Naming Ionic Compounds Ionic compounds Compounds are created













- Slides: 17

Naming Ionic Compounds

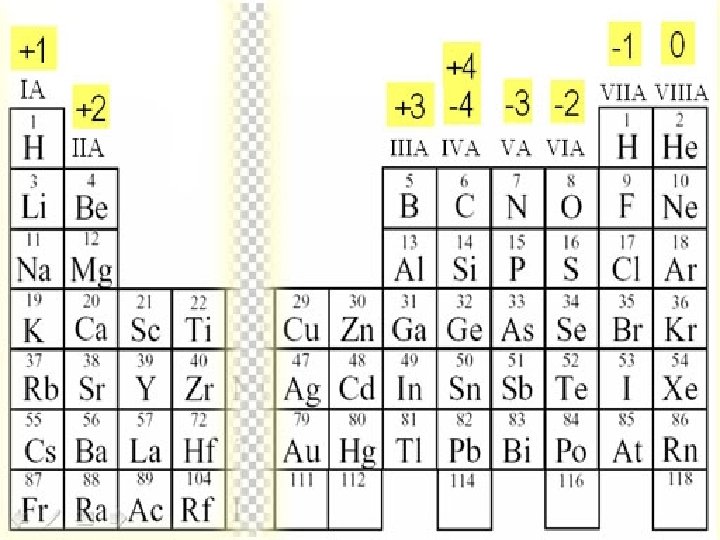
Ionic compounds ► Compounds are created by a combination of charged ions. ► Generally they contain a metal bonded to a nonmetal ► the positive ion (cations) and the negative ion (anions) can be thought of as the first and last names of the compound

► The first part of the name will come from the left side in periodic table ► Followed by naming the second element which will be further to the right

2. Cations keep their name § Ex. Li Lithium (stays the same!)

Anions change the last few letters to have the suffix –ide ► Ex. O oxide

4. Positive first, negative last Ex. Li. O ► Lithium Oxide ►

Writing Ionic Formulas ► Write the symbols for the elements in the compound. ► Remember the ending "ide" is used for fluoride to show that it is a negative ion of fluorine. Example: Barium Fluorine

Barium Fluoride ►Look up the oxidation numbers of the elements involved and write them as superscripts to the right of the elemental symbols.


2+ Ba F ►Note that when no number accompanies a charge symbol, as in the case of fluoride above, the charge value is understood to be "1".

2+ Ba F ► Use the correct combination of ions to produce a compound with a net charge of zero. ► Right now the net charge is 1+ (2+) + (1 -) = +1 F do we need to - ► How many more balance out the positive ion?

►Two fluoride ions will cancel out one barium ion. (2+) + 2(1 -) = 0 ►we use a subscript of two after the symbol for fluorine to show the ratio of 1: 2. Ba. F 2

Writing Ionic Formulas 2+ 3 Mg N ►What is this compounds net charge? (2+) + (3 -) = 1►How can we get this to have a net charge of zero? 3(2+) +2(3 -) = 0

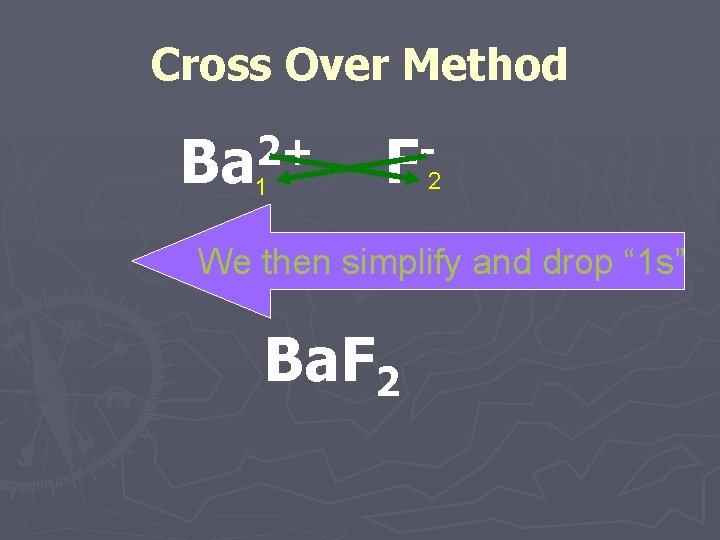
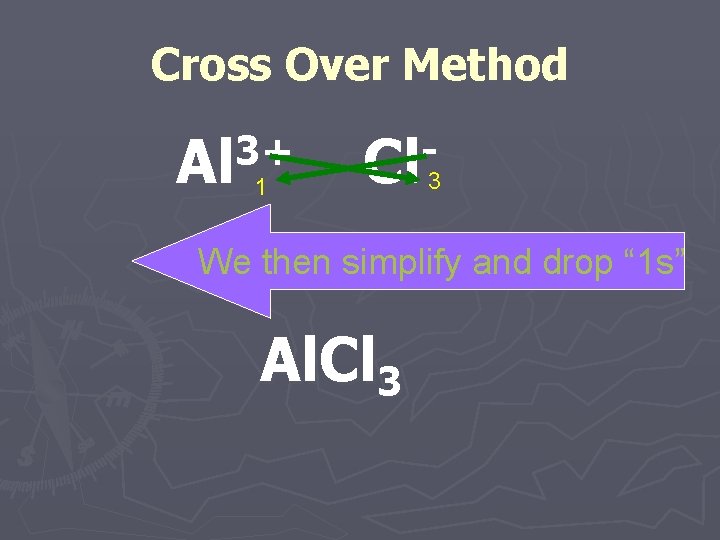
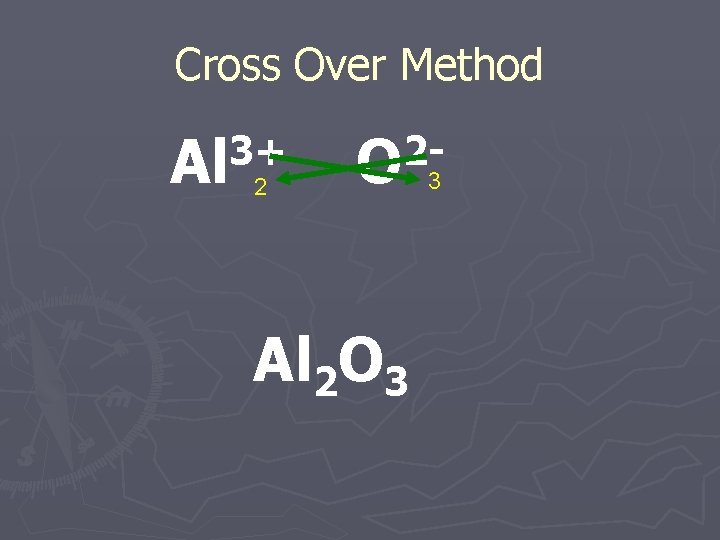
Cross Over Method ►You can simply take the ionic charge for the positively charged ion and make it the subscript for the negatively charged ion and vice versa.

Cross Over Method 2+ Ba F 1 2 We then simplify and drop “ 1 s” Ba. F 2

Cross Over Method 3+ Al Cl 1 3 We then simplify and drop “ 1 s” Al. Cl 3

Cross Over Method 3+ 2 Al O 2 Al 2 O 3 3