Quantitative Composition of Compounds Preparation for College Chemistry



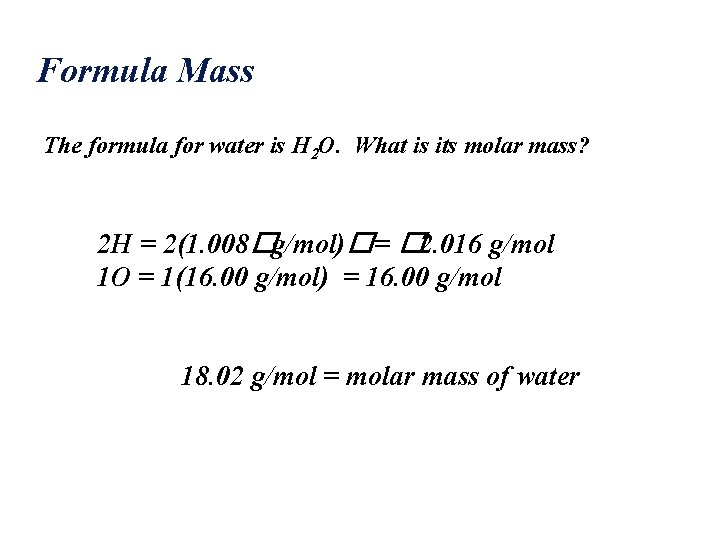


- Slides: 27

Quantitative Composition of Compounds Preparation for College Chemistry Luis Avila Columbia University Department of Chemistry

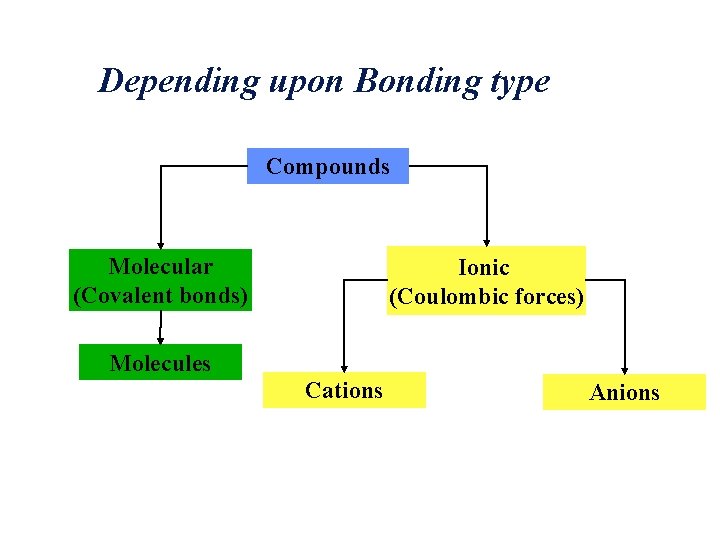
Depending upon Bonding type Compounds Molecular (Covalent bonds) Ionic (Coulombic forces) Molecules Cations Anions

Claude Berthollet: Proportions by mass of elements in a compound VARY OVER A CERTAIN RANGE Joseph Proust: Proportions by mass of elements in a compound ARE FIXED. VARIATIONS ARE DUE TO IMPURITIES. Careful experimentation lead Proust to demonstrate THE LAW OF DEFINITE PROPORTIONS (CONSTANT COMPOSITION): “The proportions by mass of the elements in a compound ARE FIXED, and do not depend on its mode of preparation. ” H 2(g) + Cl 2(g) H 2 SO 4(l) + 2 Na. Cl(s) 2 HCl(g) + Na 2 SO 4(aq)

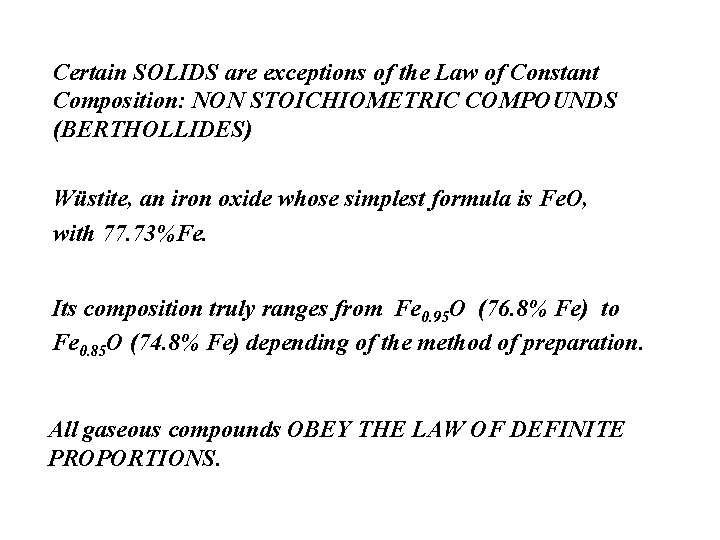
Certain SOLIDS are exceptions of the Law of Constant Composition: NON STOICHIOMETRIC COMPOUNDS (BERTHOLLIDES) Wüstite, an iron oxide whose simplest formula is Fe. O, with 77. 73%Fe. Its composition truly ranges from Fe 0. 95 O (76. 8% Fe) to Fe 0. 85 O (74. 8% Fe) depending of the method of preparation. All gaseous compounds OBEY THE LAW OF DEFINITE PROPORTIONS.

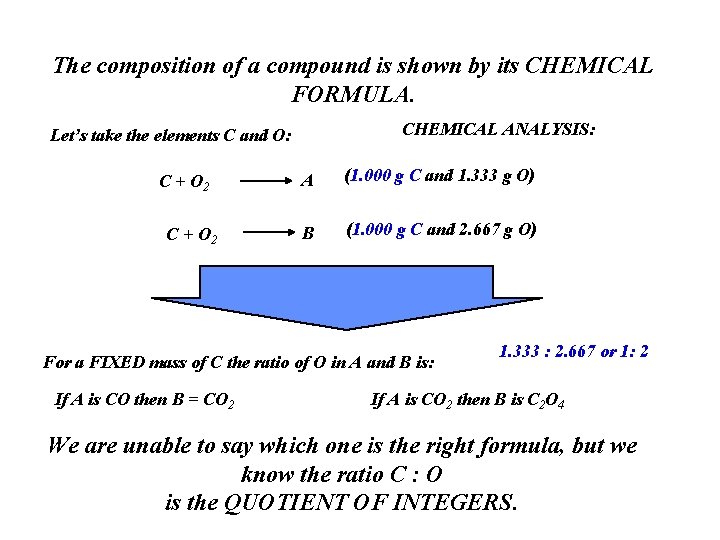
The composition of a compound is shown by its CHEMICAL FORMULA. CHEMICAL ANALYSIS: Let’s take the elements C and O: C + O 2 A (1. 000 g C and 1. 333 g O) B (1. 000 g C and 2. 667 g O) For a FIXED mass of C the ratio of O in A and B is: If A is CO then B = CO 2 1. 333 : 2. 667 or 1: 2 If A is CO 2 then B is C 2 O 4 We are unable to say which one is the right formula, but we know the ratio C : O is the QUOTIENT OF INTEGERS.

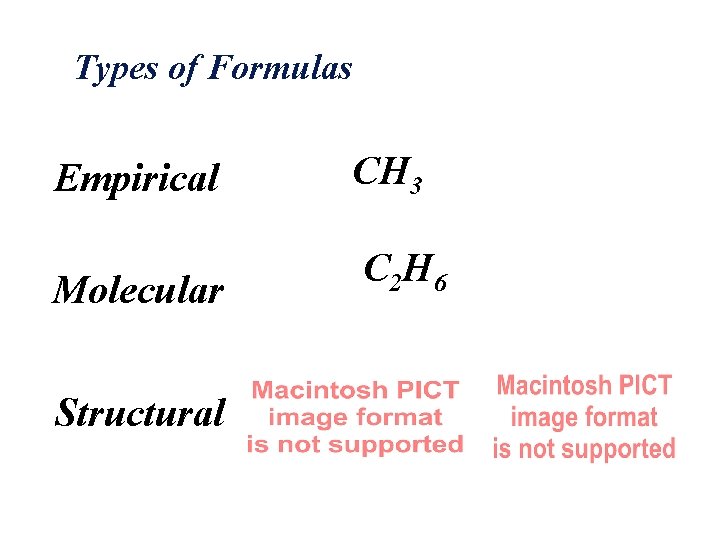
Molecules Composition Types of Formulas

Composition Held together by covalent bonds Usually made up of nonmetal atoms

Types of Formulas Empirical CH 3 Molecular C 2 H 6 Structural

Atomic and Formula Masses of Individual Atoms Meaning of Atomic Masses Formula Mass

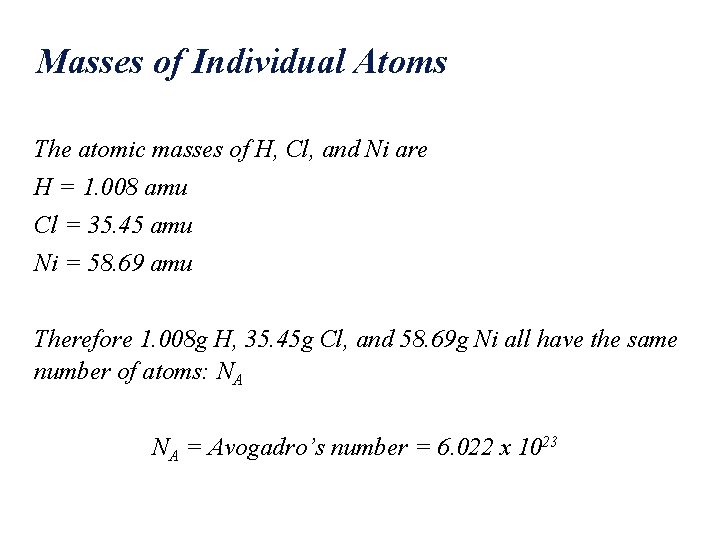
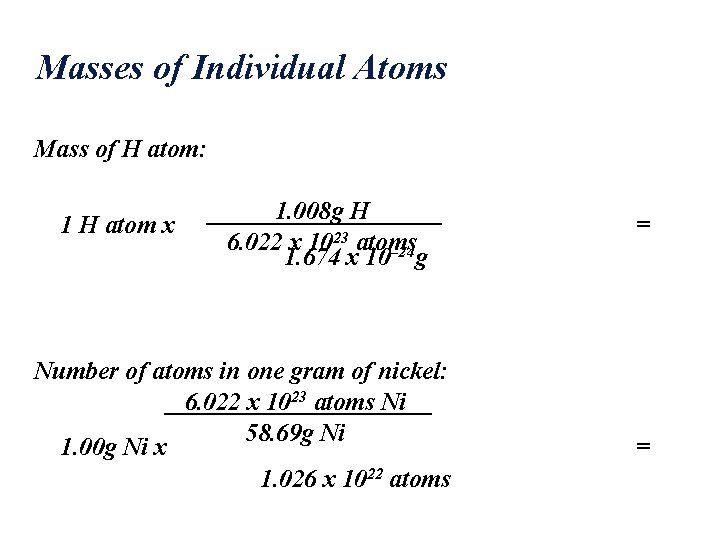
Masses of Individual Atoms The atomic masses of H, Cl, and Ni are H = 1. 008 amu Cl = 35. 45 amu Ni = 58. 69 amu Therefore 1. 008 g H, 35. 45 g Cl, and 58. 69 g Ni all have the same number of atoms: NA NA = Avogadro’s number = 6. 022 x 1023

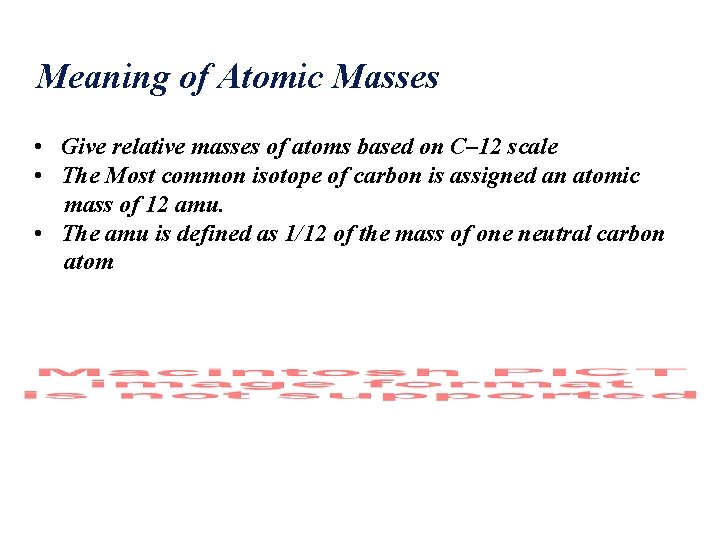
Meaning of Atomic Masses • Give relative masses of atoms based on C– 12 scale • The Most common isotope of carbon is assigned an atomic mass of 12 amu. • The amu is defined as 1/12 of the mass of one neutral carbon atom

Masses of Individual Atoms Mass of H atom: 1 H atom x 1. 008 g H 6. 022 x 1023 atoms 1. 674 x 10– 24 g Number of atoms in one gram of nickel: 6. 022 x 1023 atoms Ni 58. 69 g Ni 1. 00 g Ni x 1. 026 x 1022 atoms = =
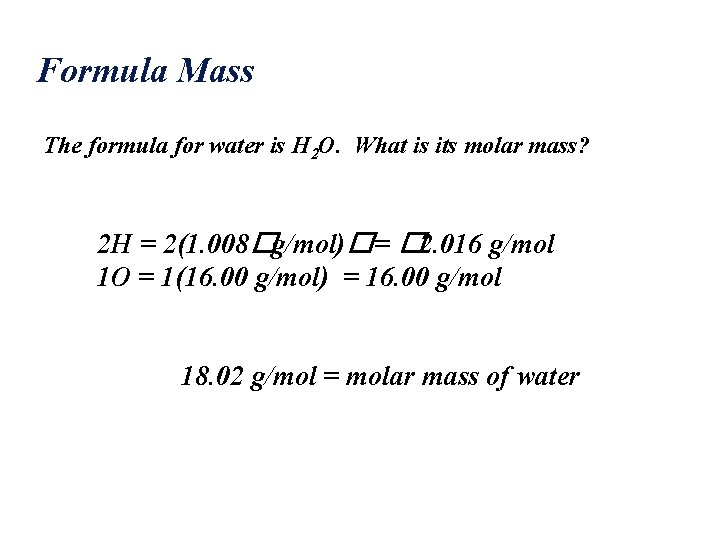
Formula Mass The formula for water is H 2 O. What is its molar mass? 2 H = 2(1. 008�g/mol)�= � 2. 016 g/mol 1 O = 1(16. 00 g/mol) = 16. 00 g/mol 18. 02 g/mol = molar mass of water

The Mole Meaning Molar Mass Mole - Mass Conversions

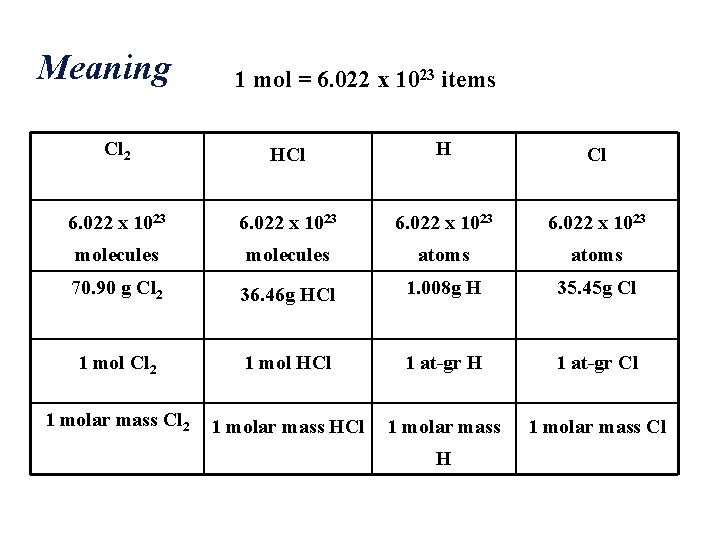
Meaning 1 mol = 6. 022 x 1023 items Cl 2 HCl H Cl 6. 022 x 1023 molecules atoms 70. 90 g Cl 2 36. 46 g HCl 1. 008 g H 35. 45 g Cl 1 mol Cl 2 1 mol HCl 1 at-gr H 1 at-gr Cl 1 molar mass Cl 2 1 molar mass HCl 1 molar mass Cl H

Molar Mass Generalizing from the previous examples, the molar mass, M, is numerically equal to the formula mass

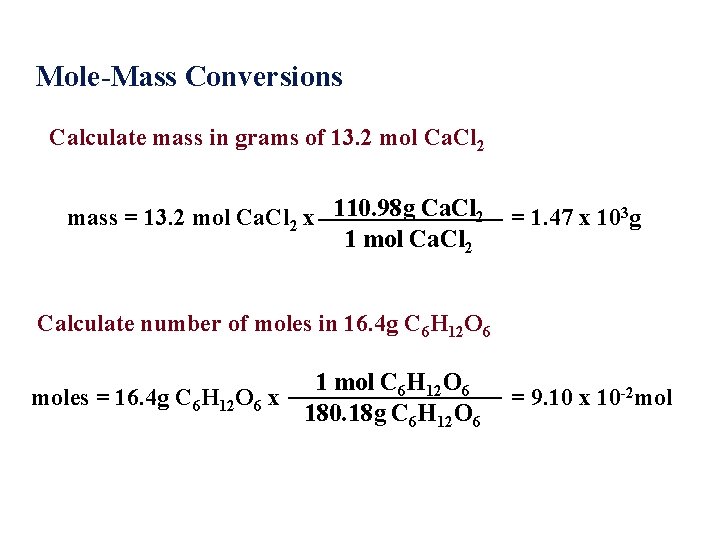
Mole-Mass Conversions Calculate mass in grams of 13. 2 mol Ca. Cl 2 mass = 13. 2 mol Ca. Cl 2 x 110. 98 g Ca. Cl 2 1 mol Ca. Cl 2 = 1. 47 x 103 g Calculate number of moles in 16. 4 g C 6 H 12 O 6 1 mol C 6 H 12 O 6 moles = 16. 4 g C 6 H 12 O 6 x 180. 18 g C 6 H 12 O 6 = 9. 10 x 10 -2 mol

Calculating Composition % Composition from Formula % Composition from Experimental Data Empirical Formula from % Composition Molecular Formula from Empirical Formula

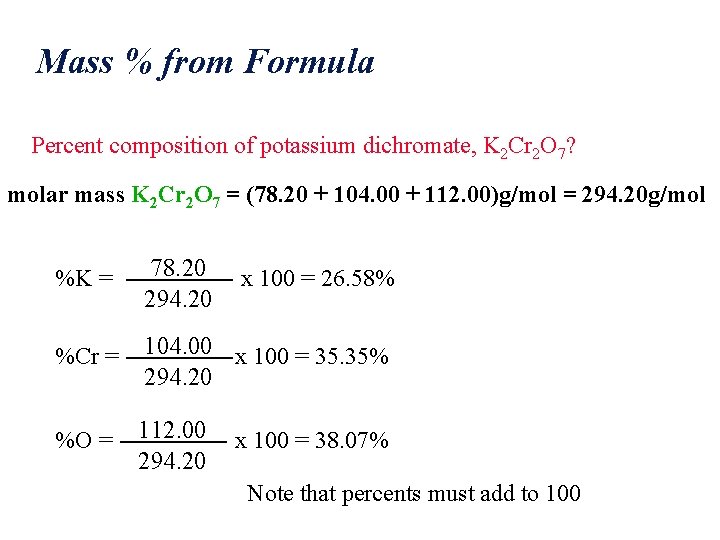
Mass % from Formula Percent composition of potassium dichromate, K 2 Cr 2 O 7? molar mass K 2 Cr 2 O 7 = (78. 20 + 104. 00 + 112. 00)g/mol = 294. 20 g/mol %K = 78. 20 294. 20 %Cr = 104. 00 x 100 = 35. 35% 294. 20 %O = 112. 00 294. 20 x 100 = 26. 58% x 100 = 38. 07% Note that percents must add to 100

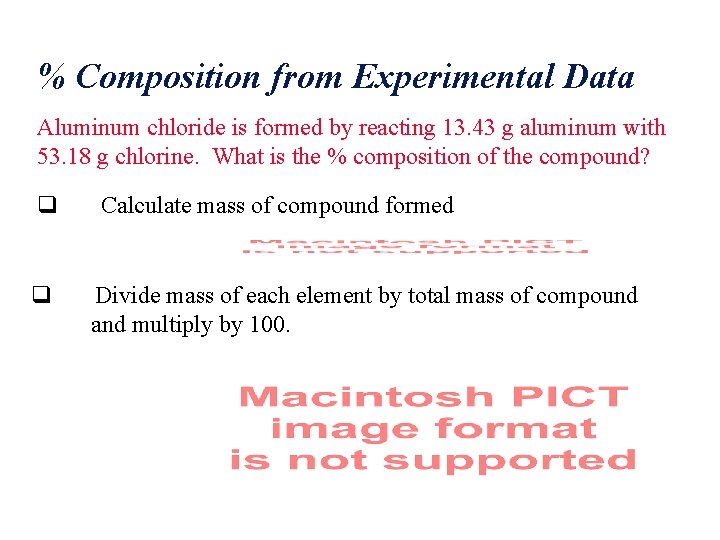
% Composition from Experimental Data Aluminum chloride is formed by reacting 13. 43 g aluminum with 53. 18 g chlorine. What is the % composition of the compound? q q Calculate mass of compound formed Divide mass of each element by total mass of compound and multiply by 100.

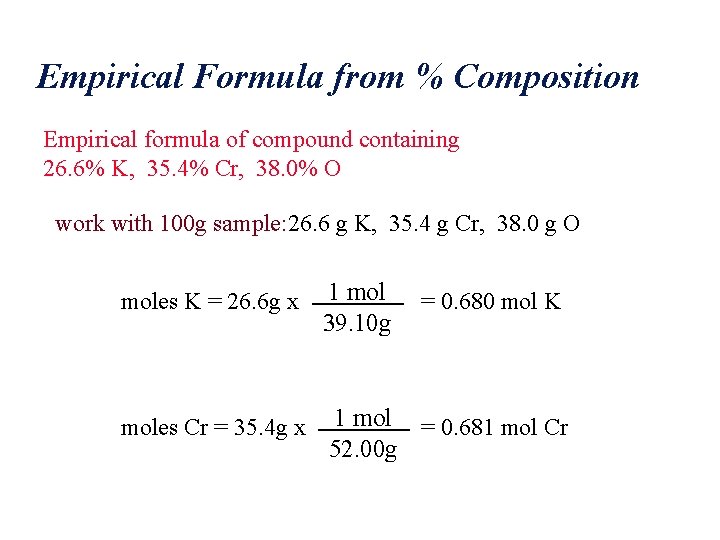
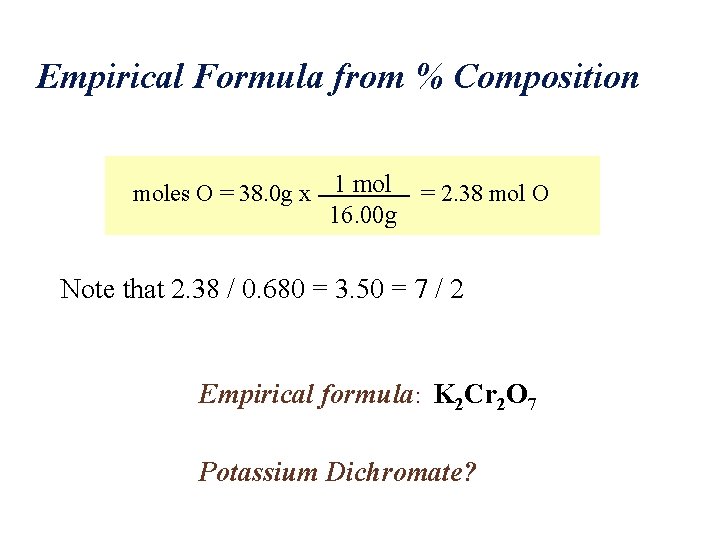
Empirical Formula from % Composition Empirical formula of compound containing 26. 6% K, 35. 4% Cr, 38. 0% O work with 100 g sample: 26. 6 g K, 35. 4 g Cr, 38. 0 g O moles K = 26. 6 g x 1 mol 39. 10 g moles Cr = 35. 4 g x 1 mol = 0. 681 mol Cr 52. 00 g = 0. 680 mol K

Empirical Formula from % Composition moles O = 38. 0 g x 1 mol = 2. 38 mol O 16. 00 g Note that 2. 38 / 0. 680 = 3. 50 = 7 / 2 Empirical formula: K 2 Cr 2 O 7 Potassium Dichromate?

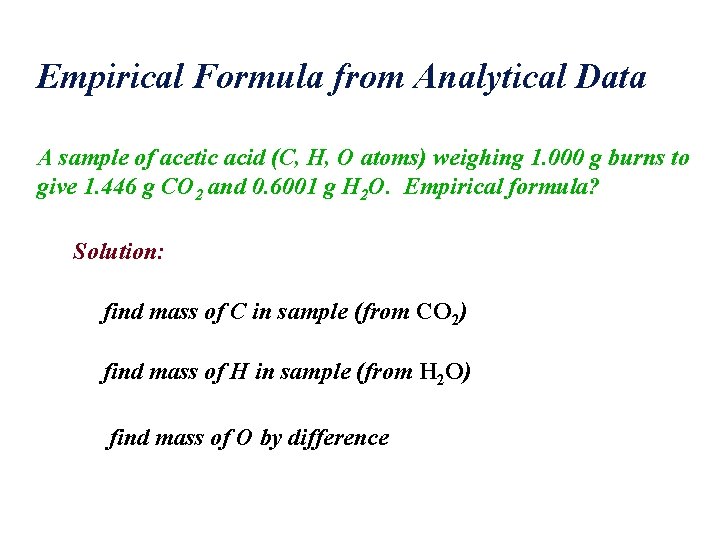
Empirical Formula from Analytical Data A sample of acetic acid (C, H, O atoms) weighing 1. 000 g burns to give 1. 446 g CO 2 and 0. 6001 g H 2 O. Empirical formula? Solution: find mass of C in sample (from CO 2) find mass of H in sample (from H 2 O) find mass of O by difference

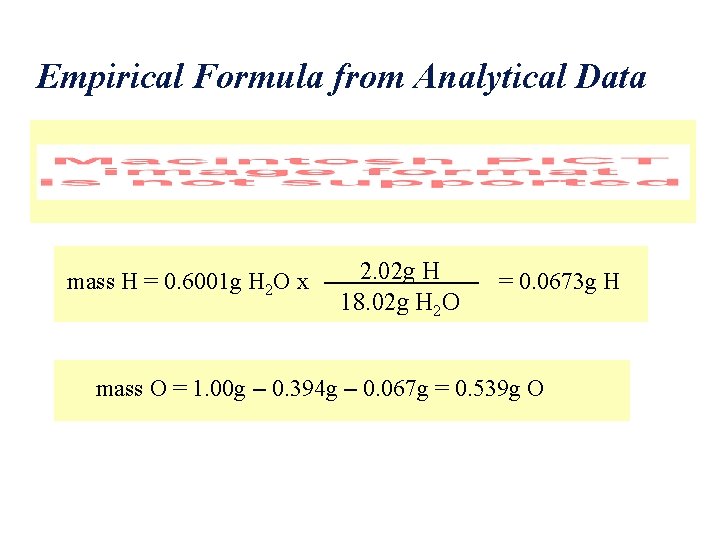
Empirical Formula from Analytical Data mass H = 0. 6001 g H 2 O x 2. 02 g H 18. 02 g H 2 O = 0. 0673 g H mass O = 1. 00 g – 0. 394 g – 0. 067 g = 0. 539 g O

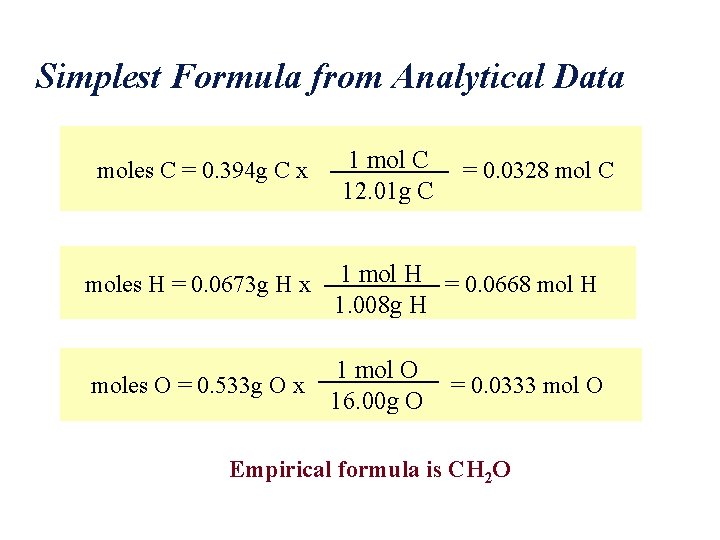
Simplest Formula from Analytical Data moles C = 0. 394 g C x 1 mol C 12. 01 g C = 0. 0328 mol C moles H = 0. 0673 g H x 1 mol H = 0. 0668 mol H 1. 008 g H moles O = 0. 533 g O x 1 mol O 16. 00 g O = 0. 0333 mol O Empirical formula is CH 2 O

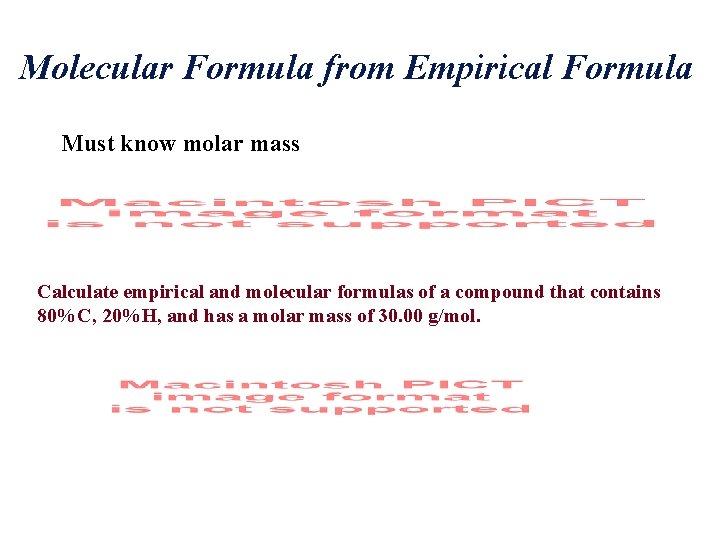
Molecular Formula from Empirical Formula Must know molar mass Calculate empirical and molecular formulas of a compound that contains 80%C, 20%H, and has a molar mass of 30. 00 g/mol.

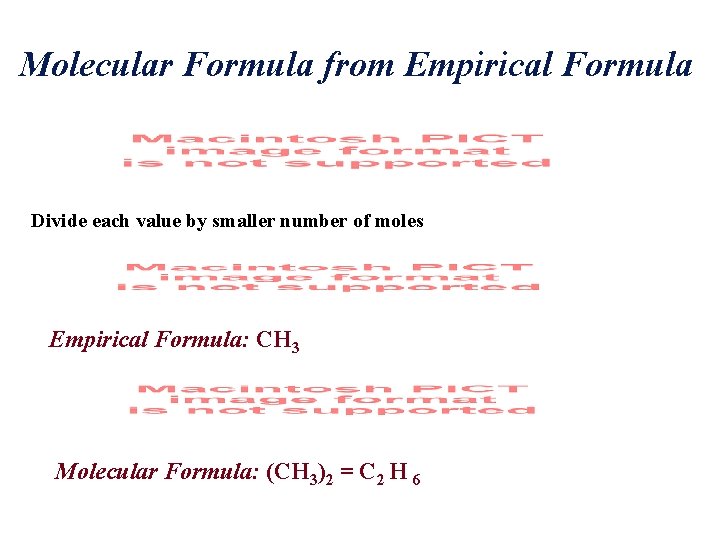
Molecular Formula from Empirical Formula Divide each value by smaller number of moles Empirical Formula: CH 3 Molecular Formula: (CH 3)2 = C 2 H 6
 Metallic bonding facts
Metallic bonding facts Quantitative analysis of organic compounds ppt
Quantitative analysis of organic compounds ppt Preparation of alicyclic compounds
Preparation of alicyclic compounds Binary compounds chemistry
Binary compounds chemistry Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Organic chemistry crash course
Organic chemistry crash course Priority of functional groups in iupac nomenclature
Priority of functional groups in iupac nomenclature Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Quantitative chemistry
Quantitative chemistry Qualitative analysis chemistry igcse
Qualitative analysis chemistry igcse Quantitative chemistry grade 11
Quantitative chemistry grade 11 Quantitative and qualitative in chemistry
Quantitative and qualitative in chemistry Chemistry percent composition
Chemistry percent composition Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Personlig tidbok
Personlig tidbok A gastrica
A gastrica Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Debattartikel struktur
Debattartikel struktur