UltravioletVisible Absorption Spectroscopy Electronic Excitation by UVVis Spectroscopy

Ultraviolet-Visible Absorption Spectroscopy
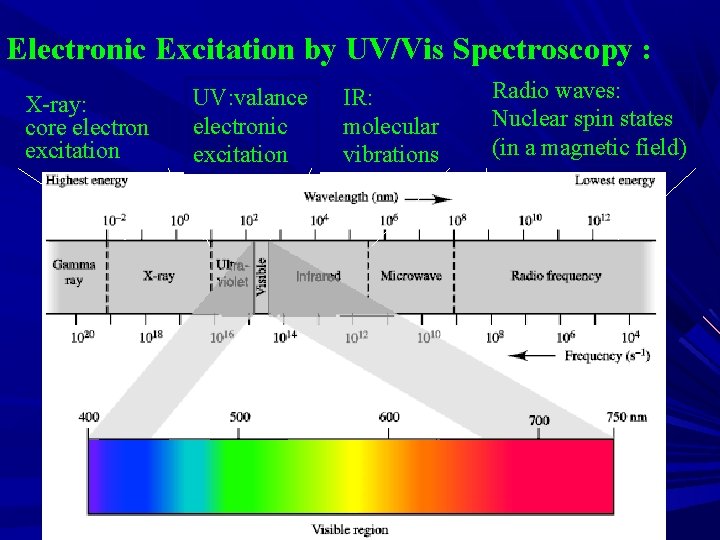
Electronic Excitation by UV/Vis Spectroscopy : X-ray: core electron excitation UV: valance electronic excitation IR: molecular vibrations Radio waves: Nuclear spin states (in a magnetic field)

Rays Frequency Wavelength γ rays 0. 01 -10 nm X rays 1020 -1016 10 -50 nm Far UV 1016 -1015 50 -200 nm Near UV 1015 -7. 5 X 1014 200 -400 nm Visible 7. 5 X 1014 4. 0 X 1014 400 -800 nm Near IR 4. 0 X 1014 -1. 2 X 1014 0. 8 -2. 5µm Mid IR 1. 2 X 1014 - 6 X 1012 2. 5 -25µm Far IR 6 X 1012 -1011 25 -400µm Micro waves 1011 -108 400 -25 cm Radiowaves 108 - 105 25 cm-1000 m

Spectroscopic Techniques UV-vis region bonding electrons Atomic Absorption UV-vis region atomic transitions (val. e-) FT-IR IR/Microwave vibrations, rotations Raman IR/UV vibrations FT-NMR Radio waves nuclear spin states X-Ray Spectroscopy X-rays inner electrons, elemental X-ray Crystallography X-rays 3 -D structure

Ultraviolet and visible spectroscopy It is used to measure the multiple bonds or atomic conjugation within the molecule. The UV-Visible region is subdivided as below – Vacuum UV: 100 -200 nm – Near UV: 200 to 400 nm – Visible region: 400 to 800 nm Vacuum UV is so named because molecule of air absorb radiation in these region. The radiation is assessable only in special vacuum equipments.
Spectroscopy: Synonyms: Spectrometry or spectrophotometry Spectroscopy is made up of Spectrum + Skopien → Given by Isaac Newton He did simple experiment V I BG YOR Sunlight

According to Newton experiment, Spectrum is a band of color or pattern of colors or arrangement or array of colors. But extended definition of spectrum is, Separation of wave or pattern of waves or arrangement /array of wave. Today, Spectrum is defined as arrangement of array or pattern of anything. Skopein: Evaluation/examination Spectroscopy: Evaluation/ Examination of spectrum Metry: Measurement Spectrometry: Measurement of spectrum Photo: EMR (Electro magnetic radiation) Spectrophotometry: Measurement of EMR spectrum

Fundamental principle of spectroscopy: Drug + EMR → Drug* + EMR* We measure difference between EMR and EMR* EMR: EMR is a radiant energy, that is transmitted through space in normal velocity Radiant energy has wave nature and being associated with electric as well as magnetic field so called EMR All EMR propagated through space with same speed (3 X 1010 cm/sec) called speed of light EMR is alternating electric field and associated magnetic field in space The two components oscillate in planes perpendicular to each other and perpendicular to the direction of propagation of radiation
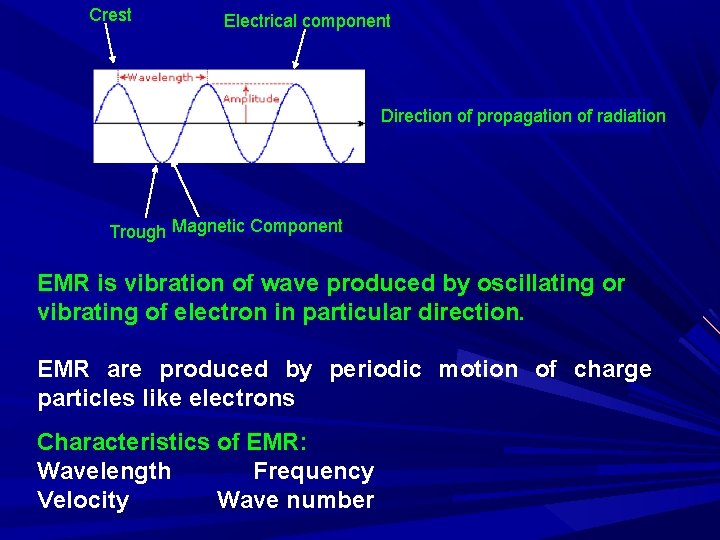
Crest Electrical component Direction of propagation of radiation Trough Magnetic Component EMR is vibration of wave produced by oscillating or vibrating of electron in particular direction. EMR are produced by periodic motion of charge particles like electrons Characteristics of EMR: Wavelength Frequency Velocity Wave number

Wavelength (λ) is defined as the distance between adjacent peaks (or crest/troughs). Designated in meters, centimeters or nanometers (10 -9 meters). Energy α 1/λ max Frequency (µ) is the number of wave cycles passes a given point in 1 sec. Expressed as cycles per second, or hertz (Hz). µ α 1/λ, , µ = c/λ Velocity (c) The distance traveled by wave in 1 sec. c= λ x µ, c= 3 x 1010 cm/sec Wavenumber (σ) – the number of waves spread in length of 1 centimeter. σ = 1/ λ • Energy α Frequency (µ) E= h µ, E = h. C/ λ H= plank const= 6. 626 x 10 -27 erg/sec

Spectroscopy: it is a branch of science deals with interaction of EMR with matter Spectroscopy can be divided in, Study at molecular/atomic level 1) Atomic spec e. g. : AAS, flame photometry Change in E at atomic level Deals with interaction of EMR with atoms which are in their lowest energy state, i. e. , ground state 2) Molecular spec e. g. : UV, IR, fluorimetry Change in E at molecular level Transition between rotational and vibrational E levels in addition to electronic transition

Study based on absorption/emission 1) Absorption Spe e. g. : UV, IR, X-ray, ESR, NMR 2) Emission Spe e. g. : flame photometry, fluorimetry Study at electric/ magnetic level • Electric Spe e. g. : UV, Colorimetry, fluorimetry (without magnetic field) 2) Magnetic Spe e. g. : NMR, ESR
UV Visible Spectroscopy also known as Molecular absorption/ electronic absorption spectroscopy Importance of UV spectra: Simple in operation High sensitivity Speedy Analysis Qualitative and quantitative application Limitation: Non Selective Molecular absorption spectrum/ UV Visible spectrum is band spectrum Atomic spectrum involves line spectrum
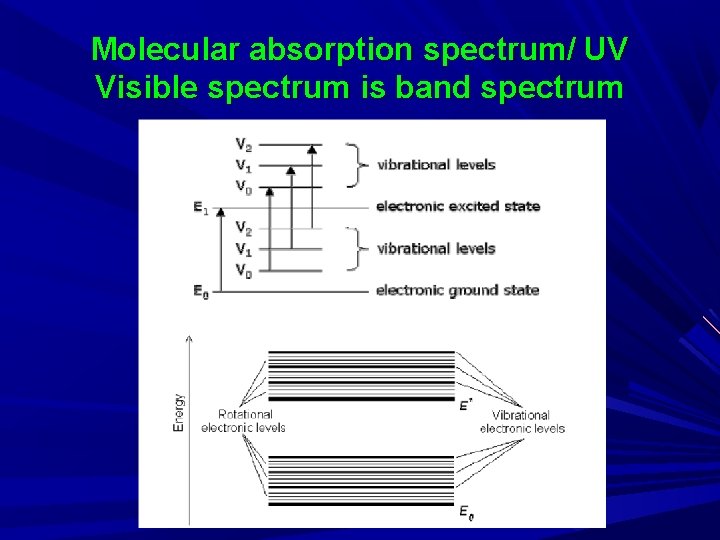
Molecular absorption spectrum/ UV Visible spectrum is band spectrum

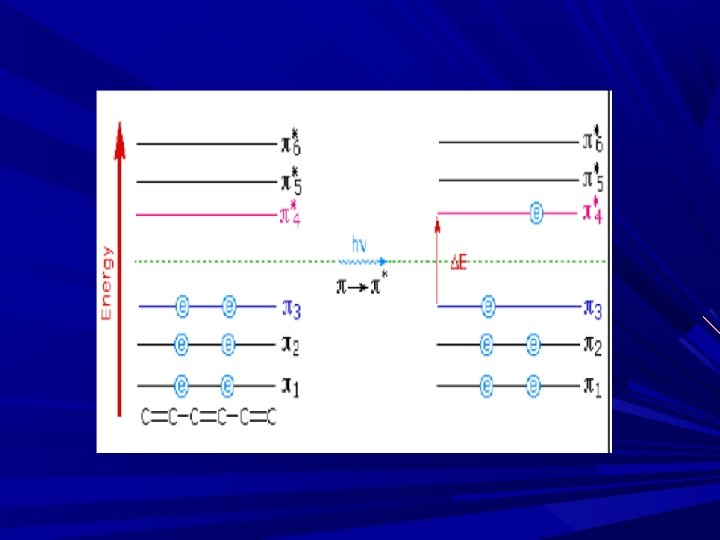
Why we get ∩ (band) and not ∏ on x-axis even though conc. is same B’se all the time energy absorbed is not the same… A molecular energy state is the sum of an electronic, vibrational, rotational, and translational component E = E electronic + E vibrational +E rotational +E translational
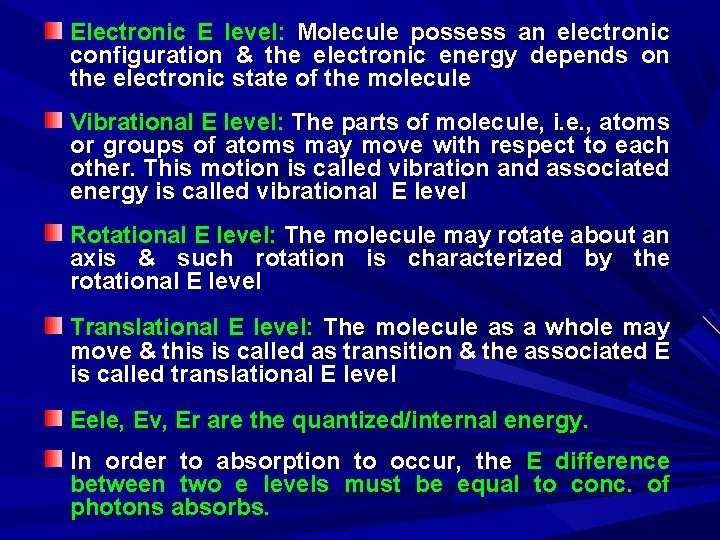
Electronic E level: Molecule possess an electronic configuration & the electronic energy depends on the electronic state of the molecule Vibrational E level: The parts of molecule, i. e. , atoms or groups of atoms may move with respect to each other. This motion is called vibration and associated energy is called vibrational E level Rotational E level: The molecule may rotate about an axis & such rotation is characterized by the rotational E level Translational E level: The molecule as a whole may move & this is called as transition & the associated E is called translational E level Eele, Ev, Er are the quantized/internal energy. In order to absorption to occur, the E difference between two e levels must be equal to conc. of photons absorbs.
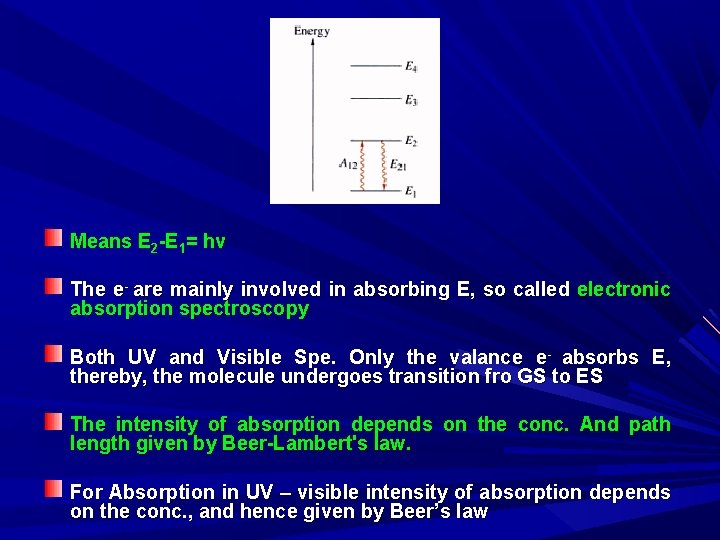
Means E 2 -E 1= hv The e- are mainly involved in absorbing E, so called electronic absorption spectroscopy Both UV and Visible Spe. Only the valance e- absorbs E, thereby, the molecule undergoes transition fro GS to ES The intensity of absorption depends on the conc. And path length given by Beer-Lambert's law. For Absorption in UV – visible intensity of absorption depends on the conc. , and hence given by Beer’s law

While in IR absorption Spe. , it concerns with jumping of e- from one vibrational E level to other vibrational E level so called Vibrational Abn. Spe. / IR Abn. Spe. For IR abn. Rotational spectrum is not important analytically. e- jump from one E level to another ----- Transition If a molecule passes from one of its allowed E level to a lower one, some of E must be released, which may be lose as radiation. ----Emission of radiation If a molecule passes from one of it’s allowed E level to higher one, some E must be absorbed, which may be absorbed as radiation -------Absorption of radiation

Valance e- : The e- which are required for bond formation present in outer most orbital. Types of valance e- : 1) σ 2) π 3) n 1) σ e- : Present in saturated hydrocarbon e. g. : paraffin ( C-C, C-H) Highly stable Required higher E for excitation and λ req. for excitation is very low Such e- do not absorbs near UV but absorbs vacuum UV radiation < 200 nm Hence the comp. ctg σ bond doesn't absorb in UV region -----transparent in UV range -----so used as a solvent. For e. g. : Hexane

2) π e-: Present in unsaturated hydrocarbon e. g. : Double or triple bond…>C=C<, -C≡C- (alkenes, alkynes, aromatic compd. , and carbonyl compds such as aldehydes and ketones, cyanides, azo compds etc. Relatively unstable and highly reactive Required less E for excitation 3) n e- : non bonding e. These e- are not involved in bond formation e. g. : S, O, N & halogen (X), such n e- are excited by UV radiation
Principle of UV radiation Any molecule has either n, σ, π or a combination of these e-. These bonding (σ and π) and non bonding (n) eabsorbs characteristic radiation undergoes transition from GS to ES By the characteristics absorption peaks, the nature of epresent, the mol. Stru. can be elucidated
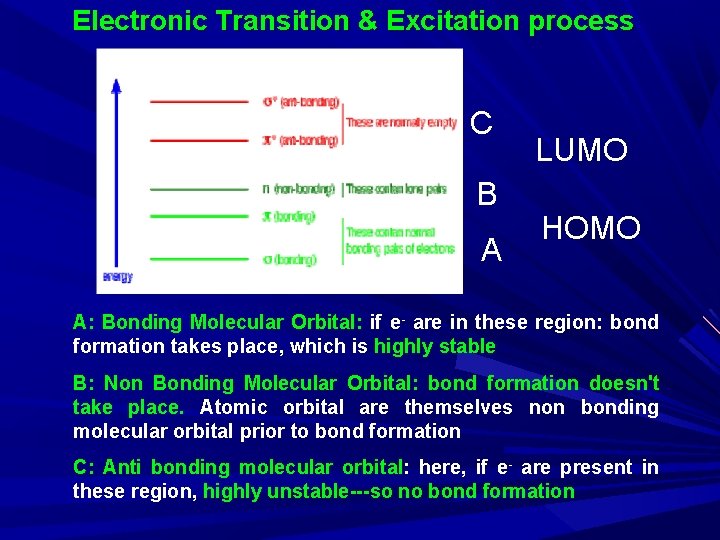
Electronic Transition & Excitation process C B A LUMO HOMO A: Bonding Molecular Orbital: if e- are in these region: bond formation takes place, which is highly stable B: Non Bonding Molecular Orbital: bond formation doesn't take place. Atomic orbital are themselves non bonding molecular orbital prior to bond formation C: Anti bonding molecular orbital: here, if e- are present in these region, highly unstable---so no bond formation
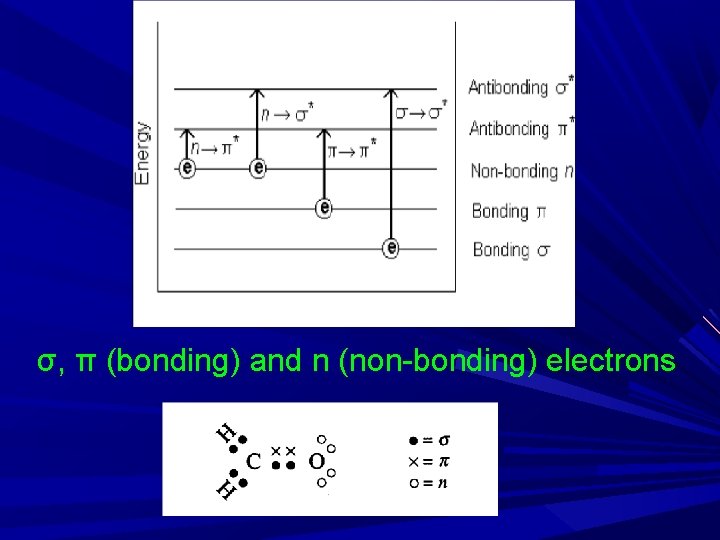
σ, π (bonding) and n (non-bonding) electrons

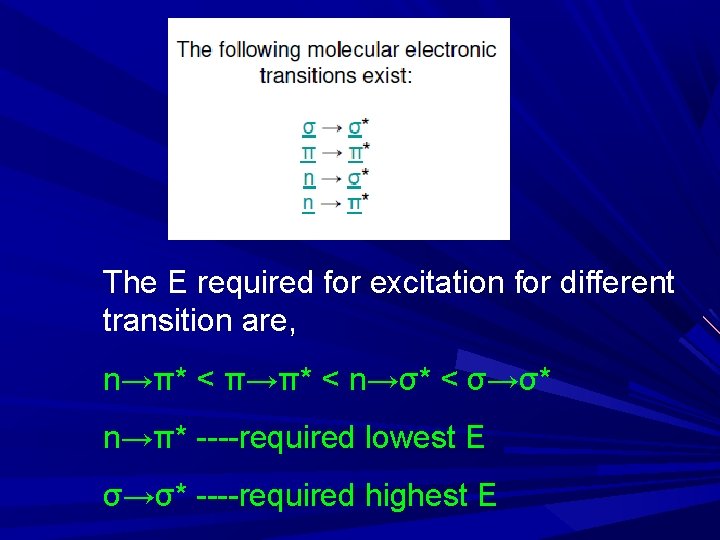
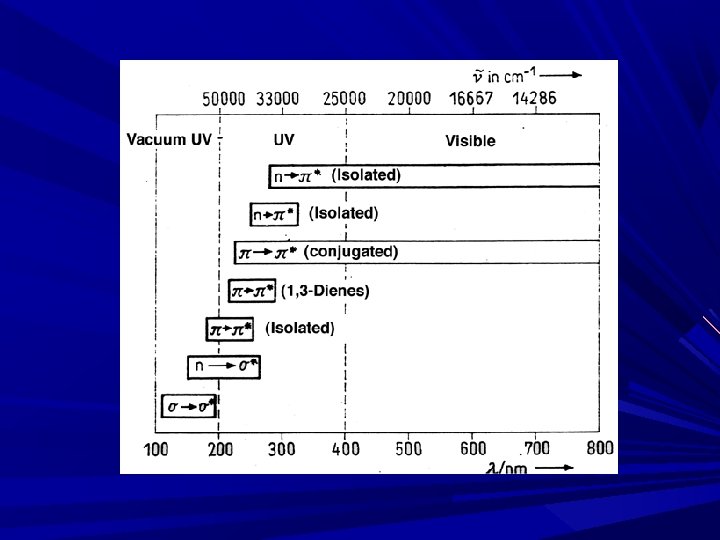
The E required for excitation for different transition are, n→π* < π→π* < n→σ* < σ→σ* n→π* ----required lowest E σ→σ* ----required highest E

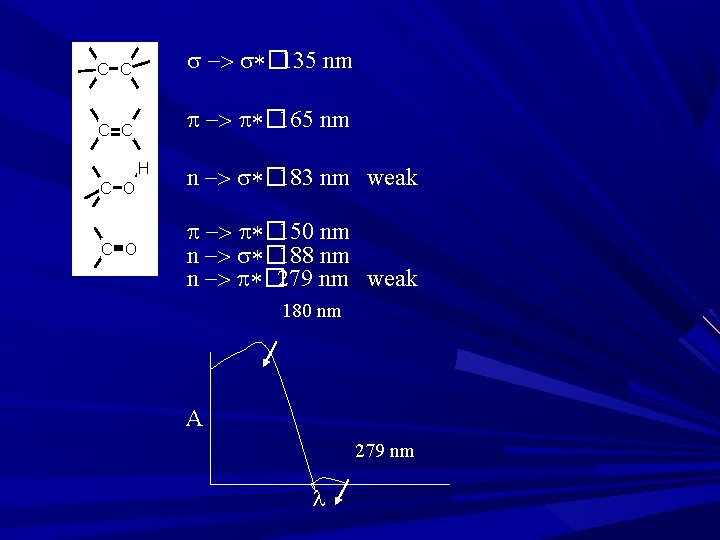
Electronic Transition σ→σ* transition: Required highest E Absorption in ~150 nm E. g. Hydrocarbons Methane: λmax = 125 nm (High E compare to ethane, Lower λ) Ethane: λmax = 135 nm (less E compare to methane, Higher λ) B’se strength of C-C bond is < C-H bonds. . Propane: maxi abs. At 135 nm… most of spectrophotometer doesn’t shows abs. < 180 -200 nm, σ→σ* not observed Exception: Cyclopropane: shows abs. at 190 nm

In far UV (Vacuum) region, Oxygen present in the air absorbs strongly, so to study σ→σ* air must be evacuated from the instrument, specially in case of Saturated HC

n→σ* transition: Saturated compd ctg. atoms with unshared pair of e- (O, N, S/ X/ non bonding e-) Majority of comps in this class doesn’t shows abs. in near UV region Transition in region of 150 -250 nm, with most abs peak < 200 nm Most commonly used solvent: Alcohols and ethers (abs < 185 nm) E. g. : Alkyl Halides The E req. for n→σ* transition ↓es with ↑es in size of halogen atom/ ↓es in the electro negativity of atom. (F, Cl, Br, I) E. g. : Methyl chloride (λmax=173) and methyl iodide (λmax=259) B’se of > electro negativity of chlorine than iodine, the n e- on chlorine atom are comparatively difficult to excite, while n e- on iodine atoms are loosely bound ε Magnitude of molar extinction coefficient ( max) for a particular absorption αnal prob. of particular electronic transition. εmax for CH 3 I= 400, εmax for CH 3 Cl= 200

n→σ* transitions are sensitive to H-bonding For E. g. : alcohols and amines forms H-bonding with solvent molecule (due to n bonding e-) So, greater E req. for excitation and hence, H-bonding shifts abs. towards shorter λ. Amines abs at higher λ as compared to alcohols B’se n bonding e- on N atoms in amine are loosely bound as compared to o atoms in alcohol (B'se higher electro negativity of O than N) E. g. : Tri methyl amine: in aq. solution doesn't shows abs due to n→σ* B'se protonated amine does not contain any n bonding e-
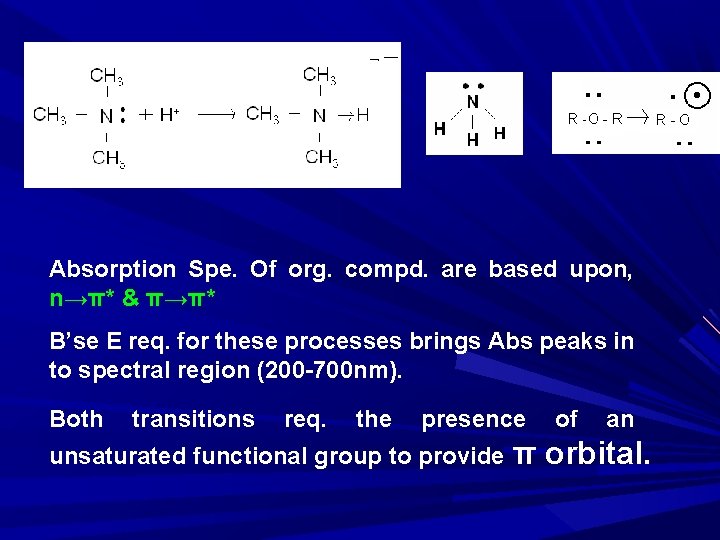
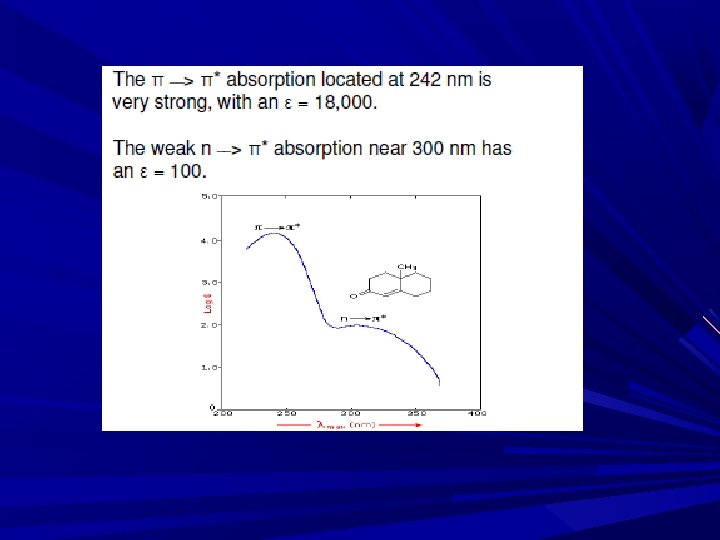
Absorption Spe. Of org. compd. are based upon, n→π* & π→π* B’se E req. for these processes brings Abs peaks in to spectral region (200 -700 nm). Both transitions req. the presence unsaturated functional group to provide π of an orbital.


π→π* transition: In simple alkenes, several transitions are available. abs. band between 170 -190 nm in un conjugated alkenes E. g. : Ethylene in vapor phase absorbs at 165 nm, & gives second band at 193 nm due to π→π*, The intensities of olefinic double bonds is independent of solvents due to non polar nature of double bond Band in π→π* transition also called as K-band is obtained in the spectra of conjugated π systems. e. g. Butadiene, Mesityl oxide K-band is also known as E-band (Ethylinic) & B band (Benzenoid)

n→π* transition: required lowest E (longer λ) The peak due to this transition also called as R-band ( longer λ) Peak seen due to: n bonding e- is present in compound ctg = or ≡. e. g. aldehyde, ketone and nitro compd. n→π*

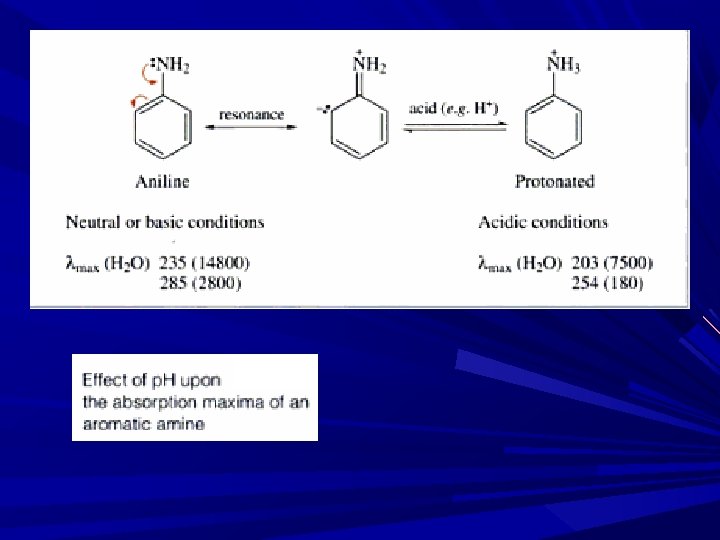
Blue shift observed with an increase in solvent polarity ( due to salvation and H-bonding to the lone pair, large shift of 30 nm) n→π* transition is characterized by taking spectrum in acid solution. E. g. : Pyridine: bands due to n→π* in pyridine disappear in acid solution b’se of the formation of bond between acidic proton and n e. C 6 H 5 N: + H+ → C 6 H 5 NH+ Peak appear Peak disappear
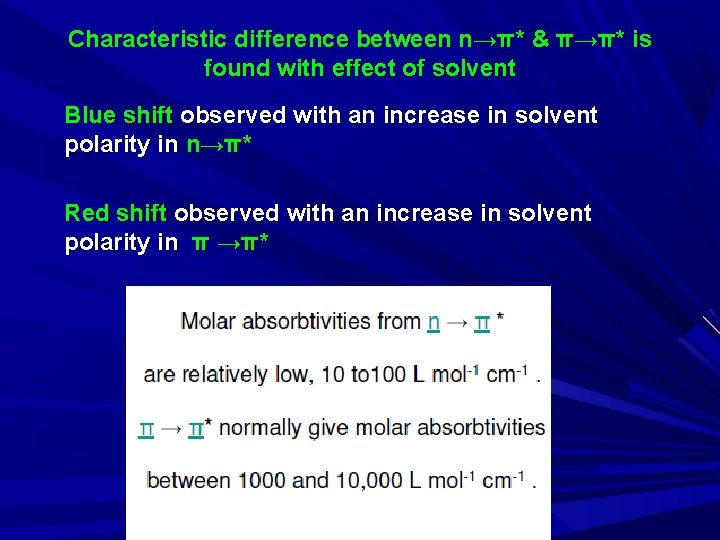
Characteristic difference between n→π* & π→π* is found with effect of solvent Blue shift observed with an increase in solvent polarity in n→π* Red shift observed with an increase in solvent polarity in π →π*
C C � 135 nm C C � 165 nm H C O n � 183 nm weak � 150 nm n � 188 nm n � 279 nm weak 180 nm A 279 nm


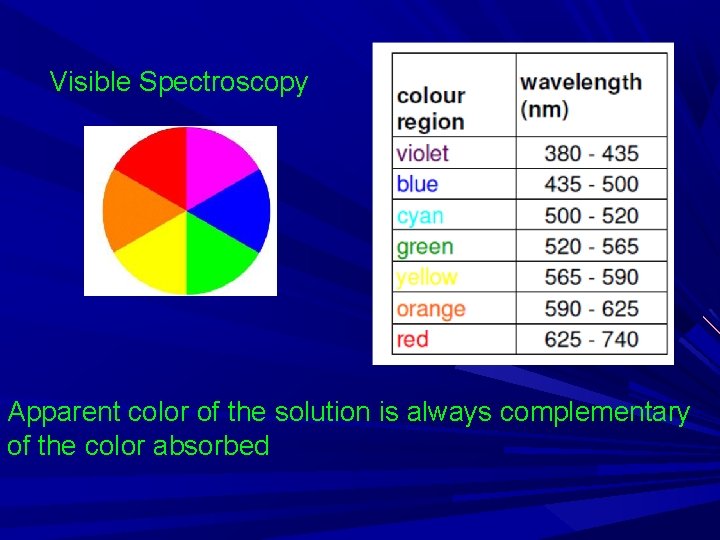
Visible Spectroscopy Apparent color of the solution is always complementary of the color absorbed
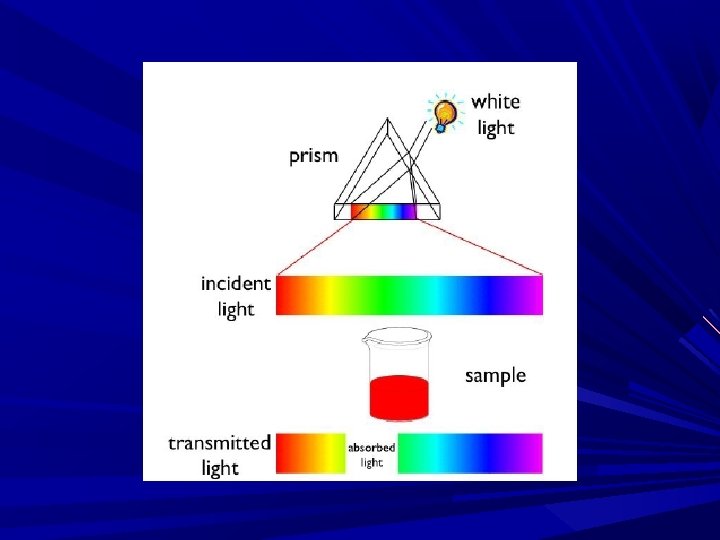

Beer’s Law: When a monochromatic radiation passed thro’ a transparent medium, a rate of decrease in intensity of radiation with concentration of medium is proportional to the intensity of the incident light. Intensity of transmission radiation decreases as the conc. of absorbing subs increases arithmetically
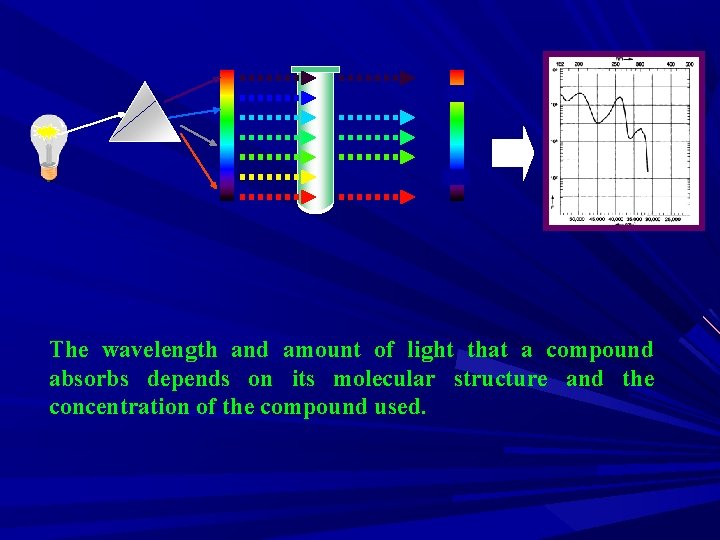
The wavelength and amount of light that a compound absorbs depends on its molecular structure and the concentration of the compound used.

where I = intensity of monochromatic light transmitted through the solution I 0 = intensity of light transmitted through the blank A = absorbance b = path length (usually in cm) a = Absorptivity, a constant for a given solution and a given wavelength C = concentration in g/100 ml
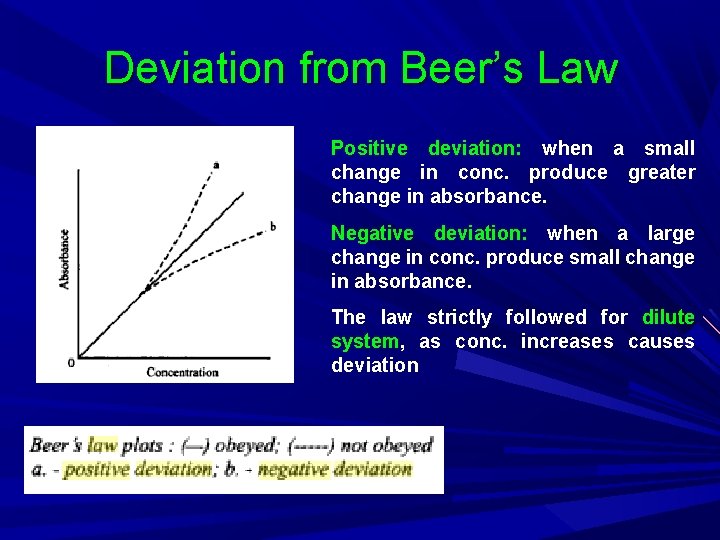
Deviation from Beer’s Law Positive deviation: when a small change in conc. produce greater change in absorbance. Negative deviation: when a large change in conc. produce small change in absorbance. The law strictly followed for dilute system, as conc. increases causes deviation

Environmental Deviation: Temp, solvent Instrumental Deviation: Relative Conc. error, Stray radiation, stability of radiation source, wavelength selector, slit control (SSW), electronics and reliability of optical parts Chemical Deviation: change in chemical equilibrium and change in p. H, ionization, presence of complexing agent, competitive metal ion reactions and conc. Dependence (hydrolysis, association, polymerization, ionization, H-bonding). Refractive index of the sample Non monochromacity of radiation Beer’s law is not applicable to suspension, coagulated particle system, impurity that fluorescence / absorbed

Methods to find Conc. By Beer’s law 1) Using Standard equation (using standard A(1%, 1 cm) and ε A= ε b c The absorbance of the solution, A, is defined as A= - log T, A= -log [I/Io], T= -log [I/Io] %T, of the solution is expressed as, A = 2. 00 – log (% T) = ε b c ε = A(1%, 1 cm) x Mol weight 10 A(1%, 1 cm)/a (Spe. absorptivity) is given than conc. Is in g/100 ml ε is given than conc. Is in mol/lit 2) Calibration Method) (by serial dilution method) 3) Using Formula method Au / As = Cu / Cs

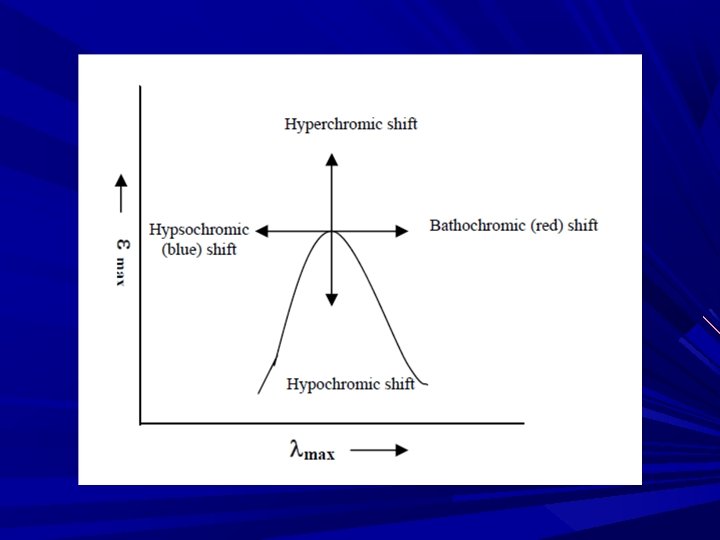
Different terminologies used in UV-Visible spectroscopy • Chromophore: A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. • Auxochrome: An auxochrome is a group of atoms attached to a chromophore which modifies the ability of that chromophore to absorb light. (hydroxyl, amino, nitro group) • Bathochromic shift: It is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength • Hypsochromic shift: It is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a shorter wavelength. • Hyperchromic shift: Increase in absorbance • Hypochromic shift: Decrease in absorbace

Important Terms in UV Spe. Chromophore 1) Dependant 2) Independent Auxochrome 1) Bathochromic gr. 2) Hypsochromic gr. Bathochromic shift (Red shift) Hypsochromic shift (Blue shift) Hyperchromic shift Hypochromic shift End absorption
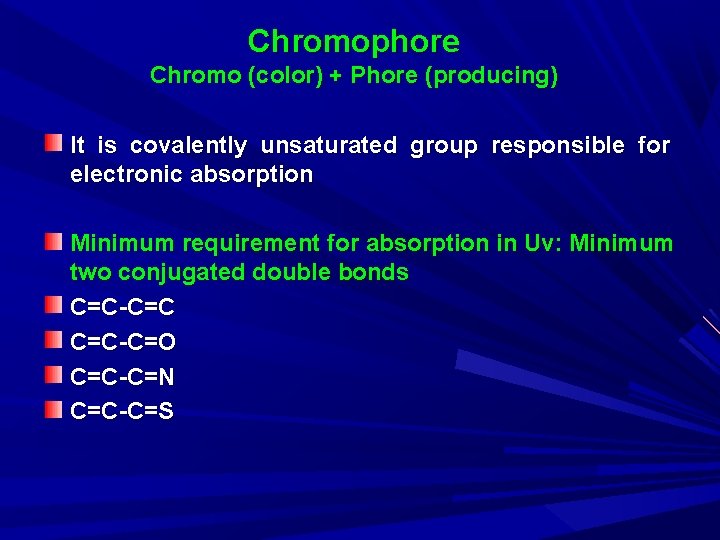
Chromophore Chromo (color) + Phore (producing) It is covalently unsaturated group responsible for electronic absorption Minimum requirement for absorption in Uv: Minimum two conjugated double bonds C=C-C=C C=C-C=O C=C-C=N C=C-C=S

Independant: single chromophore is sufficient to impart color to compd. e. g: -N=N-, -NO-, o & p quinoid gr. Dependent: More than one chromophore is required to produced color in chromogen >C=O, C=C e. g. : CH 3 COCH 3 COCOCOCH 3 Colorless (acetone) Yellow (Diacetyl keton) Orange (Tri keto pentane) Structures having same chromophoric group shows same absorption in UV and can’t analyzed by UV, so UV is non selective method

Auxochrome Auxo: Auxiliary Auxochrome is a functional group having non bonding electrons which itself has no absorption but when attached to chromophoric group, enhance the absorption properties of chromophoric groups An auxochrome contains unshared pair of electrons e. g. : NH 3, SH, RNH 2, OH Benzene Nitro benzene P-nitro aniline -no chrom. group -NO 2 chromophore -NO 2 and -NH 2 (auxo. ) -colorless -pale yellow - dark yellow Auxochrome: two types 1) Bathochromic groups: deepen the color of chromogen, shift to longer λ, e. g. : 1°, 2°, 3° amine 2) Hypsochromic groups: lighten the color of chromogen ,
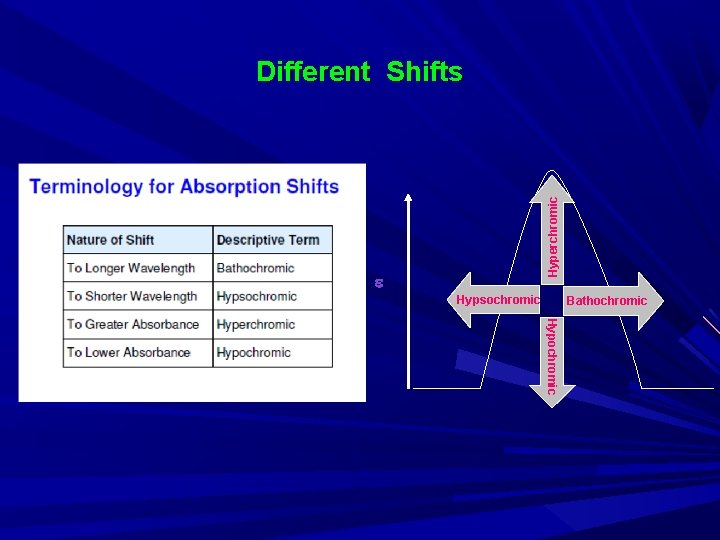
Hyperchromic Different Shifts Hypsochromic Bathochromic Hypochromic
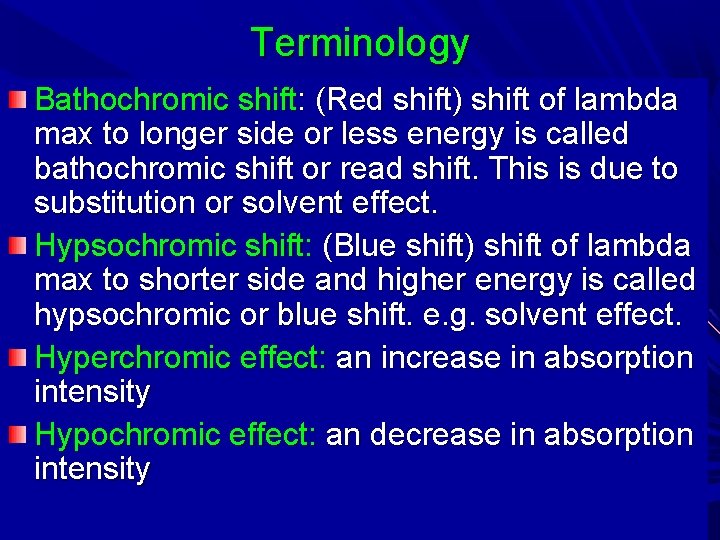
Terminology Bathochromic shift: (Red shift) shift of lambda max to longer side or less energy is called bathochromic shift or read shift. This is due to substitution or solvent effect. Hypsochromic shift: (Blue shift) shift of lambda max to shorter side and higher energy is called hypsochromic or blue shift. e. g. solvent effect. Hyperchromic effect: an increase in absorption intensity Hypochromic effect: an decrease in absorption intensity
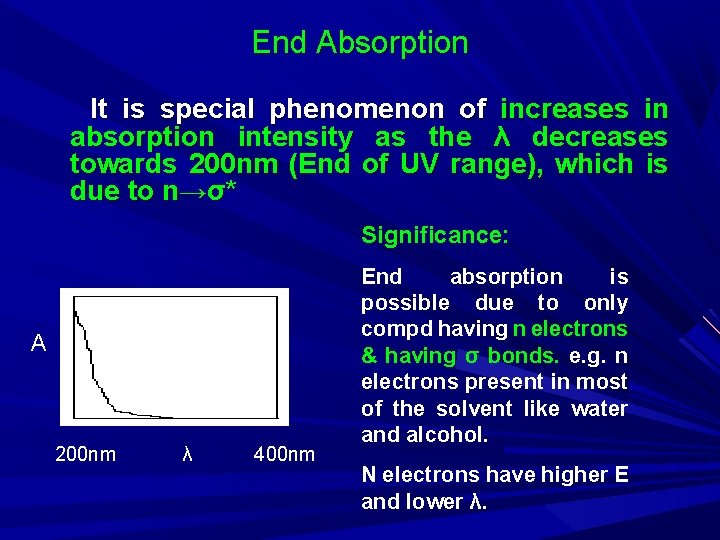
End Absorption It is special phenomenon of increases in absorption intensity as the λ decreases towards 200 nm (End of UV range), which is due to n→σ* Significance: A 200 nm λ 400 nm End absorption is possible due to only compd having n electrons & having σ bonds. e. g. n electrons present in most of the solvent like water and alcohol. N electrons have higher E and lower λ.
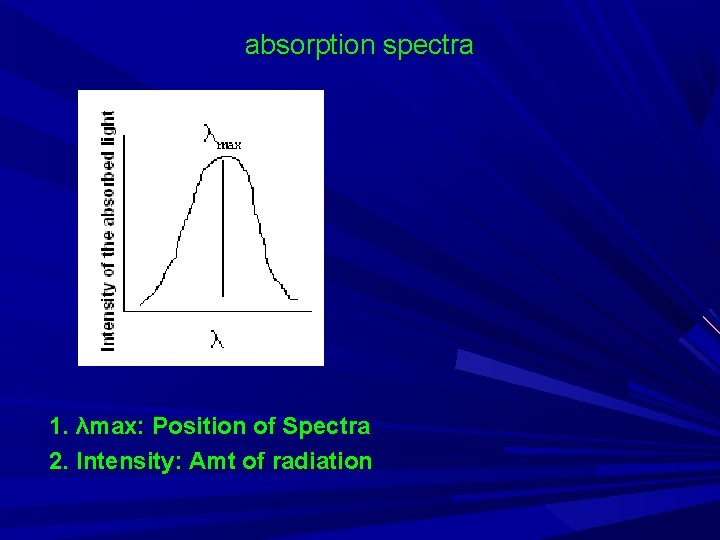
absorption spectra 1. λmax: Position of Spectra 2. Intensity: Amt of radiation
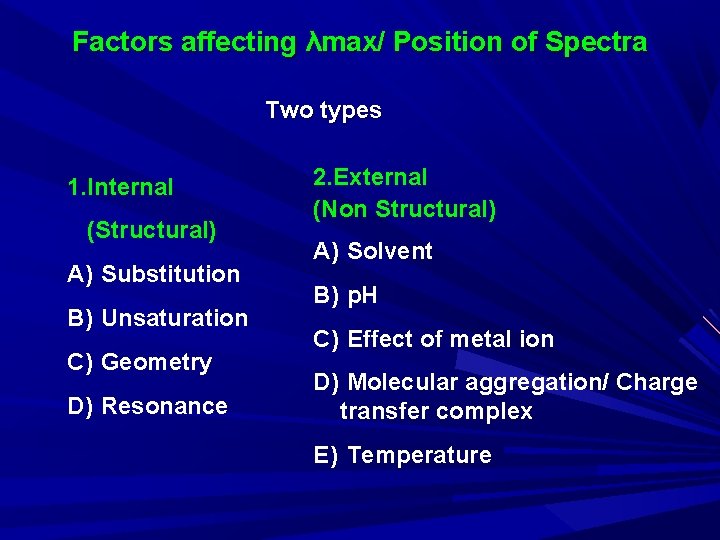
Factors affecting λmax/ Position of Spectra Two types 1. Internal (Structural) A) Substitution B) Unsaturation C) Geometry D) Resonance 2. External (Non Structural) A) Solvent B) p. H C) Effect of metal ion D) Molecular aggregation/ Charge transfer complex E) Temperature
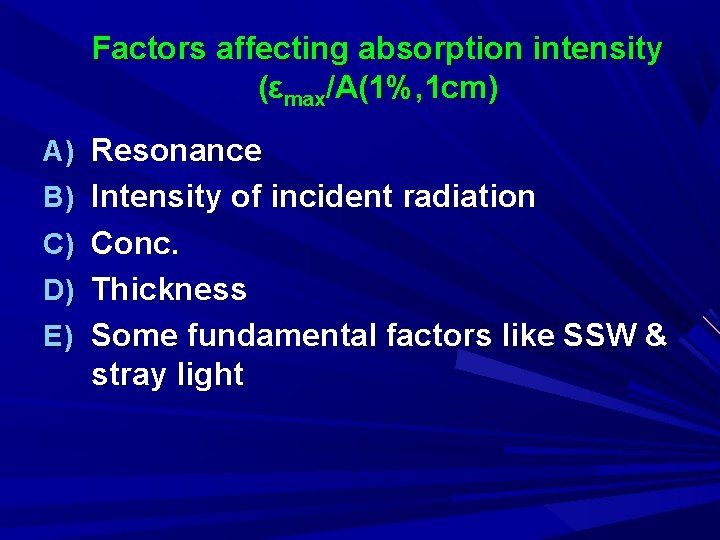
Factors affecting absorption intensity (εmax/A(1%, 1 cm) A) Resonance B) Intensity of incident radiation C) Conc. D) Thickness E) Some fundamental factors like SSW & stray light




- Slides: 66