, 2/e 98 Copyright 2011 Pearson Education, Inc.

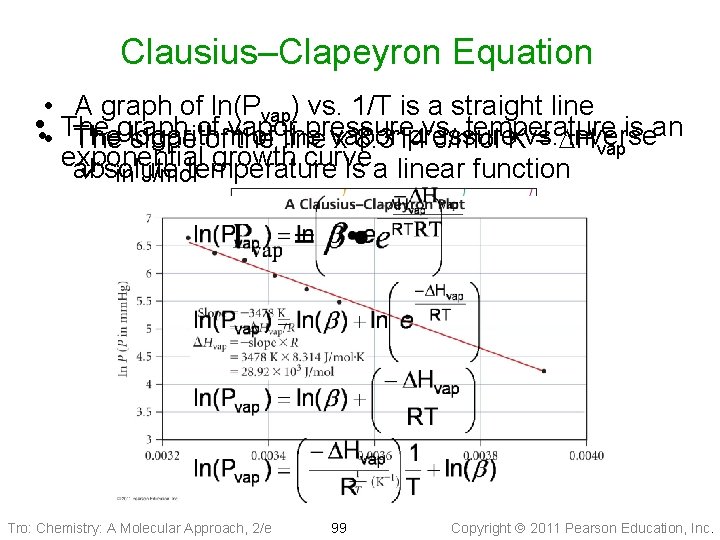
Clausius–Clapeyron Equation • A graph of ln(Pvap) vs. 1/T is a straight line • • • The pressure vs. temperature is an Thegraph logarithm of line the vapor pressure vs. inverse slopeof ofvapor the x 8. 314 J/mol∙K = DH vap exponential growth curve absolute ü in J/moltemperature is a linear function Tro: Chemistry: A Molecular Approach, 2/e 99 Copyright 2011 Pearson Education, Inc.

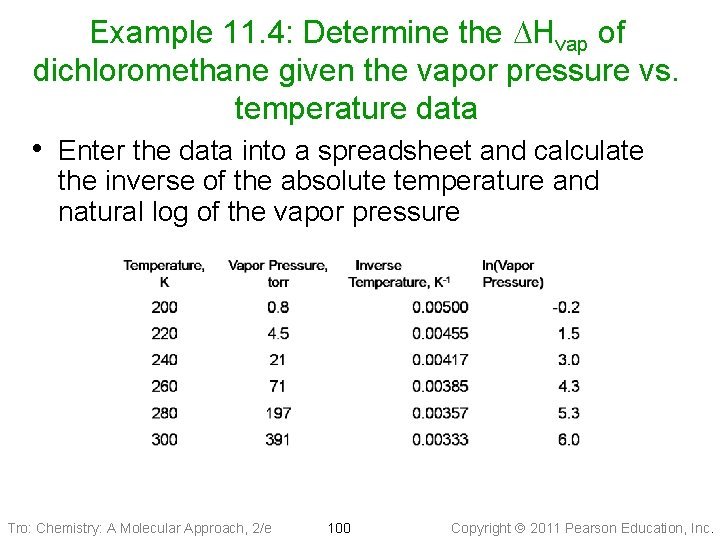
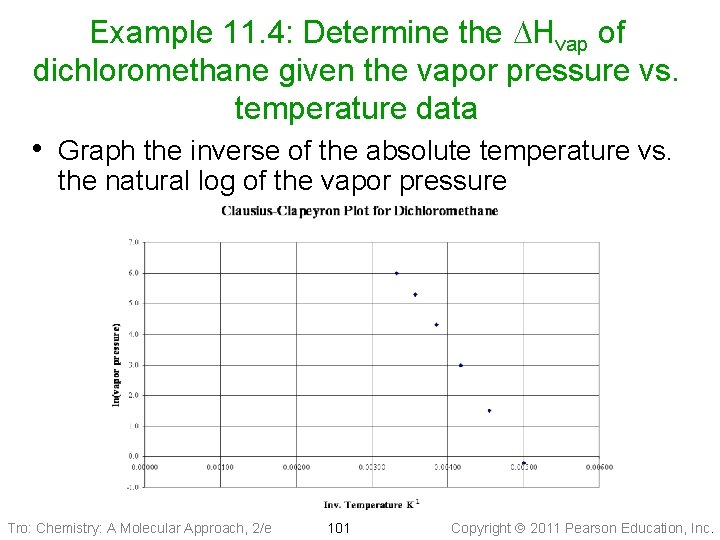
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Enter the data into a spreadsheet and calculate the inverse of the absolute temperature and natural log of the vapor pressure Tro: Chemistry: A Molecular Approach, 2/e 100 Copyright 2011 Pearson Education, Inc.

Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Graph the inverse of the absolute temperature vs. the natural log of the vapor pressure Tro: Chemistry: A Molecular Approach, 2/e 101 Copyright 2011 Pearson Education, Inc.

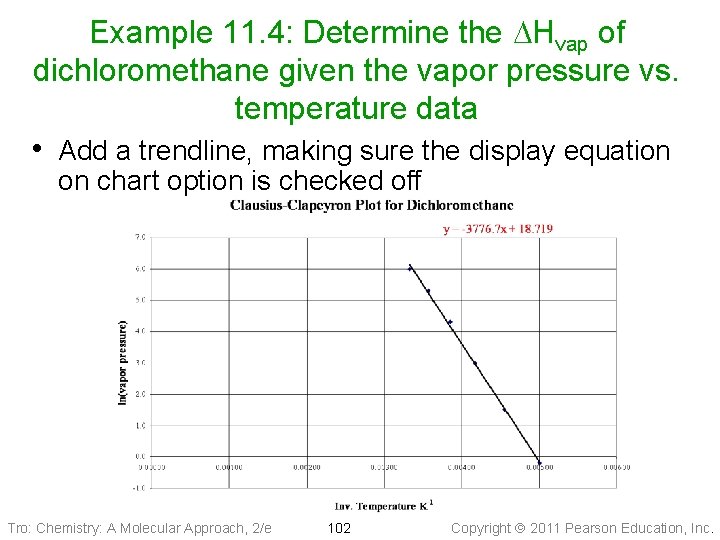
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Add a trendline, making sure the display equation on chart option is checked off Tro: Chemistry: A Molecular Approach, 2/e 102 Copyright 2011 Pearson Education, Inc.

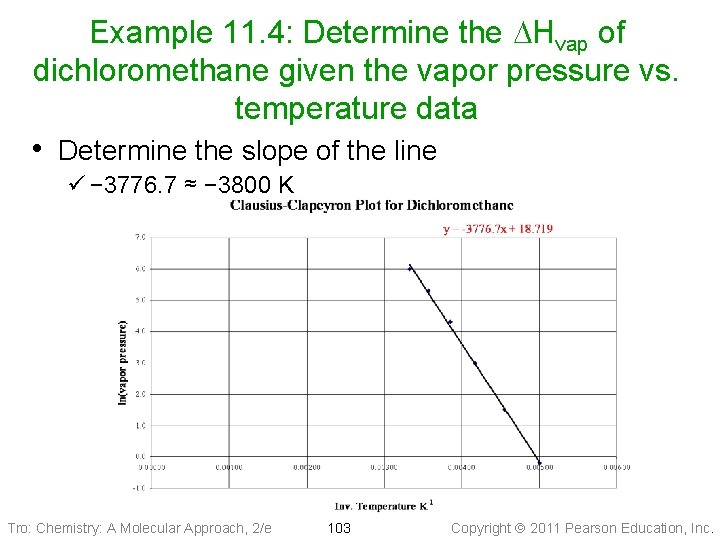
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Determine the slope of the line ü − 3776. 7 ≈ − 3800 K Tro: Chemistry: A Molecular Approach, 2/e 103 Copyright 2011 Pearson Education, Inc.

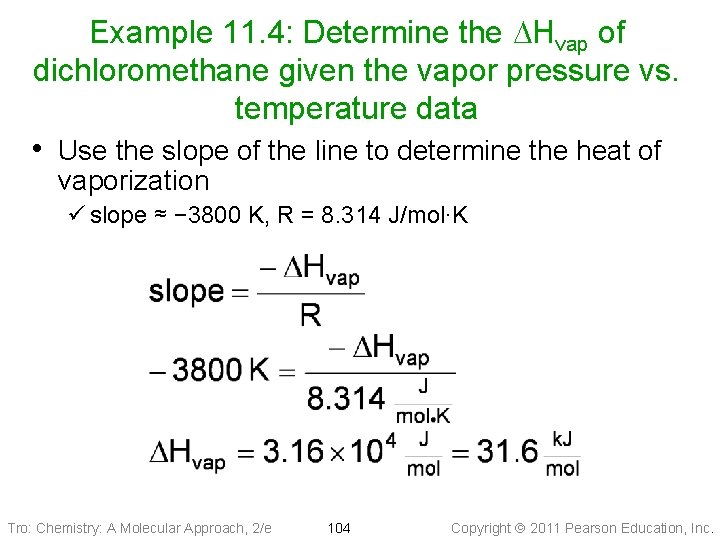
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Use the slope of the line to determine the heat of vaporization ü slope ≈ − 3800 K, R = 8. 314 J/mol∙K Tro: Chemistry: A Molecular Approach, 2/e 104 Copyright 2011 Pearson Education, Inc.

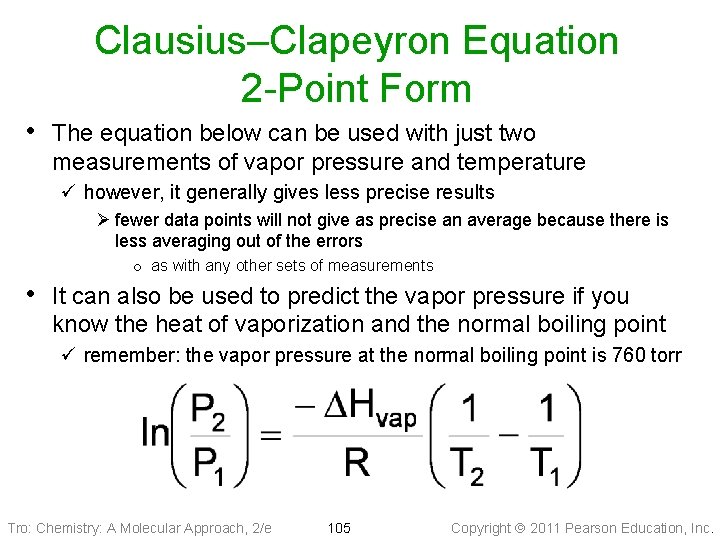
Clausius–Clapeyron Equation 2 -Point Form • The equation below can be used with just two measurements of vapor pressure and temperature ü however, it generally gives less precise results Ø fewer data points will not give as precise an average because there is less averaging out of the errors o as with any other sets of measurements • It can also be used to predict the vapor pressure if you know the heat of vaporization and the normal boiling point ü remember: the vapor pressure at the normal boiling point is 760 torr Tro: Chemistry: A Molecular Approach, 2/e 105 Copyright 2011 Pearson Education, Inc.

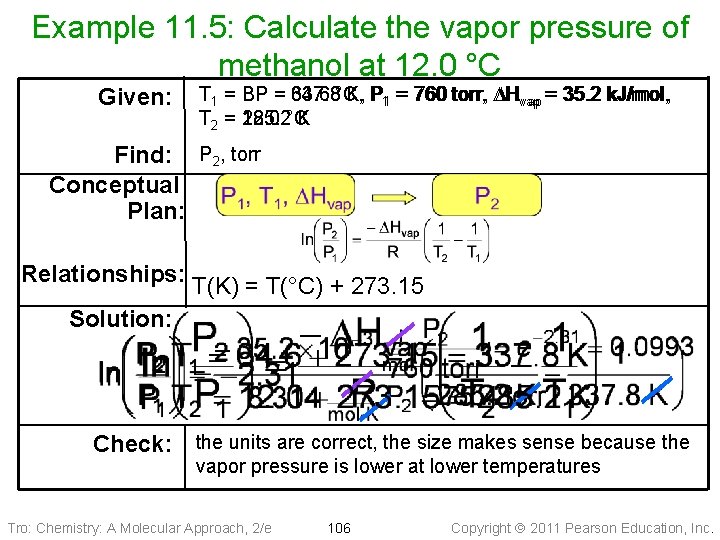
Example 11. 5: Calculate the vapor pressure of methanol at 12. 0 °C Given: T 1 = BP = 337. 8 64. 6 °C, K, P P 11 == 760 torr, DH DHvap = 35. 2 k. J/mol, vap = T 2 = 285. 2 12. 0 °C K Find: P 2, torr Conceptual Plan: Relationships: T(K) = T(°C) + 273. 15 Solution: Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures Tro: Chemistry: A Molecular Approach, 2/e 106 Copyright 2011 Pearson Education, Inc.

Practice – Determine the vapor pressure of water at 25 C (normal BP = 100. 0 C, DHvap= 40. 7 k. J/mol) Tro: Chemistry: A Molecular Approach, 2/e 107 Copyright 2011 Pearson Education, Inc.

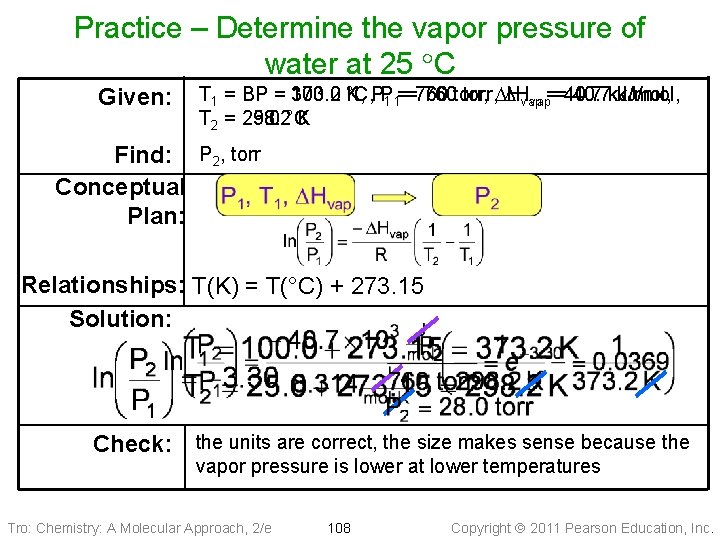
Practice – Determine the vapor pressure of water at 25 C Given: T 1 = BP = 373. 2 100. 0 K, °C, PP 1 1==760 760 torr, DH DHvap 40. 7 k. J/mol, vap==40. 7 T 2 = 298. 2 25. 0 °C K Find: P 2, torr Conceptual Plan: Relationships: T(K) = T(°C) + 273. 15 Solution: Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures Tro: Chemistry: A Molecular Approach, 2/e 108 Copyright 2011 Pearson Education, Inc.

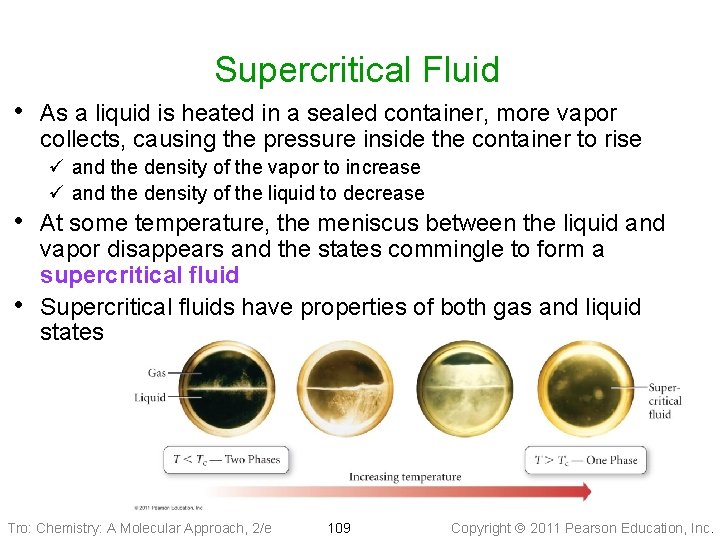
Supercritical Fluid • As a liquid is heated in a sealed container, more vapor collects, causing the pressure inside the container to rise ü and the density of the vapor to increase ü and the density of the liquid to decrease • At some temperature, the meniscus between the liquid and • vapor disappears and the states commingle to form a supercritical fluid Supercritical fluids have properties of both gas and liquid states Tro: Chemistry: A Molecular Approach, 2/e 109 Copyright 2011 Pearson Education, Inc.

The Critical Point • The temperature required to produce a • • supercritical fluid is called the critical temperature The pressure at the critical temperature is called the critical pressure At the critical temperature or higher temperatures, the gas cannot be condensed to a liquid, no matter how high the pressure gets Tro: Chemistry: A Molecular Approach, 2/e 110 Copyright 2011 Pearson Education, Inc.

Sublimation and Deposition • Molecules in the solid have thermal energy that • • • allows them to vibrate Surface molecules with sufficient energy may break free from the surface and become a gas – this process is called sublimation The capturing of vapor molecules into a solid is called deposition The solid and vapor phases exist in dynamic equilibrium in a closed container ü at temperatures below the melting point ü therefore, molecular solids have a vapor pressure solid Tro: Chemistry: A Molecular Approach, 2/e sublimation deposition 111 gas Copyright 2011 Pearson Education, Inc.

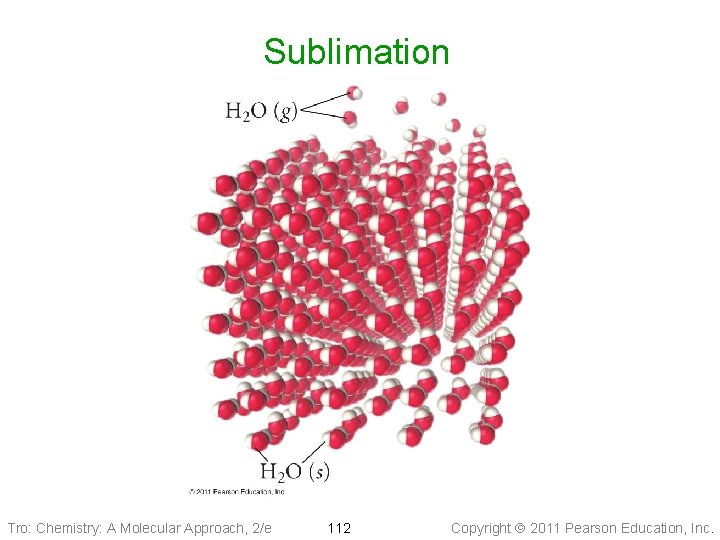
Sublimation Tro: Chemistry: A Molecular Approach, 2/e 112 Copyright 2011 Pearson Education, Inc.

Melting = Fusion • As a solid is heated, its temperature rises and • • the molecules vibrate more vigorously Once the temperature reaches the melting point, the molecules have sufficient energy to overcome some of the attractions that hold them in position and the solid melts (or fuses) The opposite of melting is freezing Tro: Chemistry: A Molecular Approach, 2/e 113 Copyright 2011 Pearson Education, Inc.

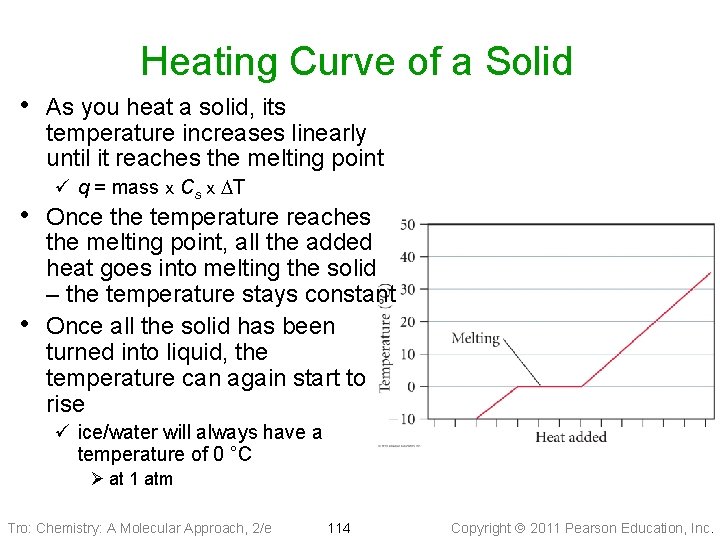
Heating Curve of a Solid • As you heat a solid, its temperature increases linearly until it reaches the melting point ü q = mass x Cs x DT • Once the temperature reaches • the melting point, all the added heat goes into melting the solid – the temperature stays constant Once all the solid has been turned into liquid, the temperature can again start to rise ü ice/water will always have a temperature of 0 °C Ø at 1 atm Tro: Chemistry: A Molecular Approach, 2/e 114 Copyright 2011 Pearson Education, Inc.

Energetics of Melting • When the high energy molecules are lost from • the solid, it lowers the average kinetic energy If energy is not drawn back into the solid its temperature will decrease – therefore, melting is an endothermic process ü and freezing is an exothermic process • Melting requires input of energy to overcome the attractions between molecules Tro: Chemistry: A Molecular Approach, 2/e 115 Copyright 2011 Pearson Education, Inc.

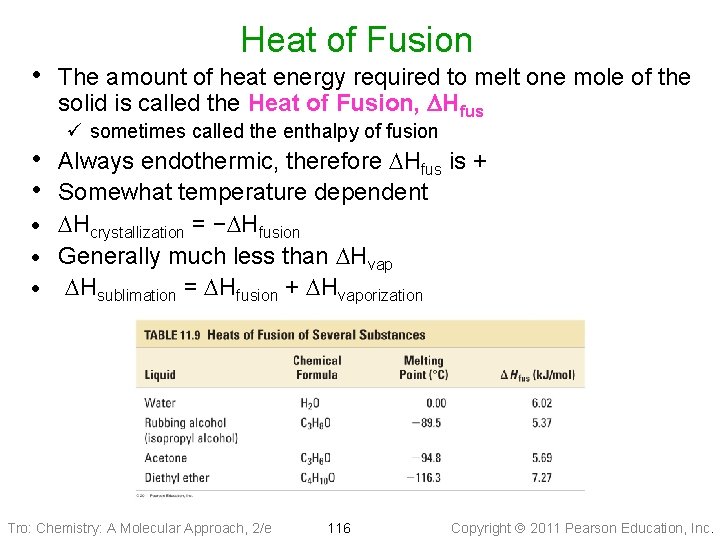
Heat of Fusion • The amount of heat energy required to melt one mole of the solid is called the Heat of Fusion, DHfus ü sometimes called the enthalpy of fusion • Always endothermic, therefore DHfus is + • Somewhat temperature dependent DHcrystallization = −DHfusion · Generally much less than DHvap · DHsublimation = DHfusion + DHvaporization · Tro: Chemistry: A Molecular Approach, 2/e 116 Copyright 2011 Pearson Education, Inc.

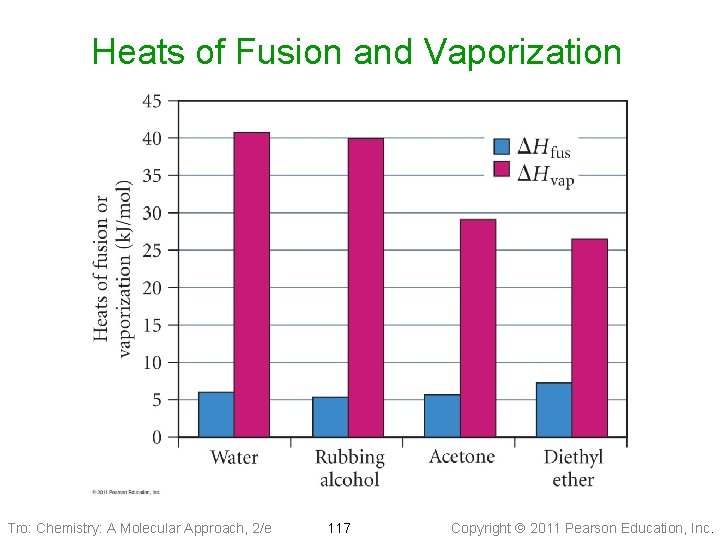
Heats of Fusion and Vaporization Tro: Chemistry: A Molecular Approach, 2/e 117 Copyright 2011 Pearson Education, Inc.

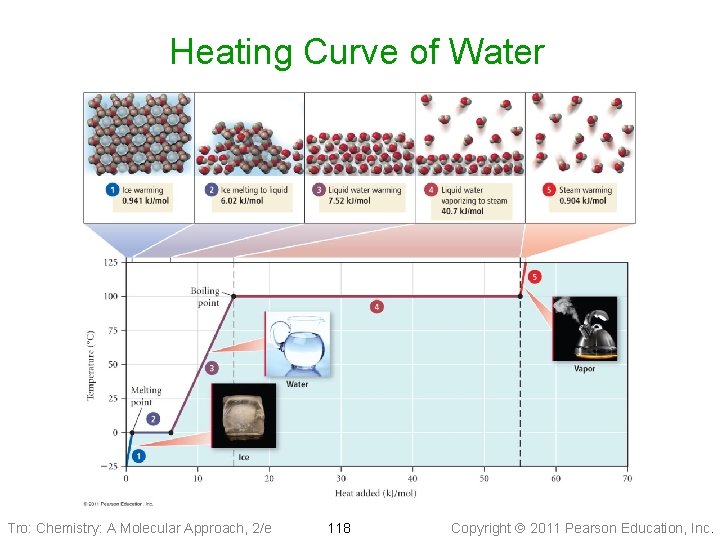
Heating Curve of Water Tro: Chemistry: A Molecular Approach, 2/e 118 Copyright 2011 Pearson Education, Inc.

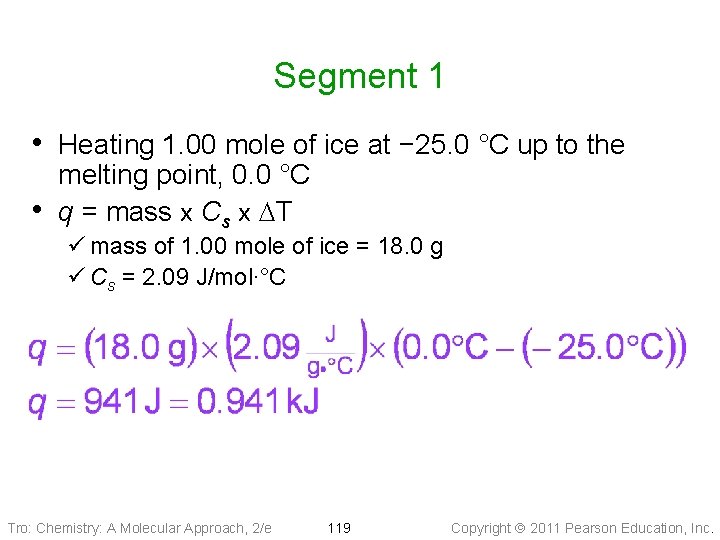
Segment 1 • Heating 1. 00 mole of ice at − 25. 0 °C up to the • melting point, 0. 0 °C q = mass x Cs x DT ü mass of 1. 00 mole of ice = 18. 0 g ü Cs = 2. 09 J/mol∙°C Tro: Chemistry: A Molecular Approach, 2/e 119 Copyright 2011 Pearson Education, Inc.

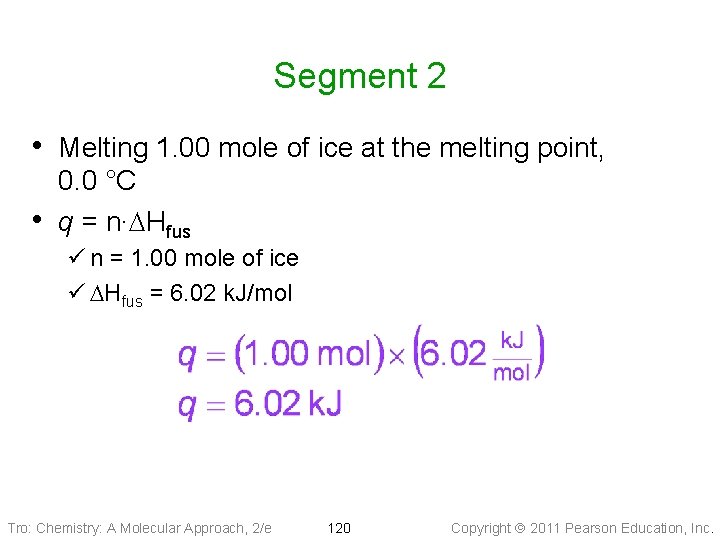
Segment 2 • Melting 1. 00 mole of ice at the melting point, • 0. 0 °C q = n∙DHfus ü n = 1. 00 mole of ice ü DHfus = 6. 02 k. J/mol Tro: Chemistry: A Molecular Approach, 2/e 120 Copyright 2011 Pearson Education, Inc.

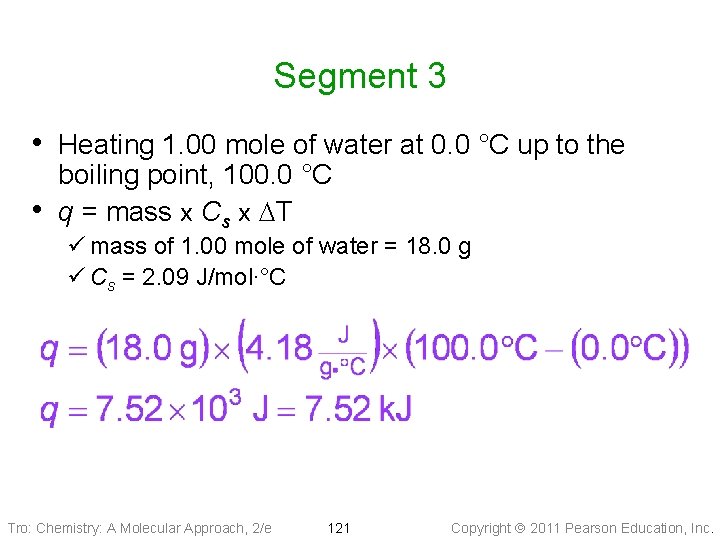
Segment 3 • Heating 1. 00 mole of water at 0. 0 °C up to the • boiling point, 100. 0 °C q = mass x Cs x DT ü mass of 1. 00 mole of water = 18. 0 g ü Cs = 2. 09 J/mol∙°C Tro: Chemistry: A Molecular Approach, 2/e 121 Copyright 2011 Pearson Education, Inc.

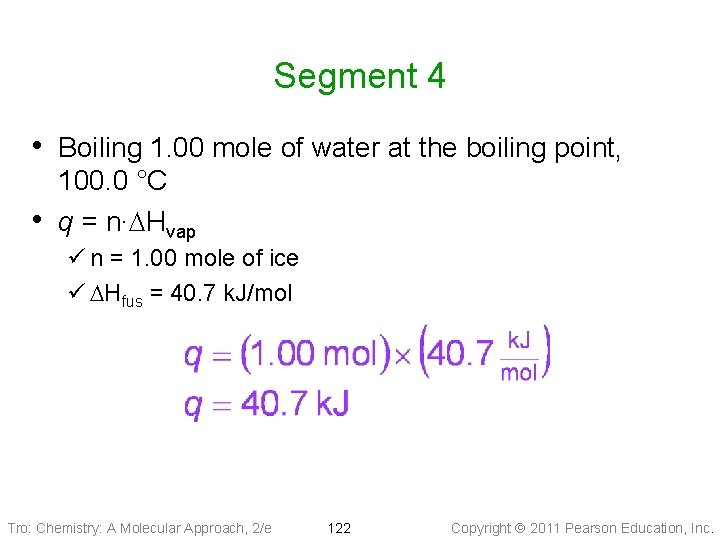
Segment 4 • Boiling 1. 00 mole of water at the boiling point, • 100. 0 °C q = n∙DHvap ü n = 1. 00 mole of ice ü DHfus = 40. 7 k. J/mol Tro: Chemistry: A Molecular Approach, 2/e 122 Copyright 2011 Pearson Education, Inc.

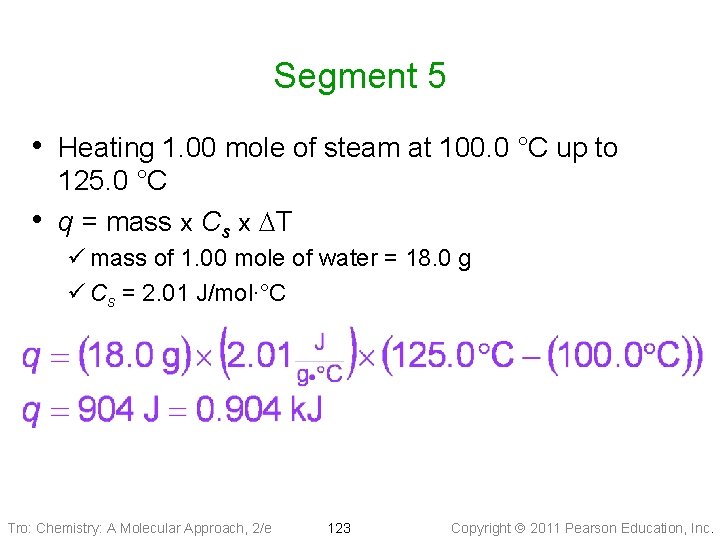
Segment 5 • Heating 1. 00 mole of steam at 100. 0 °C up to • 125. 0 °C q = mass x Cs x DT ü mass of 1. 00 mole of water = 18. 0 g ü Cs = 2. 01 J/mol∙°C Tro: Chemistry: A Molecular Approach, 2/e 123 Copyright 2011 Pearson Education, Inc.

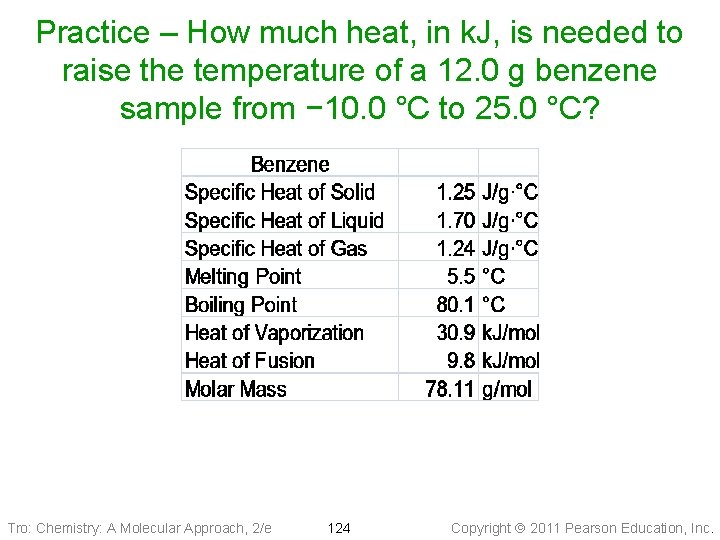
Practice – How much heat, in k. J, is needed to raise the temperature of a 12. 0 g benzene sample from − 10. 0 °C to 25. 0 °C? Tro: Chemistry: A Molecular Approach, 2/e 124 Copyright 2011 Pearson Education, Inc.

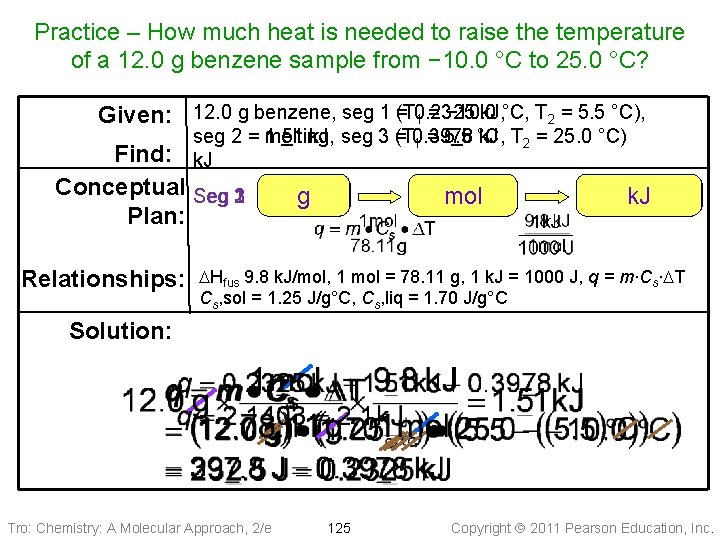
Practice – How much heat is needed to raise the temperature of a 12. 0 g benzene sample from − 10. 0 °C to 25. 0 °C? k. J, °C, T 2 = 5. 5 °C), Given: 12. 0 g benzene, seg 1 =(T 0. 2325 1 = − 10. 0 seg 2 = 1. 51 melting, k. J, seg 3 (T = 0. 3978 k. J T 2 = 25. 0 °C) 1 = 5. 5 °C, k. J Find: Conceptual Seg 231 Plan: Relationships: g mol J k. J DHfus 9. 8 k. J/mol, 1 mol = 78. 11 g, 1 k. J = 1000 J, q = m∙Cs∙DT Cs, sol = 1. 25 J/g°C, Cs, liq = 1. 70 J/g°C Solution: Tro: Chemistry: A Molecular Approach, 2/e 125 Copyright 2011 Pearson Education, Inc.

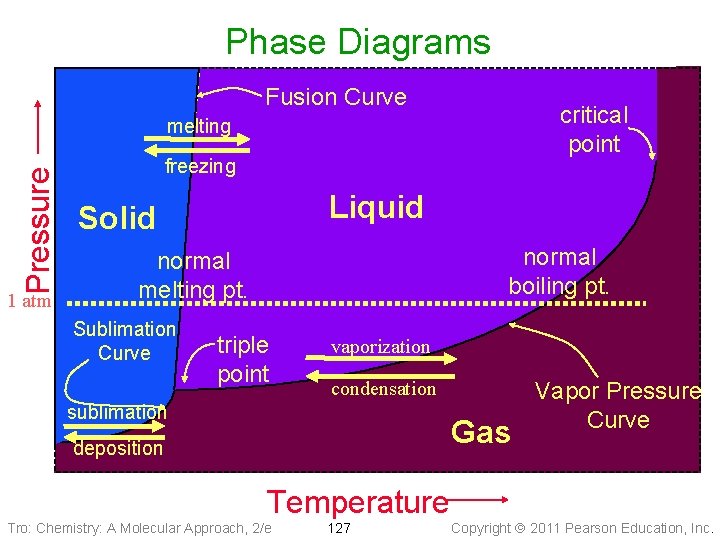
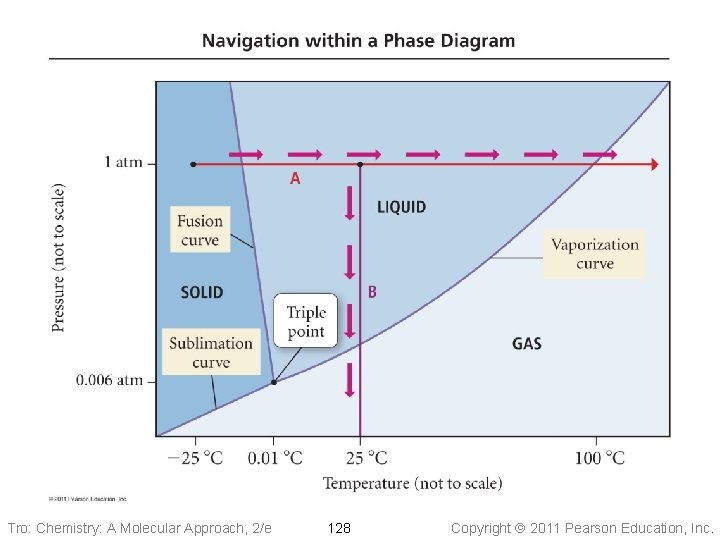
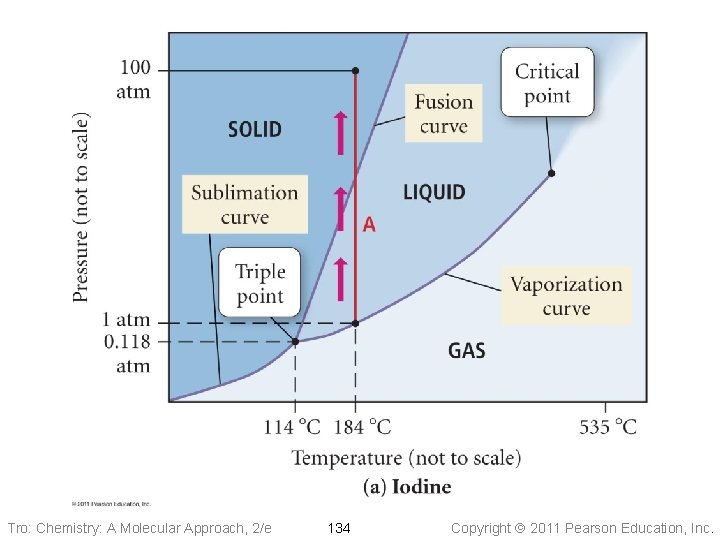
Phase Diagrams • Phase diagrams describe the different states and • • state changes that occur at various temperature/pressure conditions Regions represent states Lines represent state changes ü liquid/gas line is vapor pressure curve ü both states exist simultaneously ü critical point is the furthest point on the vapor pressure curve • Triple point is the temperature/pressure condition • where all three states exist simultaneously For most substances, freezing point increases as pressure increases Tro: Chemistry: A Molecular Approach, 2/e 126 Copyright 2011 Pearson Education, Inc.

Phase Diagrams Fusion Curve critical point Pressure melting 1 atm freezing Liquid Solid normal boiling pt. normal melting pt. Sublimation Curve triple point vaporization condensation sublimation Gas deposition Temperature Tro: Chemistry: A Molecular Approach, 2/e 127 Vapor Pressure Curve Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 128 Copyright 2011 Pearson Education, Inc.

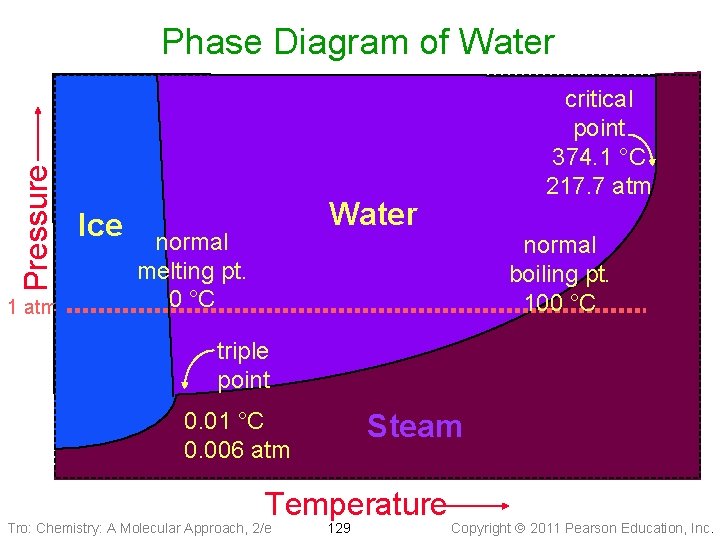
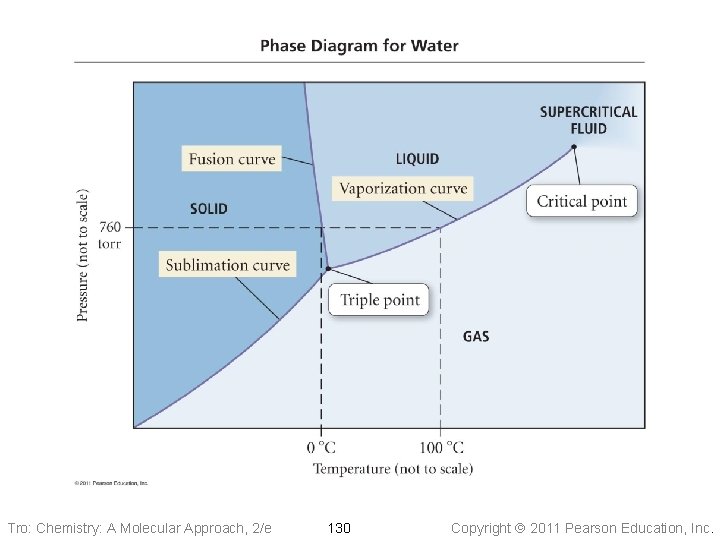
Pressure Phase Diagram of Water 1 atm Ice critical point 374. 1 °C 217. 7 atm Water normal melting pt. 0 °C normal boiling pt. 100 °C triple point 0. 01 °C 0. 006 atm Steam Temperature Tro: Chemistry: A Molecular Approach, 2/e 129 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 130 Copyright 2011 Pearson Education, Inc.

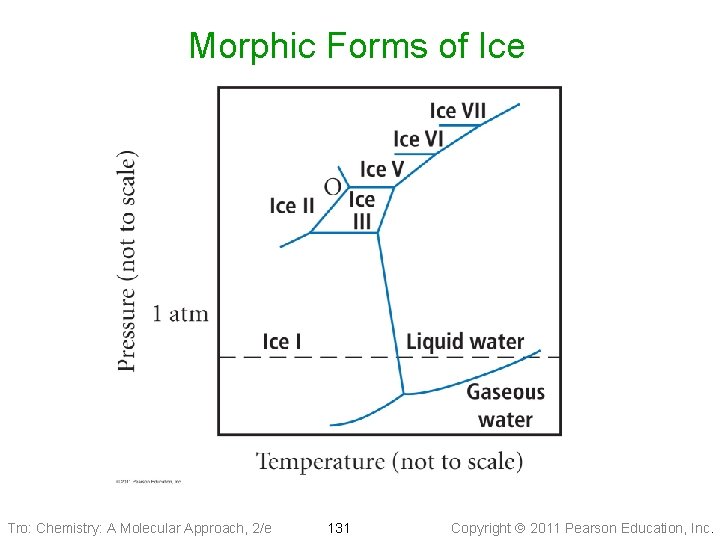
Morphic Forms of Ice Tro: Chemistry: A Molecular Approach, 2/e 131 Copyright 2011 Pearson Education, Inc.

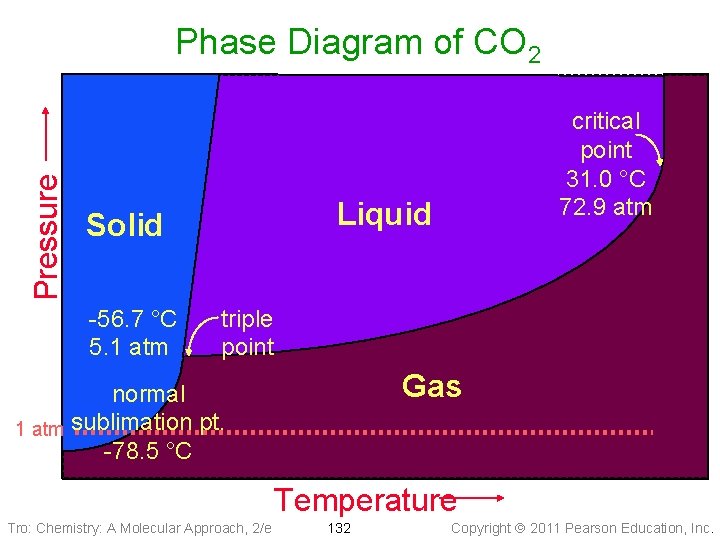
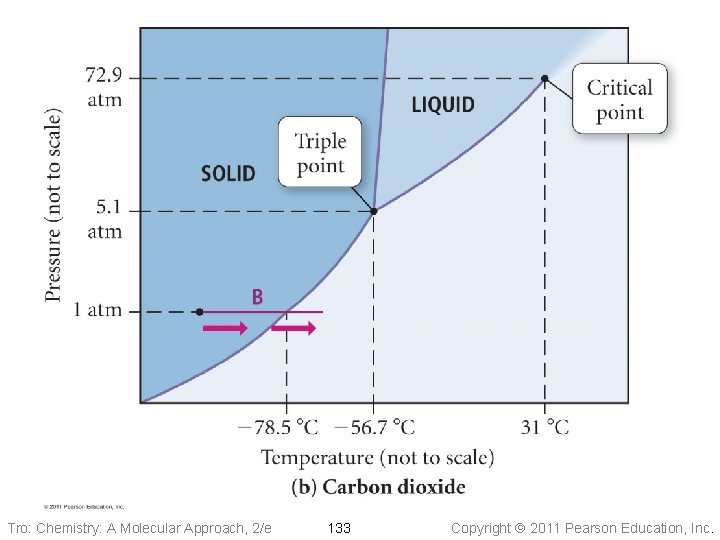
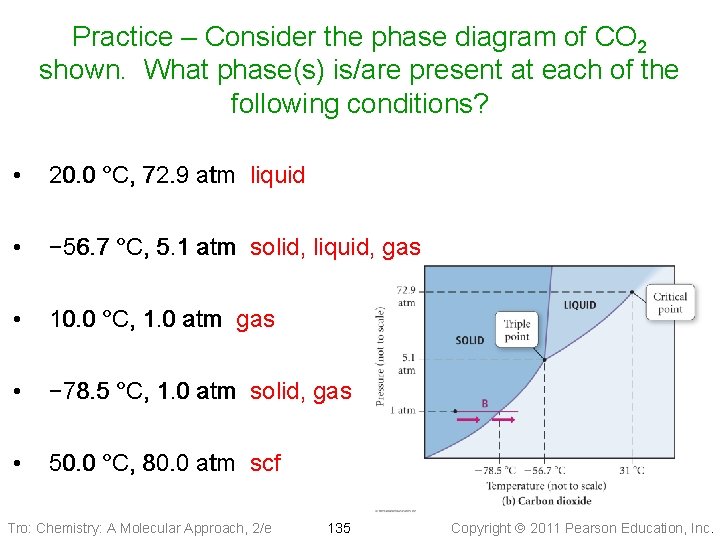
Pressure Phase Diagram of CO 2 Liquid Solid -56. 7 °C 5. 1 atm critical point 31. 0 °C 72. 9 atm triple point Gas normal 1 atm sublimation pt. -78. 5 °C Temperature Tro: Chemistry: A Molecular Approach, 2/e 132 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 133 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 134 Copyright 2011 Pearson Education, Inc.

Practice – Consider the phase diagram of CO 2 shown. What phase(s) is/are present at each of the following conditions? • 20. 0 °C, 72. 9 atm liquid • − 56. 7 °C, 5. 1 atm solid, liquid, gas • 10. 0 °C, 1. 0 atm gas • − 78. 5 °C, 1. 0 atm solid, gas • 50. 0 °C, 80. 0 atm scf Tro: Chemistry: A Molecular Approach, 2/e 135 Copyright 2011 Pearson Education, Inc.

Water – An Extraordinary Substance • Water is a liquid at room temperature ü most molecular substances with similar molar masses are gases at room temperature Ø e. g. NH 3, CH 4 ü due to H-bonding between molecules • Water is an excellent solvent – dissolving many ionic and polar molecular substances ü because of its large dipole moment ü even many small nonpolar molecules have some solubility in water Ø e. g. O 2, CO 2 • Water has a very high specific heat for a molecular substance ü moderating effect on coastal climates • Water expands when it freezes Ø at a pressure of 1 atm ü about 9% ü making ice less dense than liquid water Tro: Chemistry: A Molecular Approach, 2/e 136 Copyright 2011 Pearson Education, Inc.

Solids Properties & Structure Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

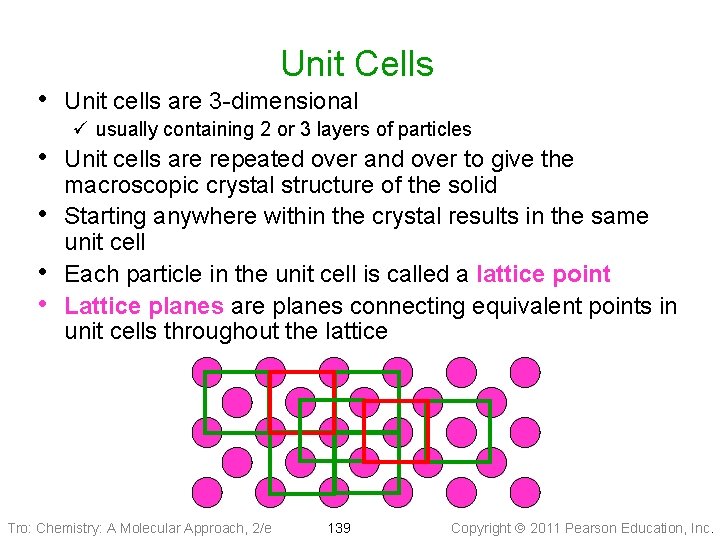
Crystal Lattice • When allowed to cool slowly, the particles in a liquid will arrange themselves to give the maximum attractive forces ü therefore minimize the energy • The result will generally be a crystalline solid • The arrangement of the particles in a • crystalline solid is called the crystal lattice The smallest unit that shows the pattern of arrangement for all the particles is called the unit cell Tro: Chemistry: A Molecular Approach, 2/e 138 Copyright 2011 Pearson Education, Inc.

Unit Cells • Unit cells are 3 -dimensional ü usually containing 2 or 3 layers of particles • Unit cells are repeated over and over to give the • • • macroscopic crystal structure of the solid Starting anywhere within the crystal results in the same unit cell Each particle in the unit cell is called a lattice point Lattice planes are planes connecting equivalent points in unit cells throughout the lattice Tro: Chemistry: A Molecular Approach, 2/e 139 Copyright 2011 Pearson Education, Inc.

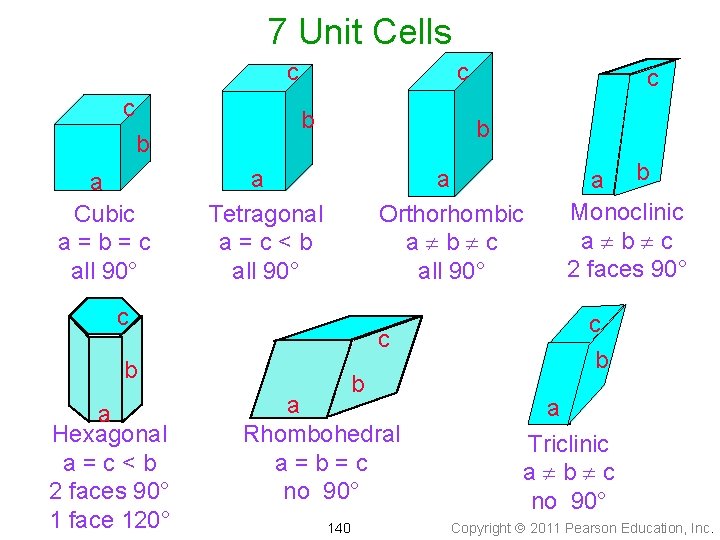
7 Unit Cells c c b a Cubic a=b=c all 90° c b b a Tetragonal a=c<b all 90° a Orthorhombic a¹b¹c all 90° c c b a Hexagonal a=c<b 2 faces 90° 1 face 120° c b a Rhombohedral a=b=c no 90° 140 a b Monoclinic a¹b¹c 2 faces 90° c b a Triclinic a¹b¹c no 90° Copyright 2011 Pearson Education, Inc.

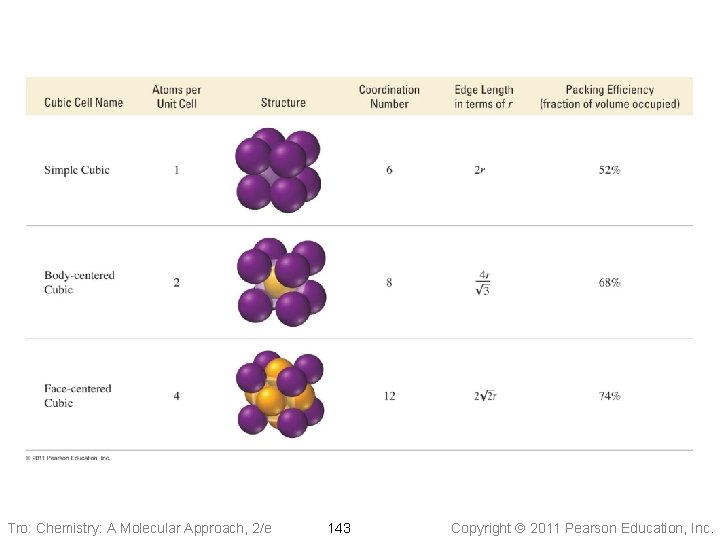
Unit Cells • The number of other particles each particle is in contact with is called its coordination number ü for ions, it is the number of oppositely charged ions an ion is in contact with • Higher coordination number means more • interaction, therefore stronger attractive forces holding the crystal together The packing efficiency is the percentage of volume in the unit cell occupied by particles ü the higher the coordination number, the more efficiently the particles are packing together Tro: Chemistry: A Molecular Approach, 2/e 141 Copyright 2011 Pearson Education, Inc.

Cubic Unit Cells • All 90° angles between corners of the unit cell • The length of all the edges are equal • If the unit cell is made of spherical particles ü ⅛ of each corner particle is within the cube ü ½ of each particle on a face is within the cube ü ¼ of each particle on an edge is within the cube Tro: Chemistry: A Molecular Approach, 2/e 142 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 143 Copyright 2011 Pearson Education, Inc.

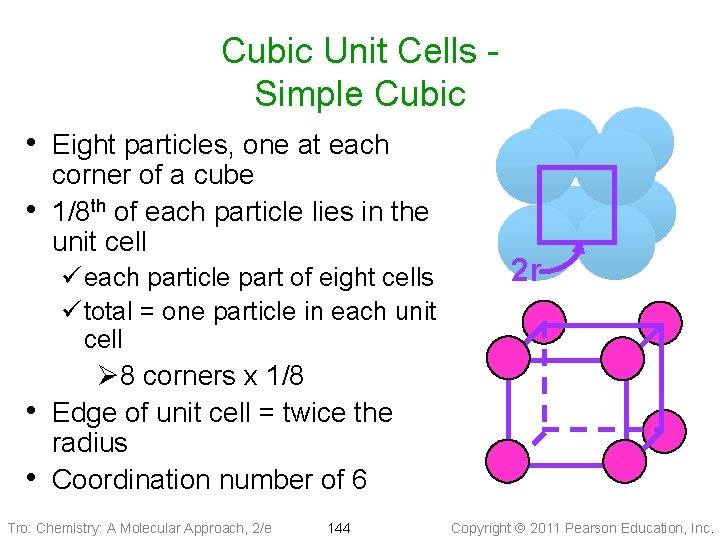
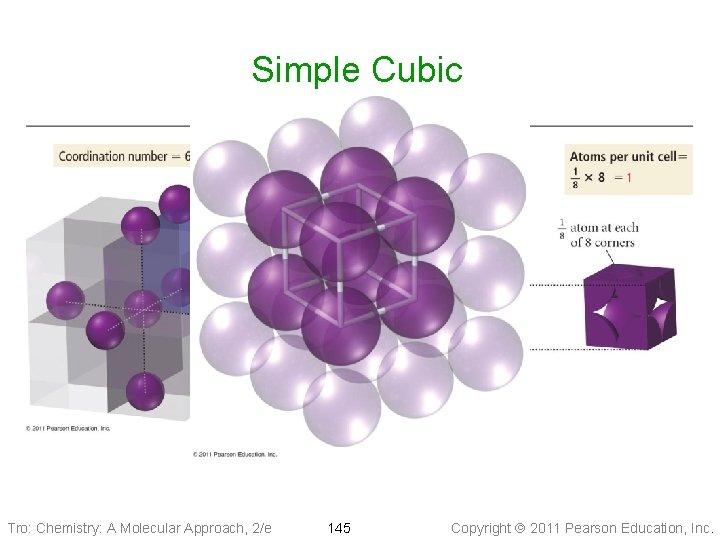
Cubic Unit Cells Simple Cubic • Eight particles, one at each • corner of a cube 1/8 th of each particle lies in the unit cell ü each particle part of eight cells ü total = one particle in each unit cell 2 r Ø 8 corners x 1/8 • Edge of unit cell = twice the radius • Coordination number of 6 Tro: Chemistry: A Molecular Approach, 2/e 144 Copyright 2011 Pearson Education, Inc.

Simple Cubic Tro: Chemistry: A Molecular Approach, 2/e 145 Copyright 2011 Pearson Education, Inc.

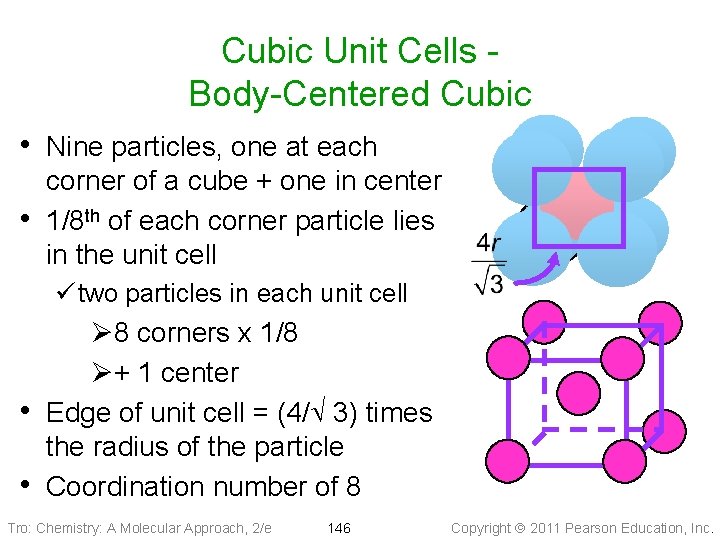
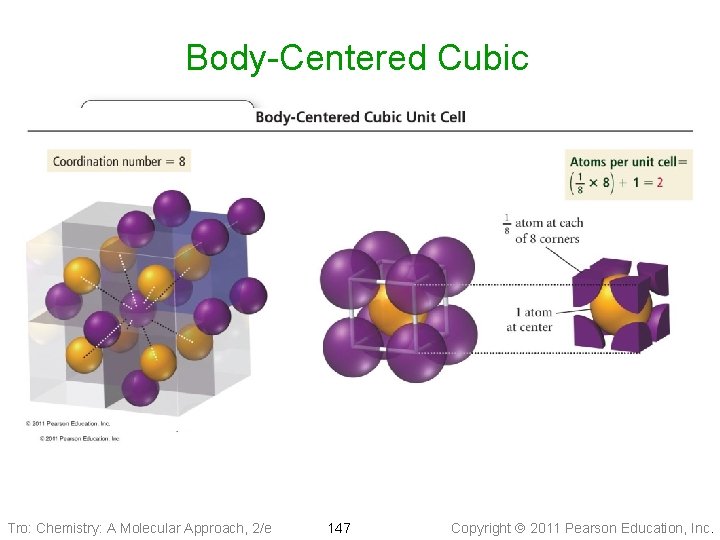
Cubic Unit Cells Body-Centered Cubic • Nine particles, one at each • corner of a cube + one in center 1/8 th of each corner particle lies in the unit cell ü two particles in each unit cell Ø 8 corners x 1/8 Ø+ 1 center • Edge of unit cell = (4/Ö 3) times the radius of the particle • Coordination number of 8 Tro: Chemistry: A Molecular Approach, 2/e 146 Copyright 2011 Pearson Education, Inc.

Body-Centered Cubic Tro: Chemistry: A Molecular Approach, 2/e 147 Copyright 2011 Pearson Education, Inc.

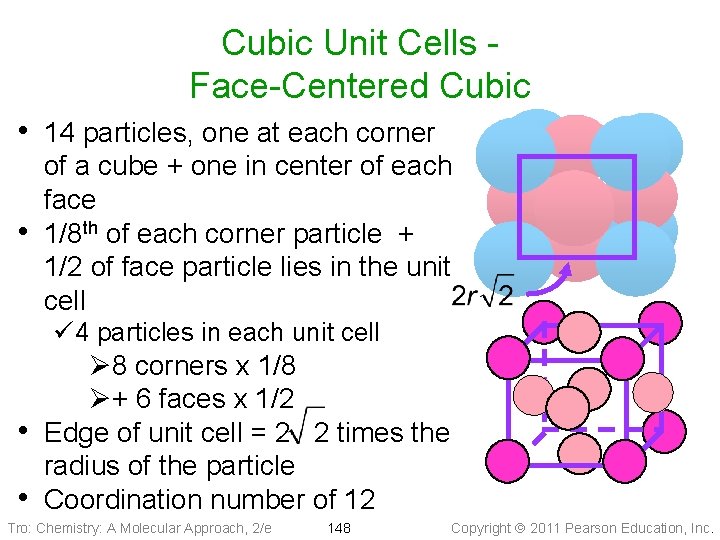
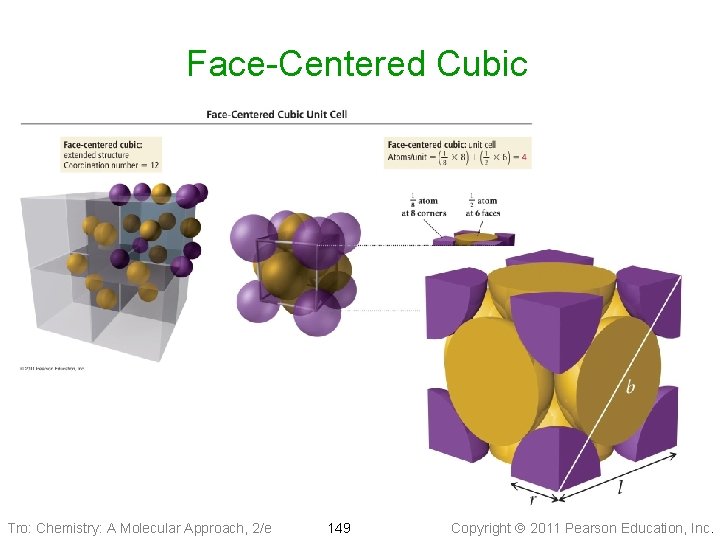
Cubic Unit Cells Face-Centered Cubic • 14 particles, one at each corner • of a cube + one in center of each face 1/8 th of each corner particle + 1/2 of face particle lies in the unit cell ü 4 particles in each unit cell Ø 8 corners x 1/8 Ø+ 6 faces x 1/2 • Edge of unit cell = 2 2 times the radius of the particle • Coordination number of 12 Tro: Chemistry: A Molecular Approach, 2/e 148 Copyright 2011 Pearson Education, Inc.

Face-Centered Cubic Tro: Chemistry: A Molecular Approach, 2/e 149 Copyright 2011 Pearson Education, Inc.

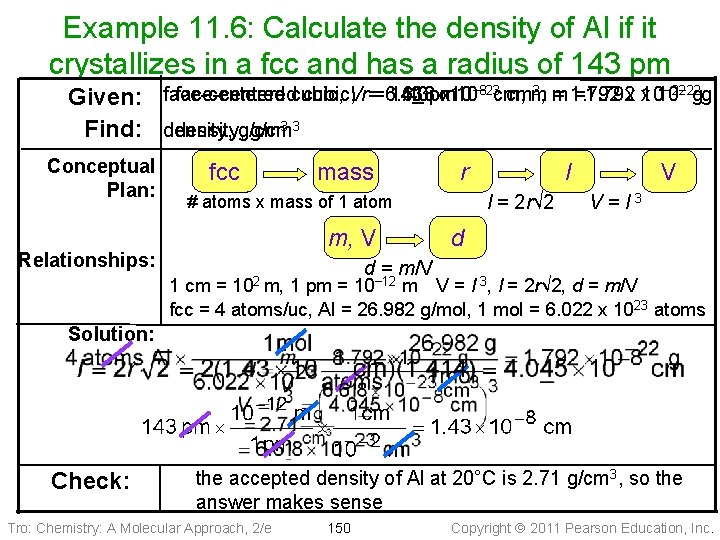
Example 11. 6: Calculate the density of Al if it crystallizes in a fcc and has a radius of 143 pm 3, m − 22 face-centeredcubic, Vr==6. 618 143 pm 1. 43 xx 10 10− 8− 23 cm, cmm = 1. 792 =1. 792 x x 1010 gg Given: face-centered 33 Find: density, g/cm Conceptual Plan: fcc mass r l = 2 r√ 2 # atoms x mass of 1 atom m, V Relationships: l V V=l 3 d d = m/V 102 m, 1 cm = 1 pm = 10− 12 m V = l 3, l = 2 r√ 2, d = m/V fcc = 4 atoms/uc, Al = 26. 982 g/mol, 1 mol = 6. 022 x 1023 atoms Solution: Check: the accepted density of Al at 20°C is 2. 71 g/cm 3, so the answer makes sense Tro: Chemistry: A Molecular Approach, 2/e 150 Copyright 2011 Pearson Education, Inc.

Practice – Estimate the density of Rb if it crystallizes in a body-centered cubic unit cell and has an atomic radius of 247. 5 pm Tro: Chemistry: A Molecular Approach, 2/e 151 Copyright 2011 Pearson Education, Inc.

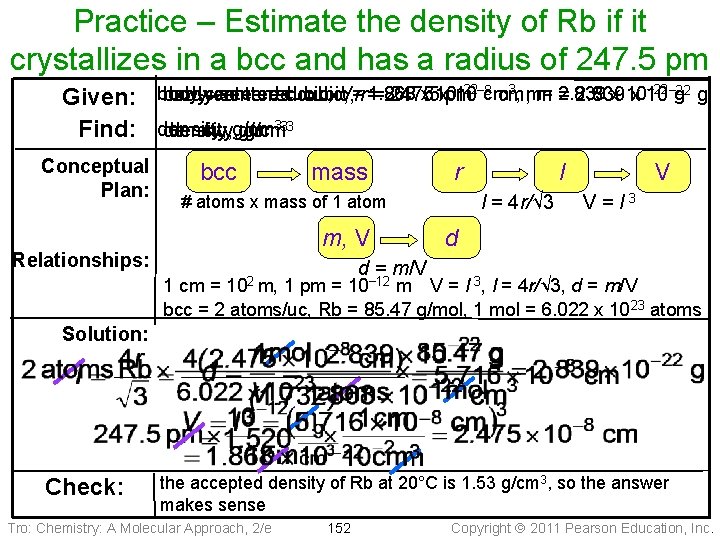
Practice – Estimate the density of Rb if it crystallizes in a bcc and has a radius of 247. 5 pm − 22− 8 3, m= 2. 839 x 10− 22 cubic, V=rr= 1. 868 x 10 cm body-centered cubic, =2. 475 247. 5 xpm 10 cm, m = 2. 839 x 10 g g Given: body-centered 333 Find: density, g/cm Conceptual Plan: bcc mass r l = 4 r/√ 3 # atoms x mass of 1 atom m, V Relationships: l V V=l 3 d d = m/V 102 m, 1 cm = 1 pm = 10− 12 m V = l 3, l = 4 r/√ 3, d = m/V bcc = 2 atoms/uc, Rb = 85. 47 g/mol, 1 mol = 6. 022 x 1023 atoms Solution: Check: the accepted density of Rb at 20°C is 1. 53 g/cm 3, so the answer makes sense Tro: Chemistry: A Molecular Approach, 2/e 152 Copyright 2011 Pearson Education, Inc.

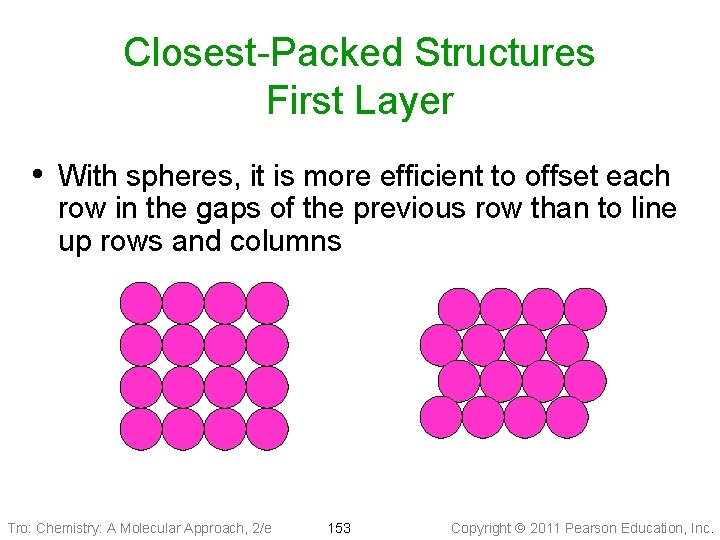
Closest-Packed Structures First Layer • With spheres, it is more efficient to offset each row in the gaps of the previous row than to line up rows and columns Tro: Chemistry: A Molecular Approach, 2/e 153 Copyright 2011 Pearson Education, Inc.

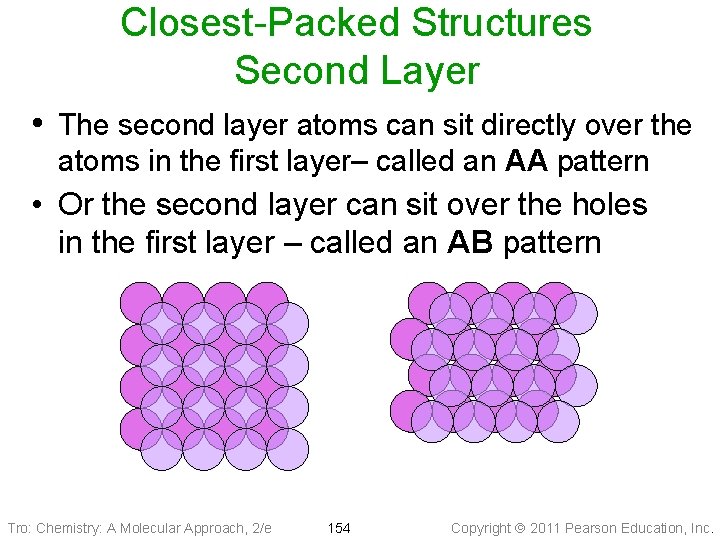
Closest-Packed Structures Second Layer • The second layer atoms can sit directly over the atoms in the first layer– called an AA pattern • Or the second layer can sit over the holes in the first layer – called an AB pattern Tro: Chemistry: A Molecular Approach, 2/e 154 Copyright 2011 Pearson Education, Inc.

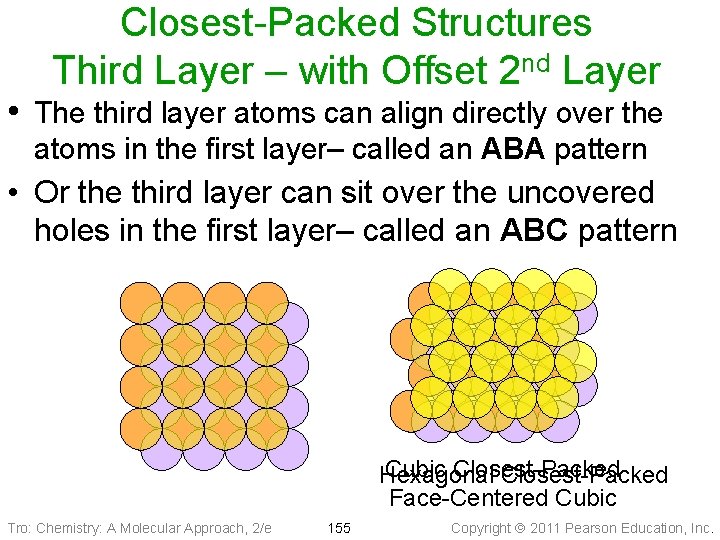
Closest-Packed Structures Third Layer – with Offset 2 nd Layer • The third layer atoms can align directly over the atoms in the first layer– called an ABA pattern • Or the third layer can sit over the uncovered holes in the first layer– called an ABC pattern Cubic Closest-Packed Hexagonal Closest-Packed Face-Centered Cubic Tro: Chemistry: A Molecular Approach, 2/e 155 Copyright 2011 Pearson Education, Inc.

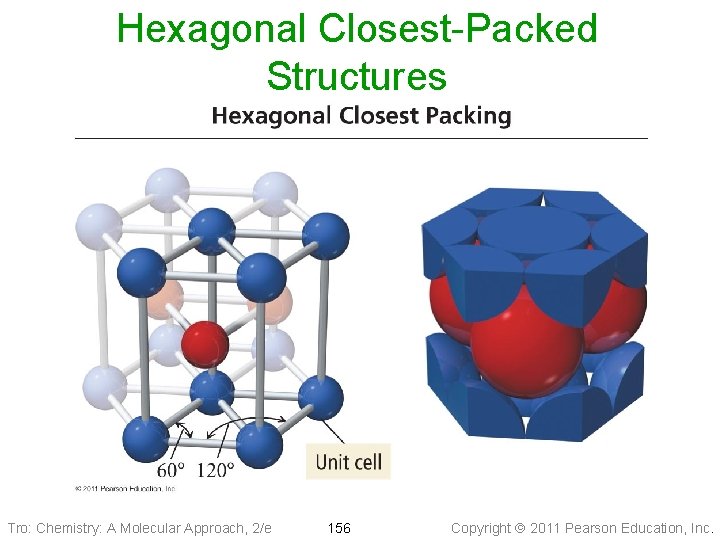
Hexagonal Closest-Packed Structures Tro: Chemistry: A Molecular Approach, 2/e 156 Copyright 2011 Pearson Education, Inc.

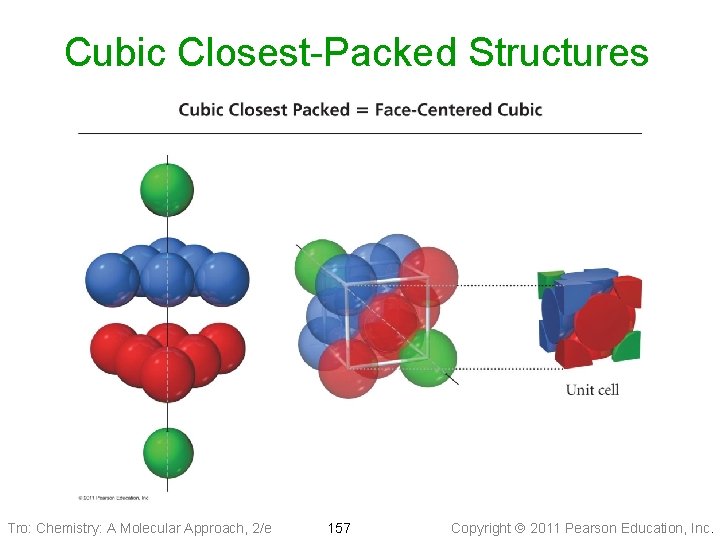
Cubic Closest-Packed Structures Tro: Chemistry: A Molecular Approach, 2/e 157 Copyright 2011 Pearson Education, Inc.

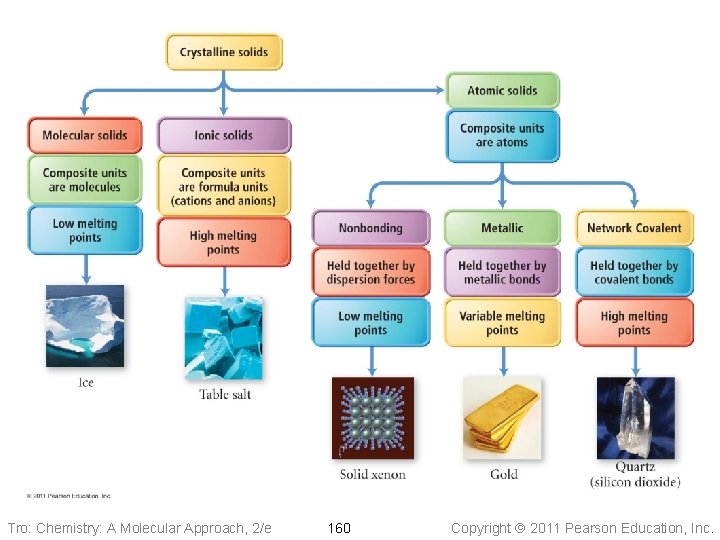
Classifying Crystalline Solids • Crystalline solids are classified by the kinds of • particles found Some of the categories are sub-classified by the kinds of attractive forces holding the particles together Tro: Chemistry: A Molecular Approach, 2/e 158 Copyright 2011 Pearson Education, Inc.

Classifying Crystalline Solids • Molecular solids are solids whose composite • • particles are molecules Ionic solids are solids whose composite particles are ions Atomic solids are solids whose composite particles are atoms ü nonbonding atomic solids are held together by dispersion forces ü metallic atomic solids are held together by metallic bonds ü network covalent atomic solids are held together by covalent bonds Tro: Chemistry: A Molecular Approach, 2/e 159 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 160 Copyright 2011 Pearson Education, Inc.

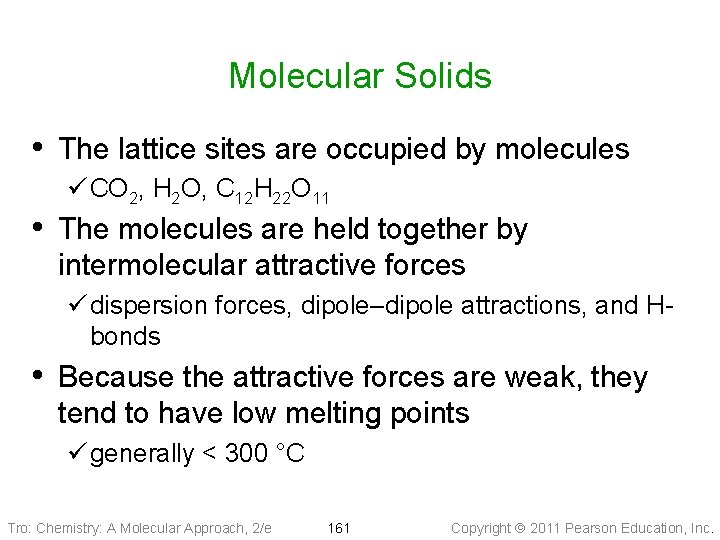
Molecular Solids • The lattice sites are occupied by molecules ü CO 2, H 2 O, C 12 H 22 O 11 • The molecules are held together by intermolecular attractive forces ü dispersion forces, dipole–dipole attractions, and Hbonds • Because the attractive forces are weak, they tend to have low melting points ü generally < 300 °C Tro: Chemistry: A Molecular Approach, 2/e 161 Copyright 2011 Pearson Education, Inc.

Ionic Solids • Lattice sites occupied by ions • Held together by attractions between oppositely charged ions ü nondirectional ü therefore every cation attracts all anions around it, and vice-versa • The coordination number represents the number of close • cation–anion interactions in the crystal The higher the coordination number, the more stable the solid ü lowers the potential energy of the solid • The coordination number depends on the relative sizes of the cations and anions that maintains charge balance ü generally, anions are larger than cations ü the number of anions that can surround the cation is limited by the size of the cation ü the closer in size the ions are, the higher the coordination number is Tro: Chemistry: A Molecular Approach, 2/e 162 Copyright 2011 Pearson Education, Inc.

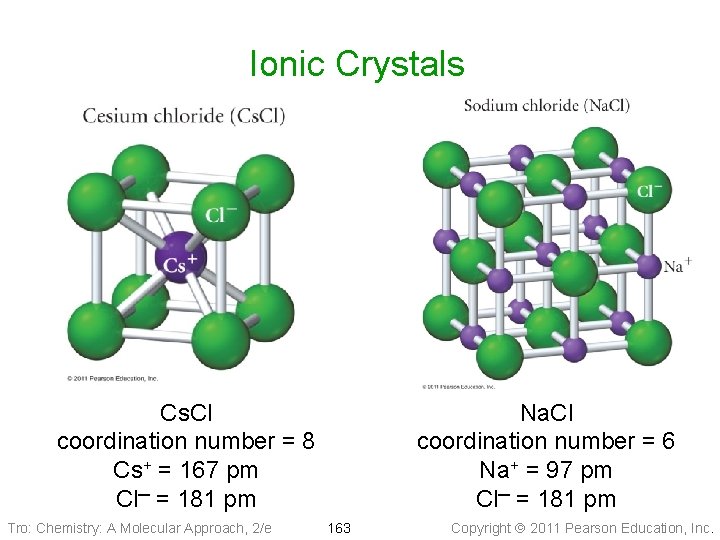
Ionic Crystals Cs. Cl coordination number = 8 Cs+ = 167 pm Cl─ = 181 pm Tro: Chemistry: A Molecular Approach, 2/e Na. Cl coordination number = 6 Na+ = 97 pm Cl─ = 181 pm 163 Copyright 2011 Pearson Education, Inc.

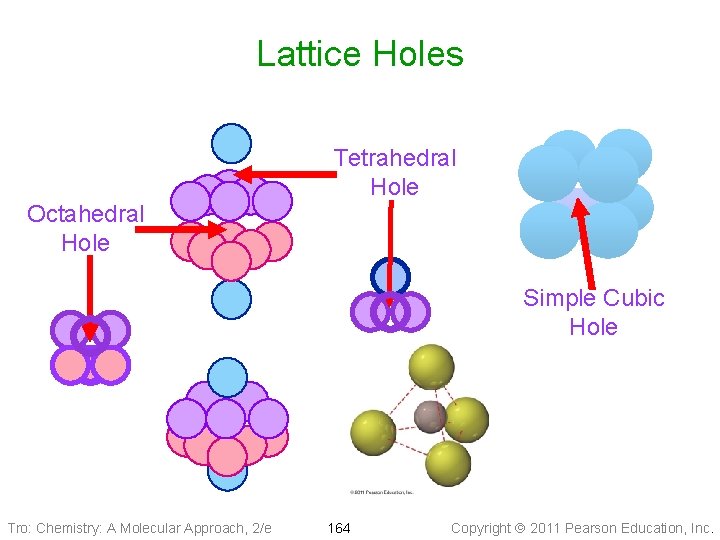
Lattice Holes Tetrahedral Hole Octahedral Hole Simple Cubic Hole Tro: Chemistry: A Molecular Approach, 2/e 164 Copyright 2011 Pearson Education, Inc.

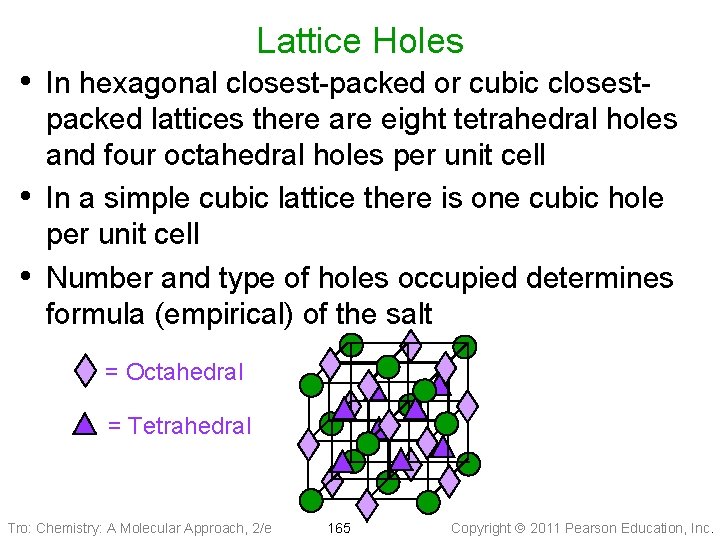
Lattice Holes • In hexagonal closest-packed or cubic closest • • packed lattices there are eight tetrahedral holes and four octahedral holes per unit cell In a simple cubic lattice there is one cubic hole per unit cell Number and type of holes occupied determines formula (empirical) of the salt = Octahedral = Tetrahedral Tro: Chemistry: A Molecular Approach, 2/e 165 Copyright 2011 Pearson Education, Inc.

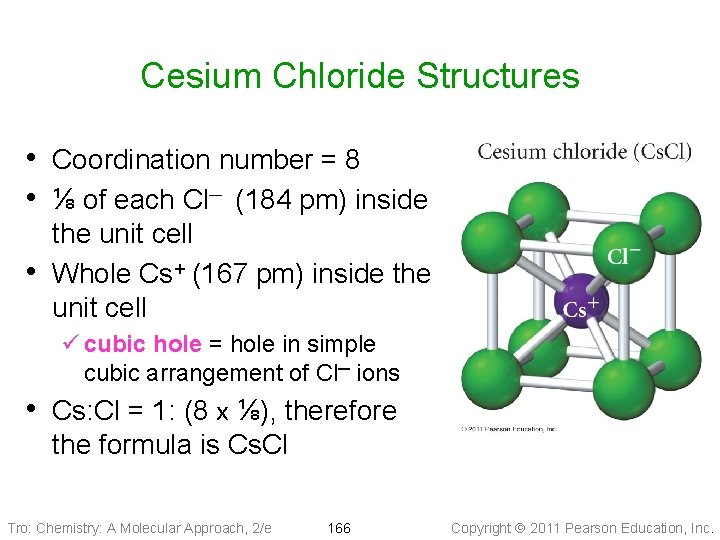
Cesium Chloride Structures • Coordination number = 8 • ⅛ of each Cl─ (184 pm) inside • the unit cell Whole Cs+ (167 pm) inside the unit cell ü cubic hole = hole in simple cubic arrangement of Cl─ ions • Cs: Cl = 1: (8 x ⅛), therefore the formula is Cs. Cl Tro: Chemistry: A Molecular Approach, 2/e 166 Copyright 2011 Pearson Education, Inc.

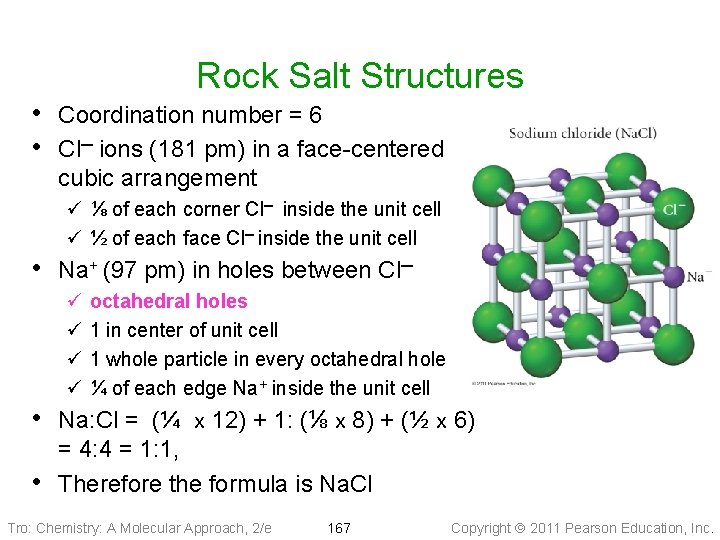
Rock Salt Structures • Coordination number = 6 • Cl─ ions (181 pm) in a face-centered cubic arrangement ü ⅛ of each corner Cl─ inside the unit cell ü ½ of each face Cl─ inside the unit cell • Na+ (97 pm) in holes between Cl─ ü ü octahedral holes 1 in center of unit cell 1 whole particle in every octahedral hole ¼ of each edge Na+ inside the unit cell • Na: Cl = (¼ x 12) + 1: (⅛ x 8) + (½ x 6) • = 4: 4 = 1: 1, Therefore the formula is Na. Cl Tro: Chemistry: A Molecular Approach, 2/e 167 Copyright 2011 Pearson Education, Inc.

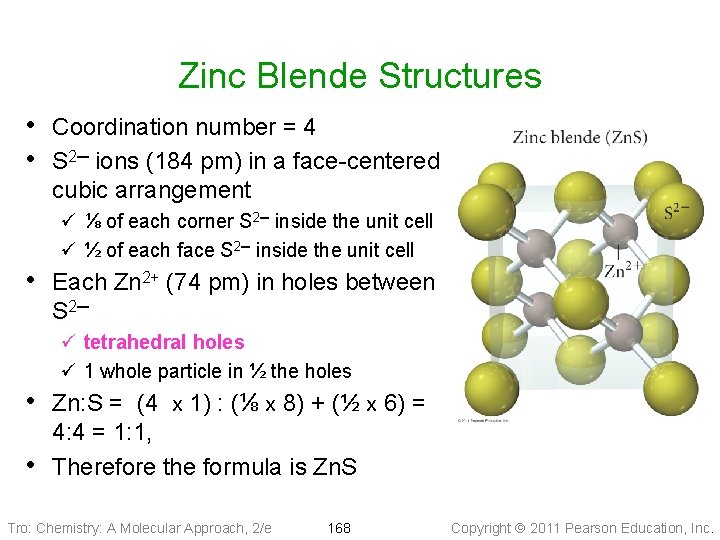
Zinc Blende Structures • Coordination number = 4 • S 2─ ions (184 pm) in a face-centered cubic arrangement ü ⅛ of each corner S 2─ inside the unit cell ü ½ of each face S 2─ inside the unit cell • Each Zn 2+ (74 pm) in holes between S 2─ ü tetrahedral holes ü 1 whole particle in ½ the holes • Zn: S = (4 x 1) : (⅛ x 8) + (½ x 6) = • 4: 4 = 1: 1, Therefore the formula is Zn. S Tro: Chemistry: A Molecular Approach, 2/e 168 Copyright 2011 Pearson Education, Inc.

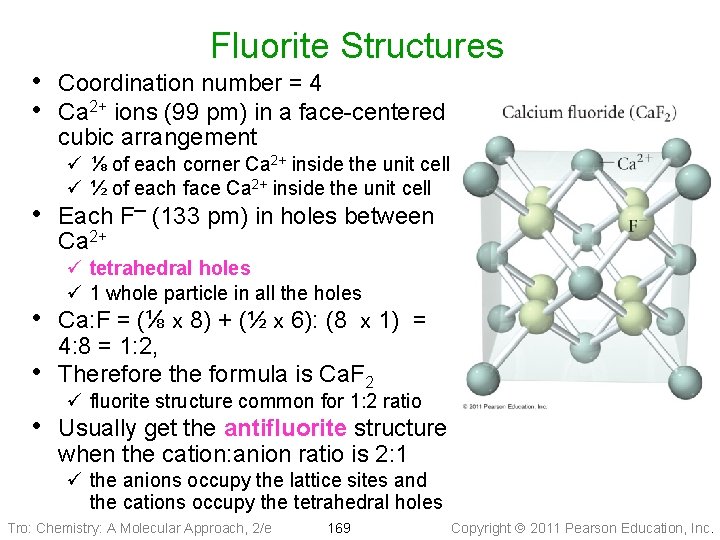
Fluorite Structures • Coordination number = 4 • Ca 2+ ions (99 pm) in a face-centered cubic arrangement ü ⅛ of each corner Ca 2+ inside the unit cell ü ½ of each face Ca 2+ inside the unit cell • Each F─ (133 pm) in holes between Ca 2+ ü tetrahedral holes ü 1 whole particle in all the holes • Ca: F = (⅛ x 8) + (½ x 6): (8 x 1) = • 4: 8 = 1: 2, Therefore the formula is Ca. F 2 ü fluorite structure common for 1: 2 ratio • Usually get the antifluorite structure when the cation: anion ratio is 2: 1 ü the anions occupy the lattice sites and the cations occupy the tetrahedral holes Tro: Chemistry: A Molecular Approach, 2/e 169 Copyright 2011 Pearson Education, Inc.

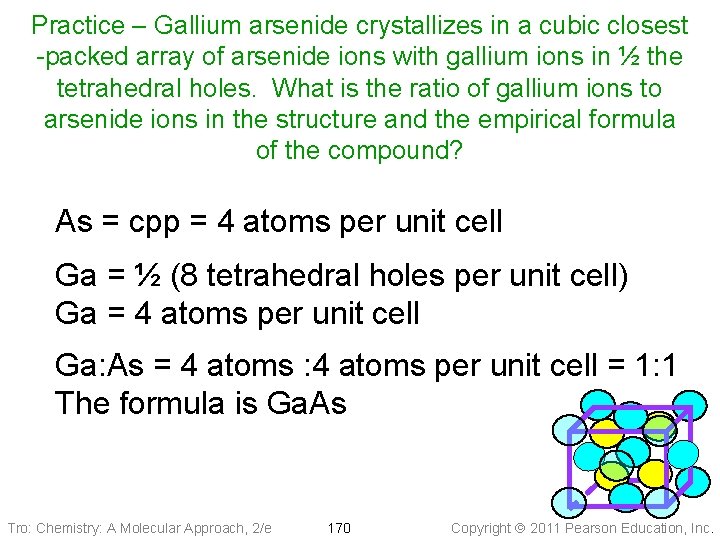
Practice – Gallium arsenide crystallizes in a cubic closest -packed array of arsenide ions with gallium ions in ½ the tetrahedral holes. What is the ratio of gallium ions to arsenide ions in the structure and the empirical formula of the compound? As = cpp = 4 atoms per unit cell Ga = ½ (8 tetrahedral holes per unit cell) Ga = 4 atoms per unit cell Ga: As = 4 atoms : 4 atoms per unit cell = 1: 1 The formula is Ga. As Tro: Chemistry: A Molecular Approach, 2/e 170 Copyright 2011 Pearson Education, Inc.

Nonbonding Atomic Solids • Noble gases in solid form • Solid held together by weak dispersion forces ü very low melting • Tend to arrange atoms in closest-packed structure ü either hexagonal cp or cubic cp ü maximizes attractive forces and minimizes energy Tro: Chemistry: A Molecular Approach, 2/e 171 Copyright 2011 Pearson Education, Inc.

Metallic Atomic Solids • Solid held together by metallic bonds ü strength varies with sizes and charges of cations Øcoulombic attractions • Melting point varies • Mostly closest-packed arrangements of the lattice points ü cations Tro: Chemistry: A Molecular Approach, 2/e 172 Copyright 2011 Pearson Education, Inc.

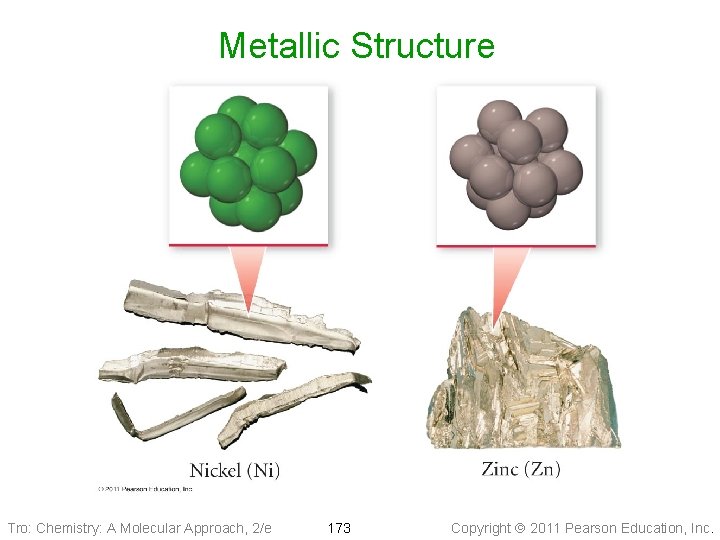
Metallic Structure Tro: Chemistry: A Molecular Approach, 2/e 173 Copyright 2011 Pearson Education, Inc.

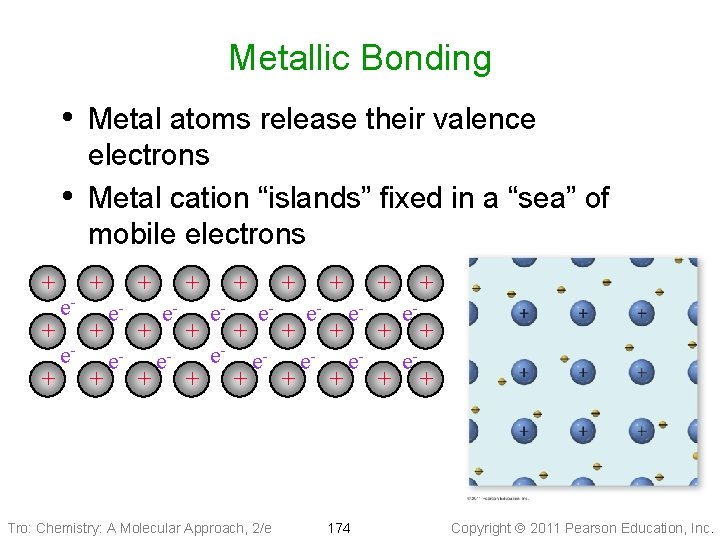
Metallic Bonding • Metal atoms release their valence • + + + ee- electrons Metal cation “islands” fixed in a “sea” of mobile electrons + + ee- + + + ee- Tro: Chemistry: A Molecular Approach, 2/e + + + ee- 174 + + + ee- + + Copyright 2011 Pearson Education, Inc.

Network Covalent Solids • Atoms attached to their nearest neighbors by • • covalent bonds Because of the directionality of the covalent bonds, these do not tend to form closest-packed arrangements in the crystal Because of the strength of the covalent bonds, these have very high melting points ü generally > 1000 °C • Dimensionality of the network affects other physical properties Tro: Chemistry: A Molecular Approach, 2/e 175 Copyright 2011 Pearson Education, Inc.

The Diamond Structure: a 3 -Dimensional Network • The carbon atoms in a diamond each have four covalent bonds to surrounding atoms ü sp 3 ü tetrahedral geometry • This effectively makes each crystal one giant molecule held together by covalent bonds ü you can follow a path of covalent bonds from any atom to every other atom Tro: Chemistry: A Molecular Approach, 2/e 176 Copyright 2011 Pearson Education, Inc.

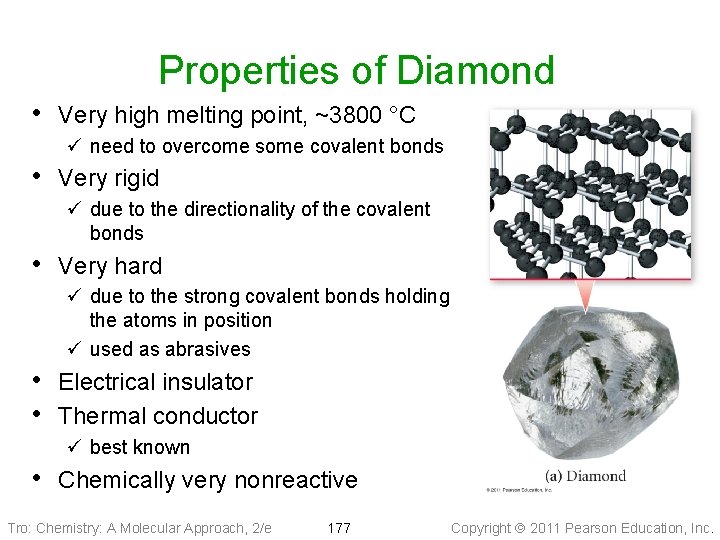
Properties of Diamond • Very high melting point, ~3800 °C ü need to overcome some covalent bonds • Very rigid ü due to the directionality of the covalent bonds • Very hard ü due to the strong covalent bonds holding the atoms in position ü used as abrasives • Electrical insulator • Thermal conductor ü best known • Chemically very nonreactive Tro: Chemistry: A Molecular Approach, 2/e 177 Copyright 2011 Pearson Education, Inc.

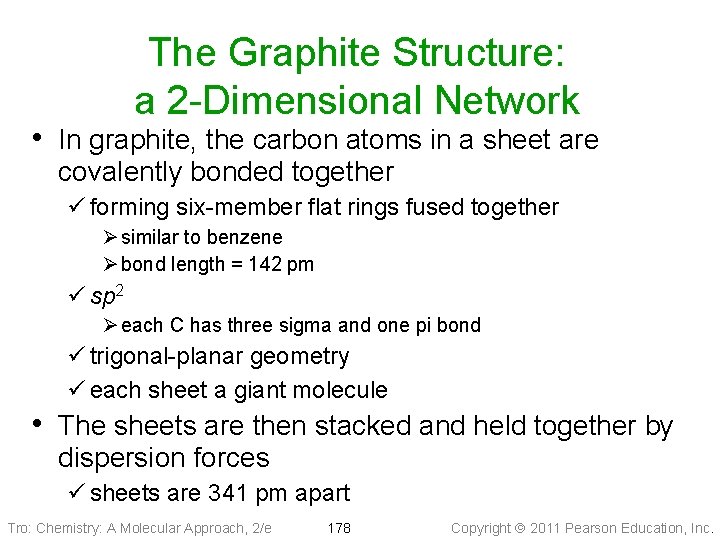
The Graphite Structure: a 2 -Dimensional Network • In graphite, the carbon atoms in a sheet are covalently bonded together ü forming six-member flat rings fused together Ø similar to benzene Ø bond length = 142 pm ü sp 2 Ø each C has three sigma and one pi bond ü trigonal-planar geometry ü each sheet a giant molecule • The sheets are then stacked and held together by dispersion forces ü sheets are 341 pm apart Tro: Chemistry: A Molecular Approach, 2/e 178 Copyright 2011 Pearson Education, Inc.

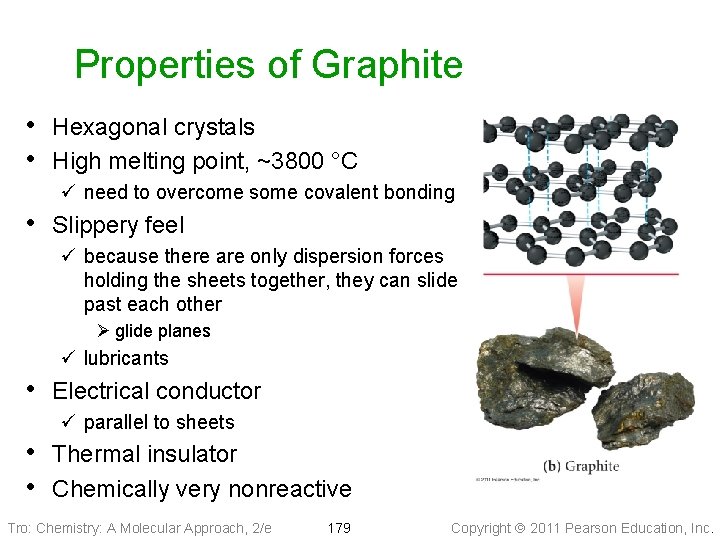
Properties of Graphite • Hexagonal crystals • High melting point, ~3800 °C ü need to overcome some covalent bonding • Slippery feel ü because there are only dispersion forces holding the sheets together, they can slide past each other Ø glide planes ü lubricants • Electrical conductor ü parallel to sheets • Thermal insulator • Chemically very nonreactive Tro: Chemistry: A Molecular Approach, 2/e 179 Copyright 2011 Pearson Education, Inc.

Silicates • ~90% of Earth’s crust • Extended arrays of Si O ü sometimes with Al substituted for Si – aluminosilicates • Glass is the amorphous form Tro: Chemistry: A Molecular Approach, 2/e 180 Copyright 2011 Pearson Education, Inc.

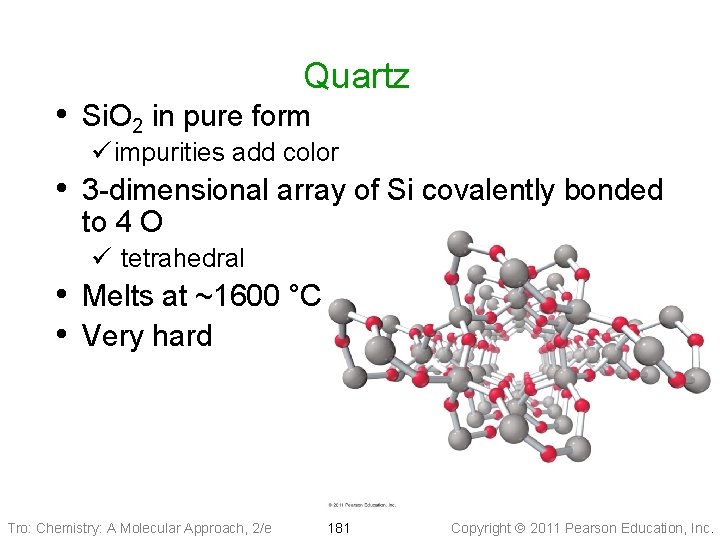
Quartz • Si. O 2 in pure form ü impurities add color • 3 -dimensional array of Si covalently bonded to 4 O ü tetrahedral • Melts at ~1600 °C • Very hard Tro: Chemistry: A Molecular Approach, 2/e 181 Copyright 2011 Pearson Education, Inc.

Micas • There are various kinds of mica that have slightly different compositions – but are all of the general form X 2 Y 4 -6 Z 8 O 20(OH, F)4 ü X is K, Na, or Ca or less commonly Ba, Rb, or Cs ü Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc. ü Z is chiefly Si or Al but also may include Fe 3+ or Ti • Minerals that are mainly 2 -dimensional arrays of Si bonded to O ü hexagonal arrangement of atoms • Sheets • Chemically stable • Thermal and electrical insulator Tro: Chemistry: A Molecular Approach, 2/e 182 Copyright 2011 Pearson Education, Inc.

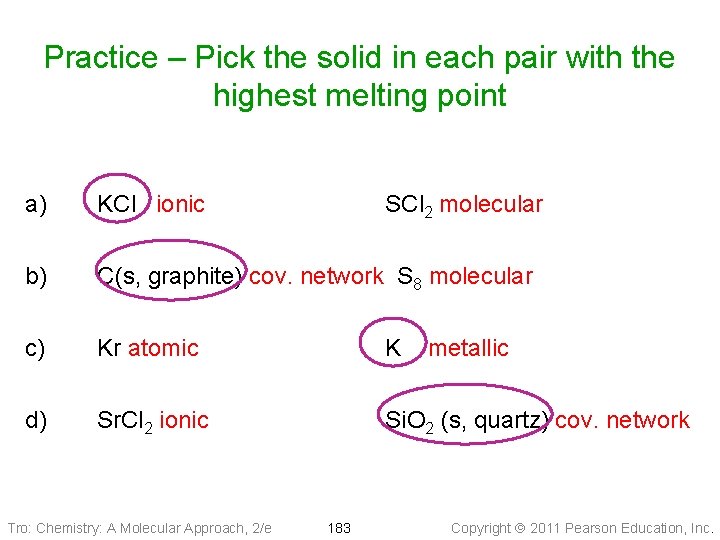
Practice – Pick the solid in each pair with the highest melting point a) KCl ionic b) C(s, graphite) cov. network S 8 molecular c) Kr atomic K d) Sr. Cl 2 ionic Si. O 2 (s, quartz) cov. network Tro: Chemistry: A Molecular Approach, 2/e SCl 2 molecular 183 metallic Copyright 2011 Pearson Education, Inc.

Band Theory • The structures of metals and covalent network • solids result in every atom’s orbitals being shared by the entire structure For large numbers of atoms, this results in a large number of molecular orbitals that have approximately the same energy; we call this an energy band Tro: Chemistry: A Molecular Approach, 2/e 184 Copyright 2011 Pearson Education, Inc.

Band Theory • When two atomic orbitals combine they • • • produce both a bonding and an antibonding molecular orbital When many atomic orbitals combine they produce a band of bonding molecular orbitals and a band of antibonding molecular orbitals The band of bonding molecular orbitals is called the valence band The band of antibonding molecular orbitals is called the conduction band Tro: Chemistry: A Molecular Approach, 2/e 185 Copyright 2011 Pearson Education, Inc.

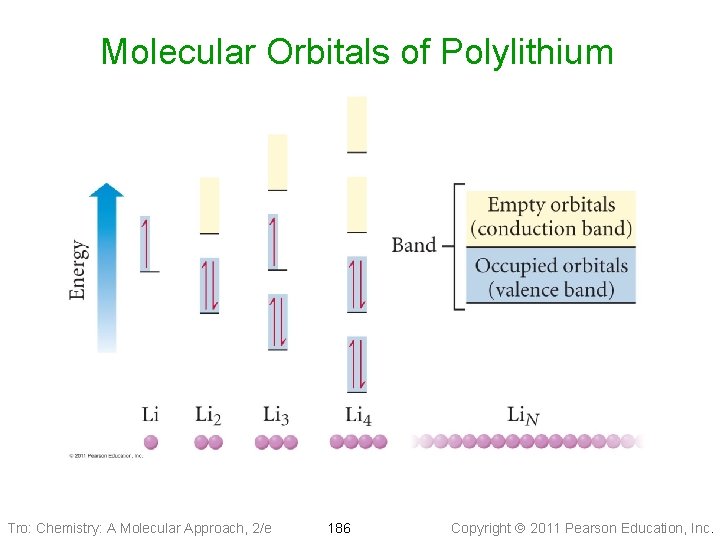
Molecular Orbitals of Polylithium Tro: Chemistry: A Molecular Approach, 2/e 186 Copyright 2011 Pearson Education, Inc.

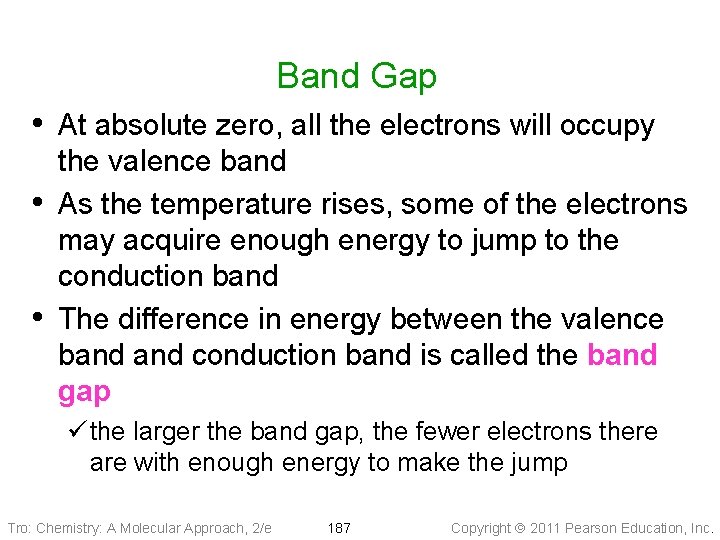
Band Gap • At absolute zero, all the electrons will occupy • • the valence band As the temperature rises, some of the electrons may acquire enough energy to jump to the conduction band The difference in energy between the valence band conduction band is called the band gap ü the larger the band gap, the fewer electrons there are with enough energy to make the jump Tro: Chemistry: A Molecular Approach, 2/e 187 Copyright 2011 Pearson Education, Inc.

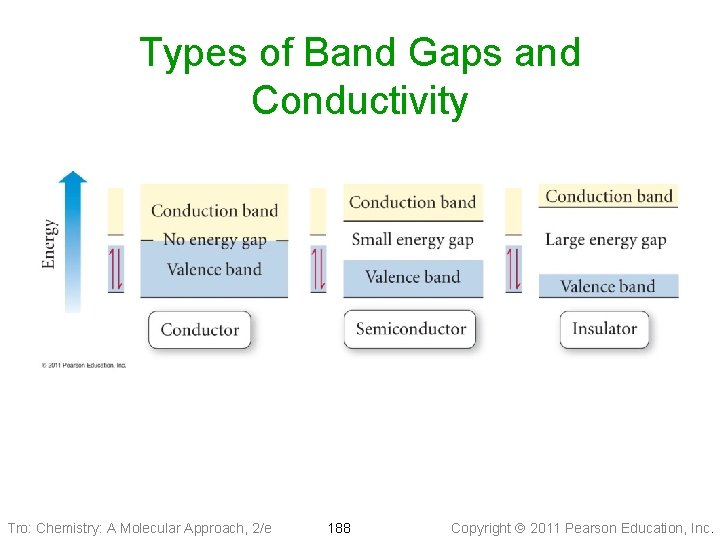
Types of Band Gaps and Conductivity Tro: Chemistry: A Molecular Approach, 2/e 188 Copyright 2011 Pearson Education, Inc.

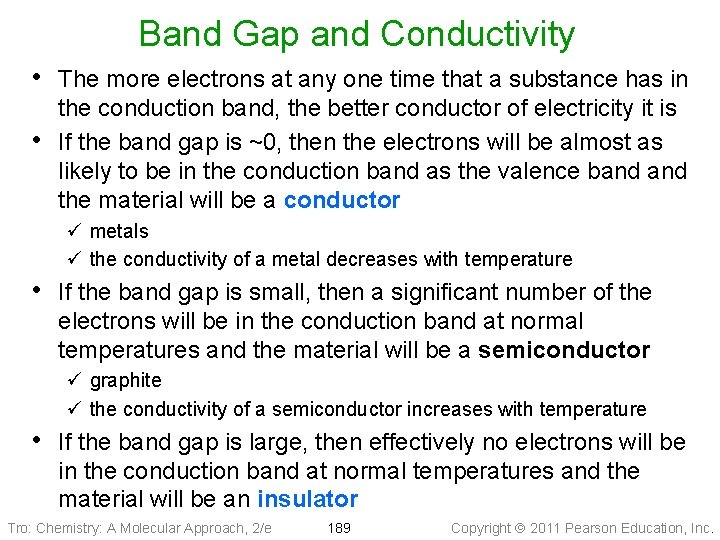
Band Gap and Conductivity • The more electrons at any one time that a substance has in • the conduction band, the better conductor of electricity it is If the band gap is ~0, then the electrons will be almost as likely to be in the conduction band as the valence band the material will be a conductor ü metals ü the conductivity of a metal decreases with temperature • If the band gap is small, then a significant number of the electrons will be in the conduction band at normal temperatures and the material will be a semiconductor ü graphite ü the conductivity of a semiconductor increases with temperature • If the band gap is large, then effectively no electrons will be in the conduction band at normal temperatures and the material will be an insulator Tro: Chemistry: A Molecular Approach, 2/e 189 Copyright 2011 Pearson Education, Inc.

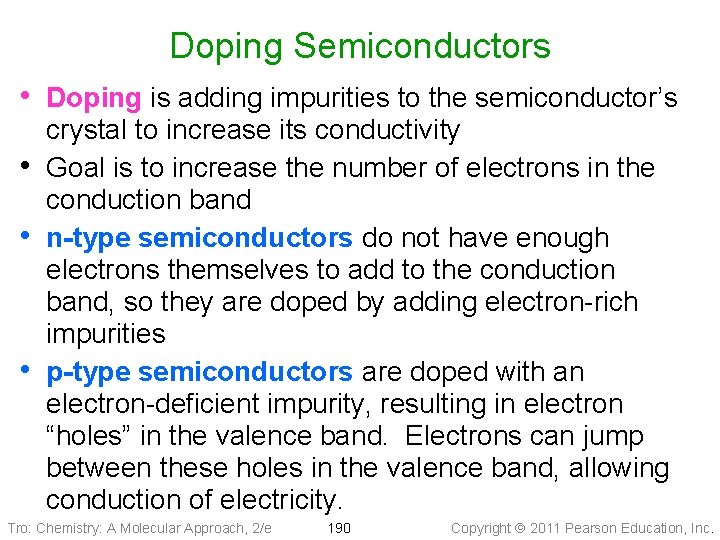
Doping Semiconductors • Doping is adding impurities to the semiconductor’s • • • crystal to increase its conductivity Goal is to increase the number of electrons in the conduction band n-type semiconductors do not have enough electrons themselves to add to the conduction band, so they are doped by adding electron-rich impurities p-type semiconductors are doped with an electron-deficient impurity, resulting in electron “holes” in the valence band. Electrons can jump between these holes in the valence band, allowing conduction of electricity. Tro: Chemistry: A Molecular Approach, 2/e 190 Copyright 2011 Pearson Education, Inc.

Diodes • When a p-type semiconductor adjoins an n-type • • semiconductor, the result is an p-n junction Electricity can flow across the p-n junction in only one direction – this is called a diode This also allows the accumulation of electrical energy – called an amplifier Tro: Chemistry: A Molecular Approach, 2/e 191 Copyright 2011 Pearson Education, Inc.
 Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Melting and boiling point of oxygen
Melting and boiling point of oxygen Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry molecular geometry
Ap chemistry molecular geometry Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Differentiate between virtual circuit and datagram network
Differentiate between virtual circuit and datagram network Theoretical models of counseling
Theoretical models of counseling Fine grained screening
Fine grained screening Multi approach avoidance conflict
Multi approach avoidance conflict Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Definition of research approach
Definition of research approach Traditional approach in system analysis and design
Traditional approach in system analysis and design Tony wagner's seven survival skills
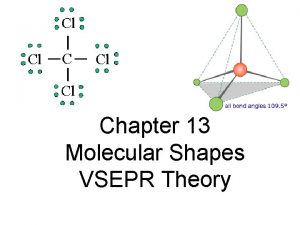
Tony wagner's seven survival skills Vsepr
Vsepr Chem shapes
Chem shapes Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Molecular biology crash course
Molecular biology crash course Molecular level vs cellular level
Molecular level vs cellular level Bathochromic shift and hypsochromic shift
Bathochromic shift and hypsochromic shift Ab6 molecular geometry
Ab6 molecular geometry Kmt law
Kmt law Properties of network covalent solids
Properties of network covalent solids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids What is the significance of a chemical formula
What is the significance of a chemical formula Criterios de amsterdam cancer de colon
Criterios de amsterdam cancer de colon Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry Molecular scissors
Molecular scissors Molecular rebar design
Molecular rebar design What is a covalent molecular substance
What is a covalent molecular substance Empirical formula of haemoglobin
Empirical formula of haemoglobin Number-average molecular weight
Number-average molecular weight Dicots
Dicots How to get empirical formula from percentages
How to get empirical formula from percentages Atomic mass of kmno4
Atomic mass of kmno4 Empirical and molecular formula
Empirical and molecular formula How to find molecular formula
How to find molecular formula Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Alkane methane structure
Alkane methane structure Naming compounds and writing formulas
Naming compounds and writing formulas Carbon pentoxide
Carbon pentoxide N srinivasan iisc passed away
N srinivasan iisc passed away Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Ch2o molecular geometry
Ch2o molecular geometry Kahoot shapes
Kahoot shapes Shapes and polarity of molecules
Shapes and polarity of molecules Molecular replacement method
Molecular replacement method Molecular replacement method
Molecular replacement method Bh3 molecular orbital diagram
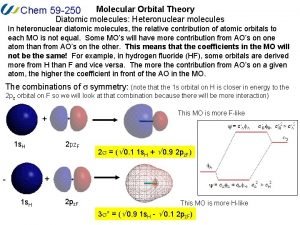
Bh3 molecular orbital diagram Molecular orbital diagram of heteronuclear diatomic
Molecular orbital diagram of heteronuclear diatomic B2 molecular orbital diagram
B2 molecular orbital diagram Simona soverini
Simona soverini What is the molecular shape of carbon tetrachloride
What is the molecular shape of carbon tetrachloride Molecular microbiology definition
Molecular microbiology definition Axe notation chart
Axe notation chart Polar molecules
Polar molecules Pf3 molecular geometry
Pf3 molecular geometry Scl molecular geometry
Scl molecular geometry Molecular geometry and bonding theories
Molecular geometry and bonding theories What is a bond order in chemistry
What is a bond order in chemistry Molecular gastronomy restaurants
Molecular gastronomy restaurants Empirical formula vs molecular formula
Empirical formula vs molecular formula Molecular pharming definition
Molecular pharming definition Concln
Concln How do you go from molecules to moles
How do you go from molecules to moles How to go from grams to moles
How to go from grams to moles Modelo cinetico molecular
Modelo cinetico molecular Osmodialysis
Osmodialysis Molécula sales minerales
Molécula sales minerales Molecular dynamics limitations
Molecular dynamics limitations Cocl2 molecular geometry
Cocl2 molecular geometry Lewis dot structure and molecular geometry
Lewis dot structure and molecular geometry Gluten molecular structure
Gluten molecular structure Kinetic molecular theory
Kinetic molecular theory Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory volume
Kinetic molecular theory volume Nocl molecular shape
Nocl molecular shape Applications of uv and visible spectroscopy
Applications of uv and visible spectroscopy Molecular replacement method
Molecular replacement method Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Bio molecular systems ltd
Bio molecular systems ltd Square planar hybridization
Square planar hybridization Biologists search the volumes of the human genome using
Biologists search the volumes of the human genome using History of molecular gastronomy
History of molecular gastronomy Lichefiaza
Lichefiaza Molecular biology evidence of evolution
Molecular biology evidence of evolution Molar mass table
Molar mass table Geometria molecular linear
Geometria molecular linear Geometria molecular
Geometria molecular Piramide trigonal
Piramide trigonal Formula for galena
Formula for galena Molecular mass
Molecular mass Atomicity
Atomicity Dan dial molecular enhancer
Dan dial molecular enhancer Iodine molecular weight
Iodine molecular weight Formulas moleculares
Formulas moleculares Covalent molecular and covalent network
Covalent molecular and covalent network Silicon carbide covalent network
Silicon carbide covalent network Carbon dioxide symbol
Carbon dioxide symbol Empirical formula of pentane
Empirical formula of pentane Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Molecular formula
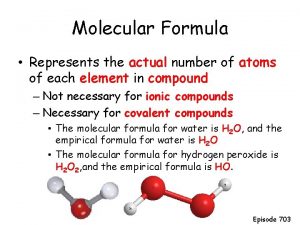
Molecular formula Emperical formula
Emperical formula Molecular vs empirical formula
Molecular vs empirical formula Empirical formula
Empirical formula Molecular formula example
Molecular formula example Cho molecular formula
Cho molecular formula Principle of fractional distillation
Principle of fractional distillation Dipole moment and polarity
Dipole moment and polarity Netmegs
Netmegs Biopolymer molecular weight determination
Biopolymer molecular weight determination Stoichiometric air fuel ratio
Stoichiometric air fuel ratio Nh4 + molecular geometry
Nh4 + molecular geometry Chemical formula covalent compounds
Chemical formula covalent compounds Covalent network vs covalent molecular
Covalent network vs covalent molecular Molecular vibration animation
Molecular vibration animation Cooh molecular geometry
Cooh molecular geometry Periodic table with molar masses
Periodic table with molar masses Molecular polarity vs bond polarity
Molecular polarity vs bond polarity Molecular biology evidence of evolution
Molecular biology evidence of evolution 4 electron domains 2 lone pairs
4 electron domains 2 lone pairs Hbr electron geometry
Hbr electron geometry Find the empirical/simplest formula fe
Find the empirical/simplest formula fe Neutral theory of molecular evolution notes
Neutral theory of molecular evolution notes Hydrosulfuric acid
Hydrosulfuric acid Molecular substance
Molecular substance Bf3 molecular orbital diagram
Bf3 molecular orbital diagram Co2 relative molecular mass
Co2 relative molecular mass Ultraviolet axn
Ultraviolet axn Chapter 16 the molecular basis of inheritance
Chapter 16 the molecular basis of inheritance Molecular weight units
Molecular weight units Dihydrogen monosulfide shape
Dihydrogen monosulfide shape Chapter 12 section 1: dna: the genetic material
Chapter 12 section 1: dna: the genetic material Chapter 12 section 1 dna the genetic material
Chapter 12 section 1 dna the genetic material Common molecules
Common molecules Io2f lewis structure
Io2f lewis structure Molecular biology of the gene chapter 10
Molecular biology of the gene chapter 10 Paraffin combustion reaction
Paraffin combustion reaction How to find molecular formula
How to find molecular formula Nh4 + molecular geometry
Nh4 + molecular geometry Electron geometry of co
Electron geometry of co Binary molecular compounds
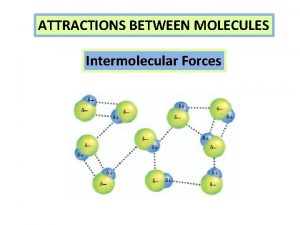
Binary molecular compounds Intermolecular forces are attractions between
Intermolecular forces are attractions between Electron domain geometry chart
Electron domain geometry chart Molecular weight unit
Molecular weight unit Atomic molecular theory
Atomic molecular theory Tienopiridinas mecanismo de acción
Tienopiridinas mecanismo de acción Alkynes
Alkynes How is orbital hybridization useful in describing molecules
How is orbital hybridization useful in describing molecules Molecular formula of ch
Molecular formula of ch Molecular friction
Molecular friction Cellular and molecular immunology
Cellular and molecular immunology Jrmin
Jrmin Ligação ionica
Ligação ionica Adhesive force
Adhesive force Diagrama de orbitais moleculares
Diagrama de orbitais moleculares Sustancia simple molecular
Sustancia simple molecular Molekul linier
Molekul linier Phosphorus with oxygen equation
Phosphorus with oxygen equation Ecuacion balanceada
Ecuacion balanceada Como convertir gramos en moles
Como convertir gramos en moles Molecular scissors that cut' dna *
Molecular scissors that cut' dna * Molar mass of potassium dichromate
Molar mass of potassium dichromate Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Biologia molecular
Biologia molecular Profilaxis antitrombótica en cirugía ortopédica
Profilaxis antitrombótica en cirugía ortopédica Binary molecular compounds def
Binary molecular compounds def Pentasulfur dinitride
Pentasulfur dinitride Molecular bio
Molecular bio Molecular rotational resonance
Molecular rotational resonance Molecule polarity worksheet answers
Molecule polarity worksheet answers Fhf- molecular orbital diagram
Fhf- molecular orbital diagram Molecular formula of c
Molecular formula of c Types of molecular farming
Types of molecular farming Genez molecular clones
Genez molecular clones Molecular biology lecture
Molecular biology lecture Mega molecular
Mega molecular Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Unidade de medida u
Unidade de medida u Drop in molecular views answer key
Drop in molecular views answer key Lesson 28: sniffing around molecular formulas answer key
Lesson 28: sniffing around molecular formulas answer key Molecular geometry and bonding theories
Molecular geometry and bonding theories Chem 150
Chem 150 Kinetic molecular theory
Kinetic molecular theory Virtual on model kit
Virtual on model kit