Percent Composition AHS CHEMISTRY GUTIERREZ 2013 2014 C


- Slides: 8

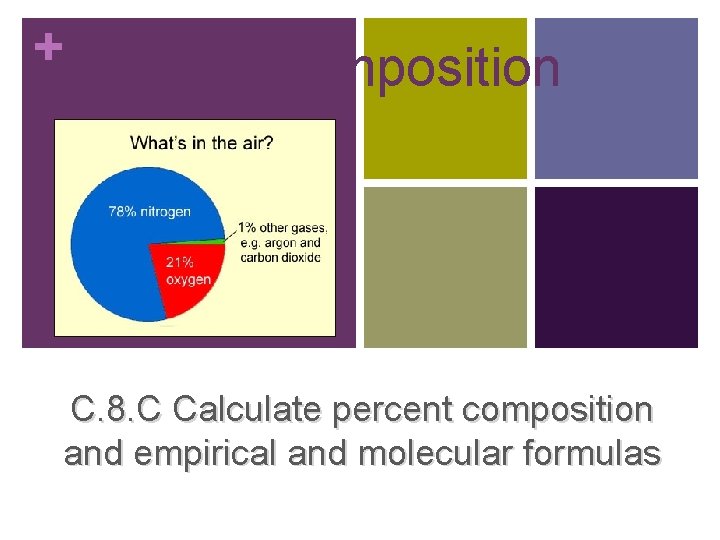
+Percent Composition AHS CHEMISTRY GUTIERREZ 2013 -2014 C. 8. C Calculate percent composition and empirical and molecular formulas

+ Compounds n Every chemical compound has a definite-a composition that is always the same wherever that compound is found. n That means that: H 2 O (water) Will always have: 2 moles of hydrogen to every 1 mole of oxygen n. If you are studying a chemical compound, you may want to find the percent composition of a certain element within that chemical compound.

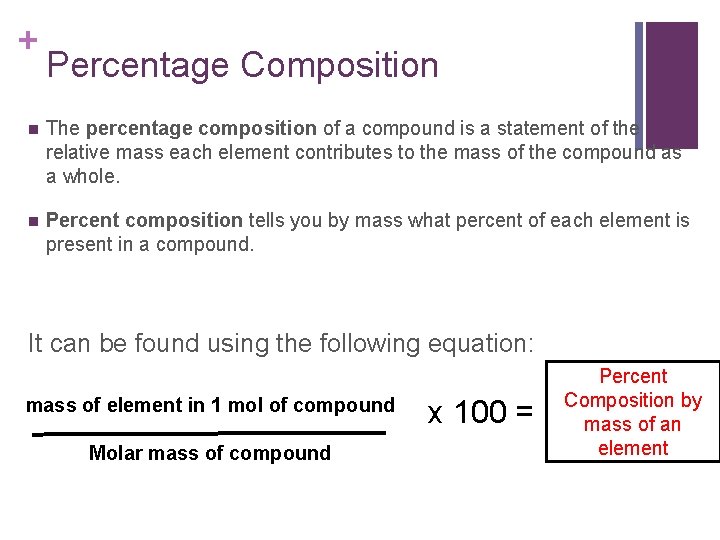
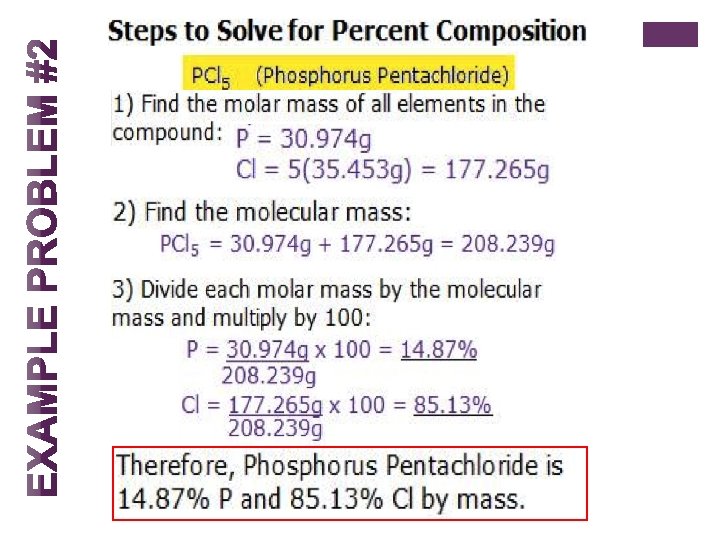
+ Percentage Composition n The percentage composition of a compound is a statement of the relative mass each element contributes to the mass of the compound as a whole. n Percent composition tells you by mass what percent of each element is present in a compound. It can be found using the following equation: mass of element in 1 mol of compound Molar mass of compound x 100 = Percent Composition by mass of an element

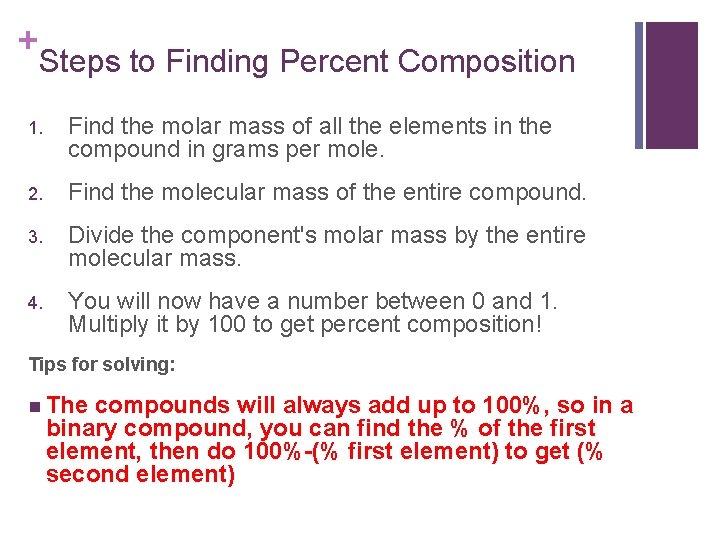
+ Steps to Finding Percent Composition 1. Find the molar mass of all the elements in the compound in grams per mole. 2. Find the molecular mass of the entire compound. 3. Divide the component's molar mass by the entire molecular mass. 4. You will now have a number between 0 and 1. Multiply it by 100 to get percent composition! Tips for solving: n The compounds will always add up to 100%, so in a binary compound, you can find the % of the first element, then do 100%-(% first element) to get (% second element)

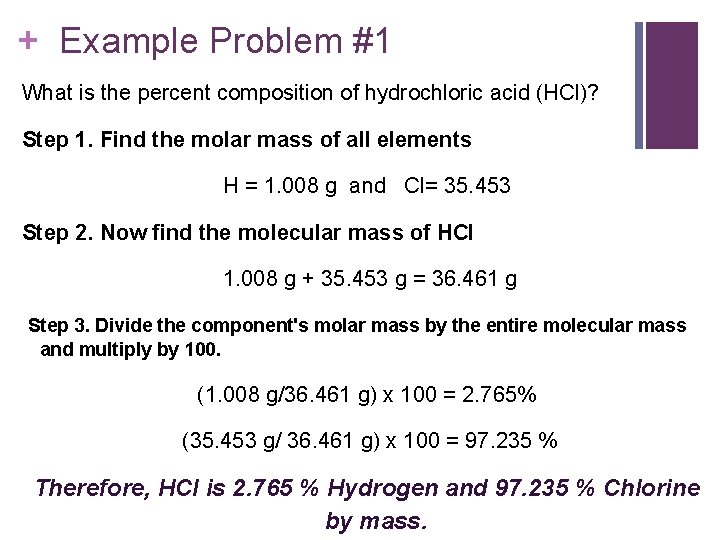
+ Example Problem #1 What is the percent composition of hydrochloric acid (HCl)? Step 1. Find the molar mass of all elements H = 1. 008 g and Cl= 35. 453 Step 2. Now find the molecular mass of HCl 1. 008 g + 35. 453 g = 36. 461 g Step 3. Divide the component's molar mass by the entire molecular mass and multiply by 100. (1. 008 g/36. 461 g) x 100 = 2. 765% (35. 453 g/ 36. 461 g) x 100 = 97. 235 % Therefore, HCl is 2. 765 % Hydrogen and 97. 235 % Chlorine by mass.


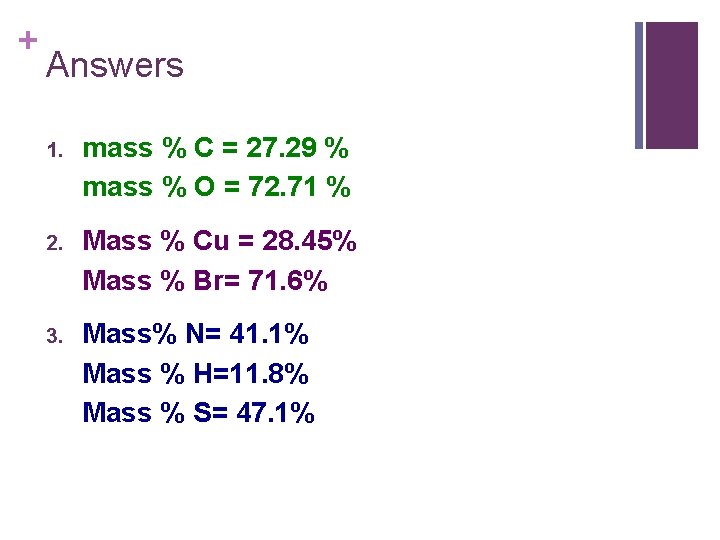
+ Now you Try! 1. What are the percent composition of carbon and oxygen in carbon dioxide, CO 2? 2. Find the percent compositions of all of the elements in the following compounds, Cu. Br. 3. Calculate the percent composition of nitrogen, hydrogen, and sulfur in (NH 4)2 S.

+ Answers 1. mass % C = 27. 29 % mass % O = 72. 71 % 2. Mass % Cu = 28. 45% Mass % Br= 71. 6% 3. Mass% N= 41. 1% Mass % H=11. 8% Mass % S= 47. 1%