Chemistry A Molecular Approach 2 nd Ed Nivaldo




























- Slides: 61

Chemistry: A Molecular Approach, 2 nd Ed. Nivaldo Tro Chapter 2 Atoms and Elements Ms. Knick HAHS Tro: Chemistry: A Molecular Approach, 2/e 1 Copyright 2011 Pearson Education, Inc.

Atomic Theory Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Let’s Take a Trip Through Time! Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Democritus 400 B. C. • There are various basic elements from which all matter is made • Everything is composed of small atoms • Some atoms are round, pointy, oily, have hooks, etc. to account for their properties Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Democritus’s Model Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

John Dalton • Introduced his ideas in 1803 • Each element is composed of • • Tro: Chemistry: A Molecular Approach, 2/e extremely small particles called atoms All the atoms of a given element are identical, but they differ from those of any other element Atoms are neither created nor destroyed in any chemical reaction Copyright 2011 Pearson Education, Inc.

Dalton’s Model Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

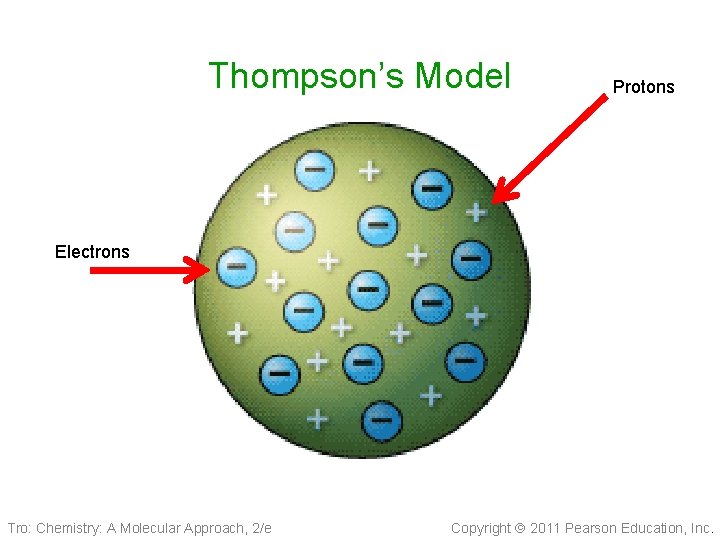
J. J. Thompson 1904 • Discovered electron • (negative particle) in the Cathode Ray Experiment Plum Pudding model 1904 ü Electrons in a soup of protons (positive charges) ü There is an equal number of positive and negative charges because the atom is neutral Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Cathode Ray Tube Experiment Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Thompson’s Model Protons Electrons Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

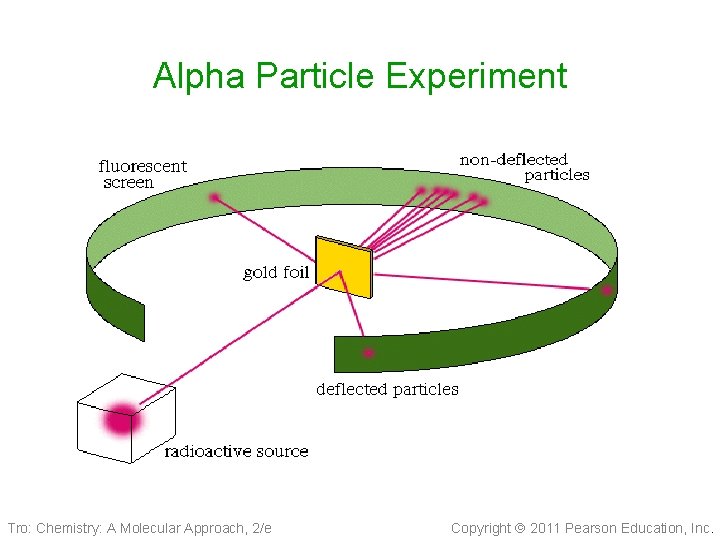
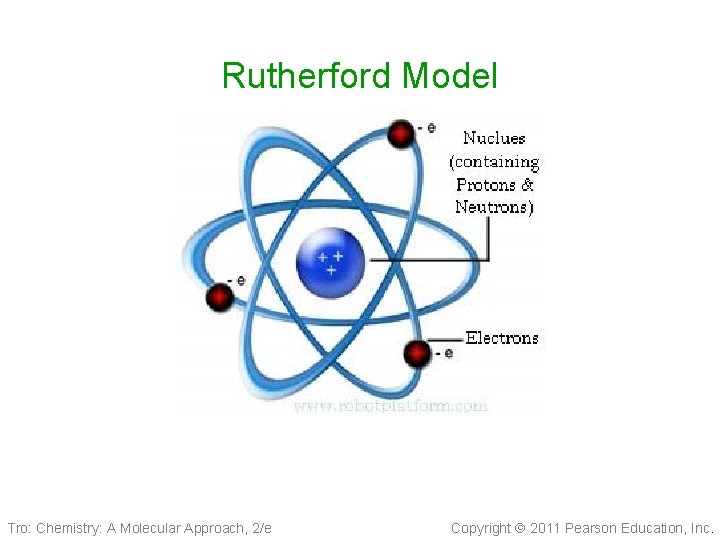
Ernest Rutherford 1910 • Nucleus Theory 1910 Tro: Chemistry: A Molecular Approach, 2/e ü alpha particle gold foil experiment • An atom’s mass is mostly in the nucleus • The nucleus has a positive charge because it contains the protons and because it is so large in mass it contains another particle called the neutrons(neutral) • Electrons in fixed orbit Copyright 2011 Pearson Education, Inc.

Alpha Particle Experiment Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Rutherford Model Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

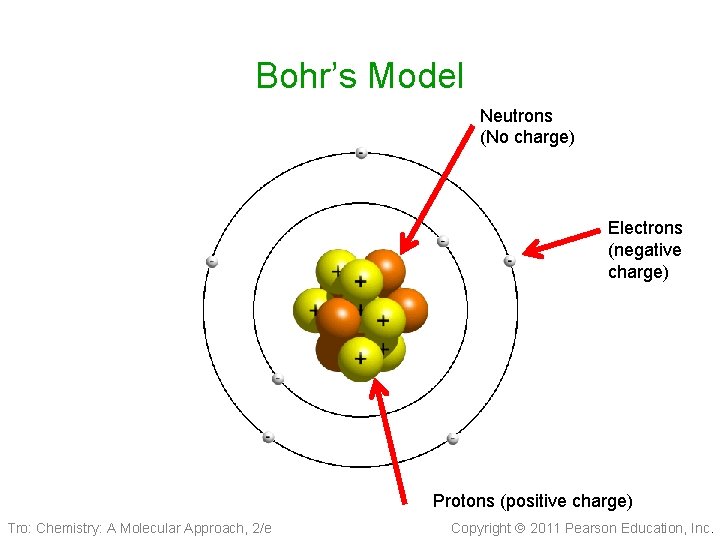
Niels Bohr 1913 • Planetary Model 1913 ü Nucleus surrounded by orbiting electrons at different energy levels ü Electrons have definite orbits • Worked on the Manhattan Project (US atomic bomb) Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Bohr’s Model Neutrons (No charge) Electrons (negative charge) Protons (positive charge) Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

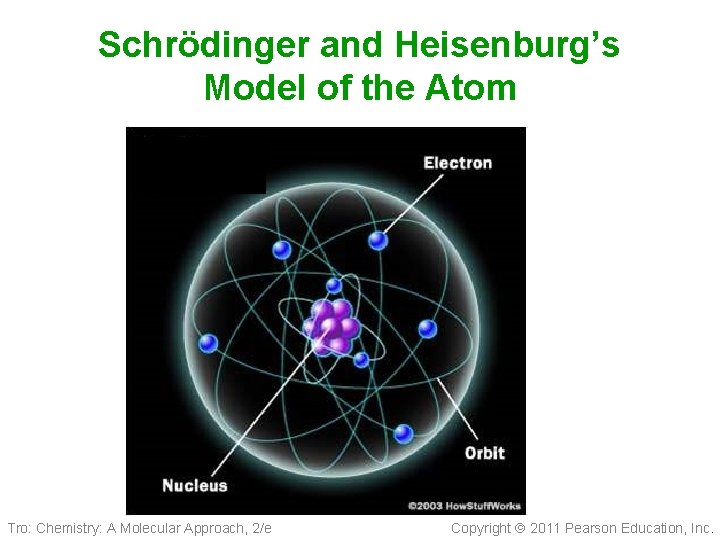
Ernst Schrödinger Werner Heisenberg • Quantum Mechanical Model 1926 ü Electrons are in probability zones called “orbitals”, not orbits and the location cannot be pinpointed and they are constantly moving (they are not moving in circular orbits) ü Schrödinger and Heisenburg’s Model of the Atom is the Model still accepted today. Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Schrödinger and Heisenburg’s Model of the Atom Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

• • Elements Each element has a unique number of protons in its nucleus The number of protons in the nucleus of an atom is called the atomic number üthe elements are arranged on the Periodic Table in order of their atomic numbers Tro: Chemistry: A Molecular Approach, 2/e 19 Copyright 2011 Pearson Education, Inc.

• Electrons The number of electrons(-) must equal the number of protons(+) in a neutral atom. Tro: Chemistry: A Molecular Approach, 2/e 20 Copyright 2011 Pearson Education, Inc.

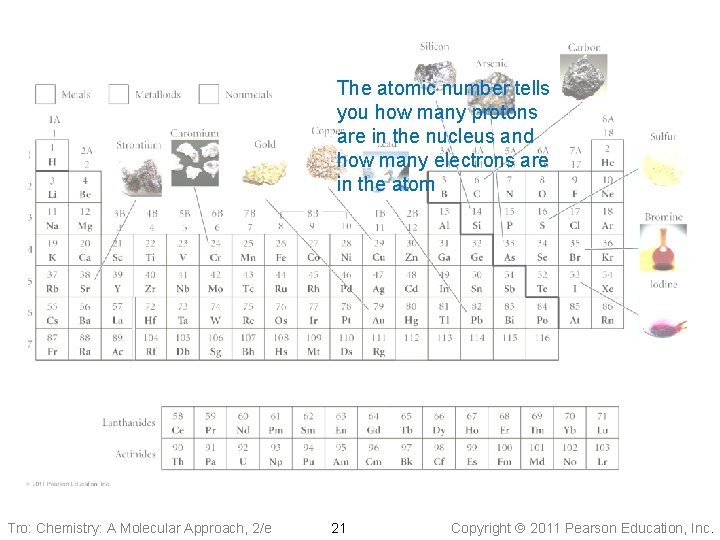
The Periodic Table of the Elements The atomic number tells you how many protons are in the nucleus and how many electrons are in the atom Tro: Chemistry: A Molecular Approach, 2/e 21 Copyright 2011 Pearson Education, Inc.

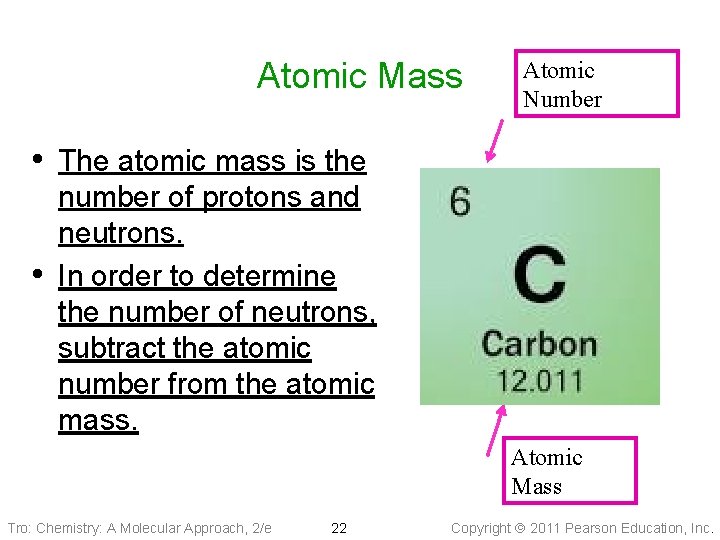
Atomic Mass Atomic Number • The atomic mass is the • number of protons and neutrons. In order to determine the number of neutrons, subtract the atomic number from the atomic mass. Atomic Mass Tro: Chemistry: A Molecular Approach, 2/e 22 Copyright 2011 Pearson Education, Inc.

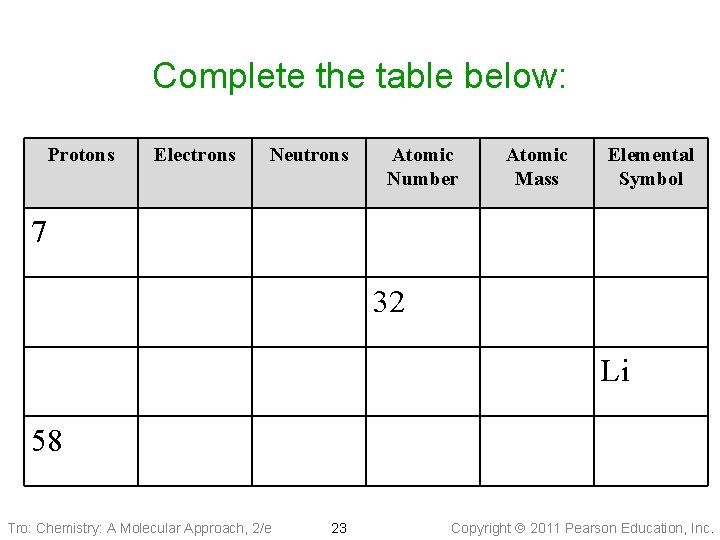
Complete the table below: Protons Electrons Neutrons Atomic Number Atomic Mass Elemental Symbol 7 32 Li 58 Tro: Chemistry: A Molecular Approach, 2/e 23 Copyright 2011 Pearson Education, Inc.

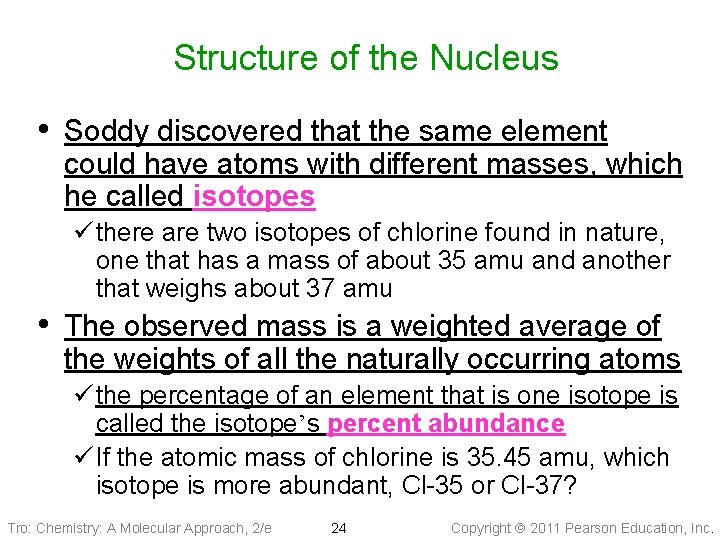
Structure of the Nucleus • Soddy discovered that the same element could have atoms with different masses, which he called isotopes ü there are two isotopes of chlorine found in nature, one that has a mass of about 35 amu and another that weighs about 37 amu • The observed mass is a weighted average of the weights of all the naturally occurring atoms ü the percentage of an element that is one isotope is called the isotope’s percent abundance ü If the atomic mass of chlorine is 35. 45 amu, which isotope is more abundant, Cl-35 or Cl-37? Tro: Chemistry: A Molecular Approach, 2/e 24 Copyright 2011 Pearson Education, Inc.

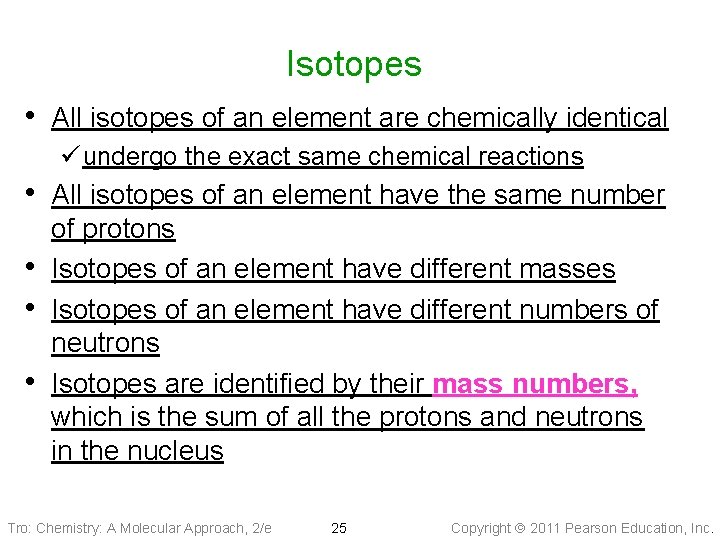
Isotopes • All isotopes of an element are chemically identical ü undergo the exact same chemical reactions • All isotopes of an element have the same number • • • of protons Isotopes of an element have different masses Isotopes of an element have different numbers of neutrons Isotopes are identified by their mass numbers, which is the sum of all the protons and neutrons in the nucleus Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc.

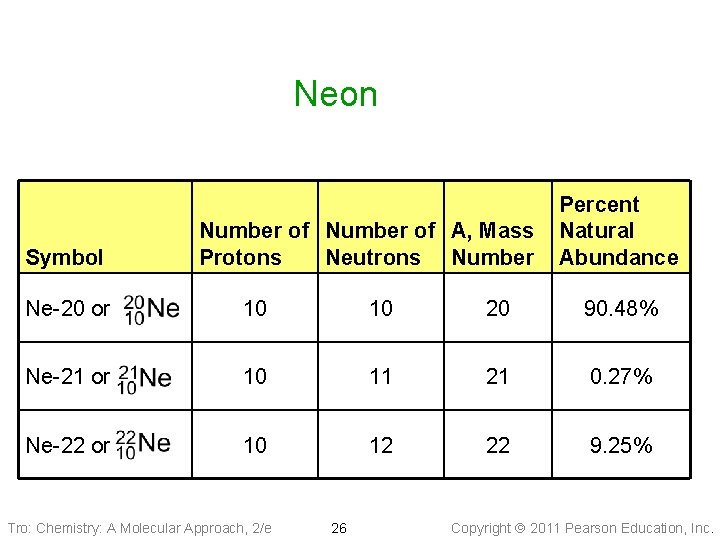
Neon Symbol Number of A, Mass Protons Neutrons Number Percent Natural Abundance Ne-20 or 10 10 20 90. 48% Ne-21 or 10 11 21 0. 27% Ne-22 or 10 12 22 9. 25% Tro: Chemistry: A Molecular Approach, 2/e 26 Copyright 2011 Pearson Education, Inc.

Practice: (EASY) Naturally occurring europium (Eu) consists of two isotopes with a mass of 151 g and 153 g. Europium-151 has an abundance of 48. 03% and Europium-153 has an abundance of 51. 97%. What is the atomic mass of europium? Tro: Chemistry: A Molecular Approach, 2/e 27 Copyright 2011 Pearson Education, Inc.

Practice: (You must think…. just a little) • Silver has two isotopes. The first isotope is 51. 83% abundant and has a mass of 106. 905 g. What is the mass of the second isotope? Tro: Chemistry: A Molecular Approach, 2/e 28 Copyright 2011 Pearson Education, Inc.

Practice: (You must think…. HARD) • An imaginary element (Atomic mass 93. 7140) has three naturally-occurring isotopes with isotopic masses of 92. 9469, 93. 2923 and 94. 9030. The abundance of the lightest isotope is 42. 38 %. What is the percentage abundance of the heaviest isotope? Tro: Chemistry: A Molecular Approach, 2/e 29 Copyright 2011 Pearson Education, Inc.

Charged Atoms • When atoms gain or lose electrons, they • • • acquire a charge Charged atoms or groups of atoms are called ions When atoms gain electrons, they become negatively charged ions, called anions When atoms lose electrons, they become positively charged ions, called cations Tro: Chemistry: A Molecular Approach, 2/e 30 Copyright 2011 Pearson Education, Inc.

Ions and Compounds • Ions behave much differently than the neutral atoms ü e. g. , the metal sodium, made of neutral Na atoms, is highly reactive and quite unstable; however, the sodium cations, Na+, found in table salt are very nonreactive and stable • Because materials such as table salt are neutral, there must be equal amounts of charge from cations and anions in them Tro: Chemistry: A Molecular Approach, 2/e 31 Copyright 2011 Pearson Education, Inc.

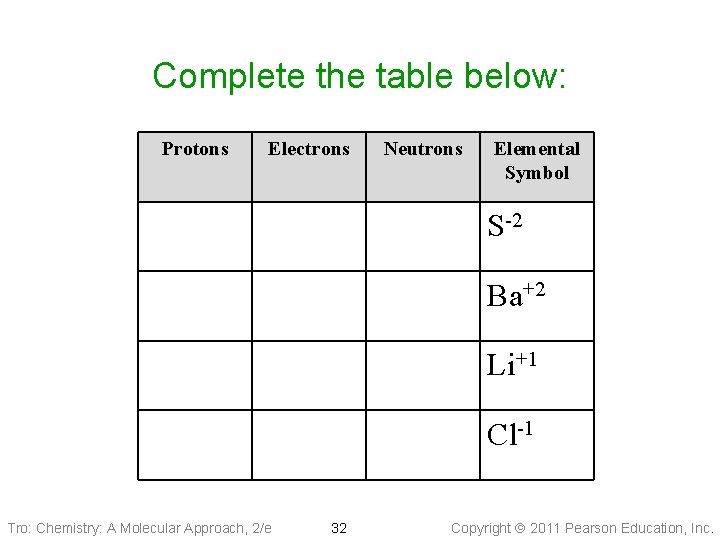
Complete the table below: Protons Electrons Neutrons Elemental Symbol S-2 Ba+2 Li+1 Cl-1 Tro: Chemistry: A Molecular Approach, 2/e 32 Copyright 2011 Pearson Education, Inc.

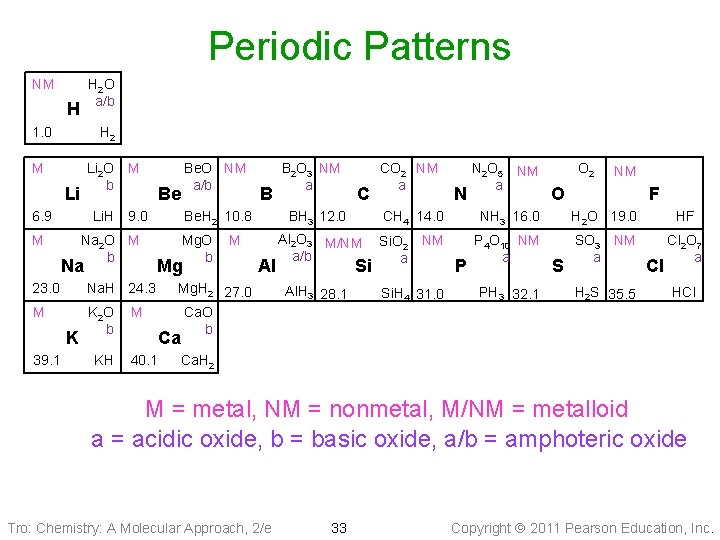
Periodic Patterns NM H 2 O a/b H 1. 0 H 2 M Li 6. 9 M Li 2 O b M Li. H 9. 0 Be Na 2 O M b Na 23. 0 Na. H 24. 3 M K 2 O b K 39. 1 KH Be. O NM a/b B Be. H 2 10. 8 Mg. O b M Mg M Al Mg. H 2 27. 0 Ca. O b B 2 O 3 NM a C CO 2 NM a N N 2 O 5 NM a O 2 NM O F BH 3 12. 0 CH 4 14. 0 NH 3 16. 0 H 2 O 19. 0 HF Al 2 O 3 M/NM a/b Si. O 2 NM a P 4 O 10 NM a SO 3 NM a Cl 2 O 7 a Al. H 3 28. 1 Si. H 4 31. 0 Si P PH 3 32. 1 S H 2 S 35. 5 Cl HCl Ca 40. 1 Ca. H 2 M = metal, NM = nonmetal, M/NM = metalloid a = acidic oxide, b = basic oxide, a/b = amphoteric oxide Tro: Chemistry: A Molecular Approach, 2/e 33 Copyright 2011 Pearson Education, Inc.

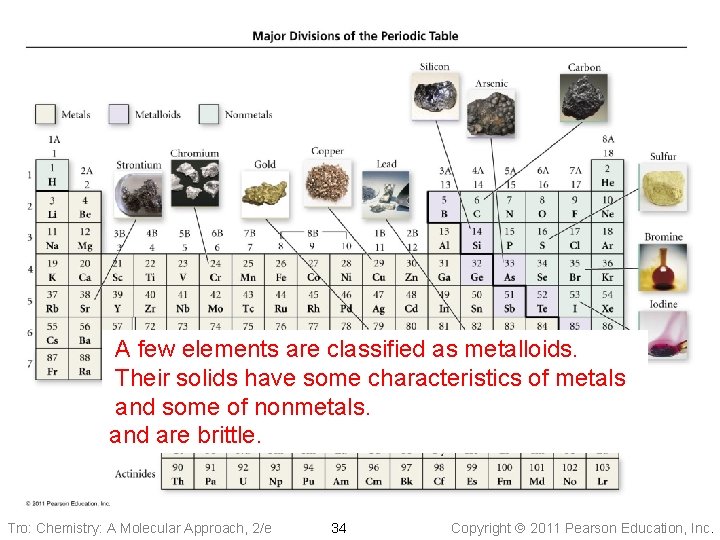
Most About A fewofelements ¾the of remaining the elements are classified asare metalloids. classified as metals. as nonmetals. They have Their solidsa. Their have reflective solids somesurface, characteristics have a non-reflective conductofheat metals and surface, electricity and some dobetter of not nonmetals. conduct than other heatelements, and electricity and are well, and malleable are brittle. and ductile Tro: Chemistry: A Molecular Approach, 2/e 34 Copyright 2011 Pearson Education, Inc.

Metals • Solids at room temperature, except Hg • Reflective surface ü shiny • Conduct heat • Conduct electricity • Malleable ü can be shaped • Ductile ü can be drawn or pulled into wires • Lose electrons and form cations in • • reactions About 75% of the elements are metals Left side of table (except H) Tro: Chemistry: A Molecular Approach, 2/e 35 Copyright 2011 Pearson Education, Inc.

Sulfur, S(s) Nonmetals • • • Found in all three states Poor conductors of heat Poor conductors of electricity Solids are brittle Gain electrons in reactions to become anions Right on the table Tro: Chemistry: A Molecular Approach, 2/e 36 Bromine, Br 2(l) Chlorine, Cl 2(g) Copyright 2011 Pearson Education, Inc.

Metalloids • Show some • properties of metals and some of nonmetals Also known as semiconductors Tro: Chemistry: A Molecular Approach, 2/e 37 Properties of Silicon shiny conducts electricity does not conduct heat well brittle Copyright 2011 Pearson Education, Inc.

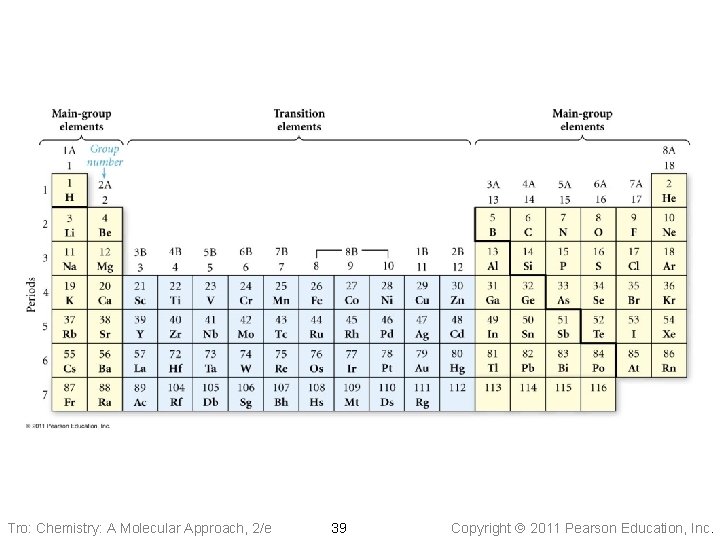
The Modern Periodic Table • Elements with similar chemical and • physical properties are in the same column Columns are called Groups or Families ü designated by a number and letter at top • Rows are called Periods • Each period shows the pattern of properties repeated in the next period Tro: Chemistry: A Molecular Approach, 2/e 38 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc.

Teach the Class: About your assigned elemental family. You must create 3 (max) Power. Point Slides about your elemental family. In order to receive full credit: • Present information in such a manner that your classmates can take notes • You must use visuals (Slide 1) • Element Examples, Location on Table (Slide 1) • Describe physical properties (Slide 2) • Describe chemical properties/reactivity and give an explanation for the behavior (Slide 3) • Submit to Miss Knick via email 3 questions about your presentation in a word document • Cite References on your word document Tro: Chemistry: A Molecular Approach, 2/e 40 Copyright 2011 Pearson Education, Inc.

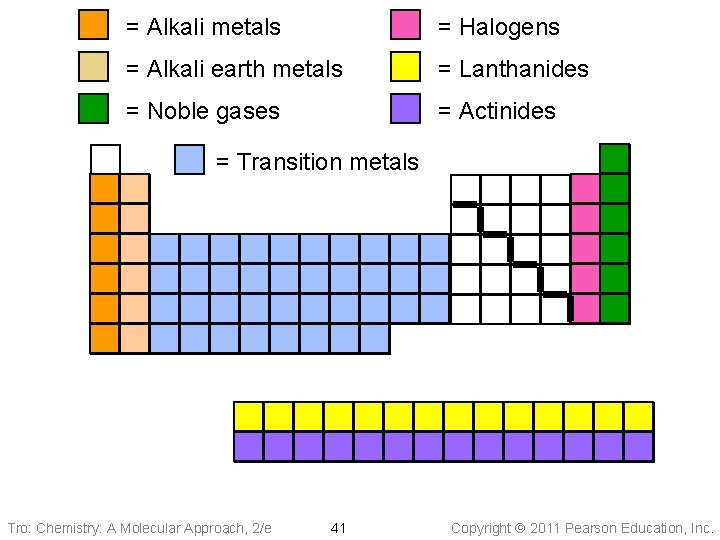
= Alkali metals = Halogens = Alkali earth metals = Lanthanides = Noble gases = Actinides = Transition metals Tro: Chemistry: A Molecular Approach, 2/e 41 Copyright 2011 Pearson Education, Inc.

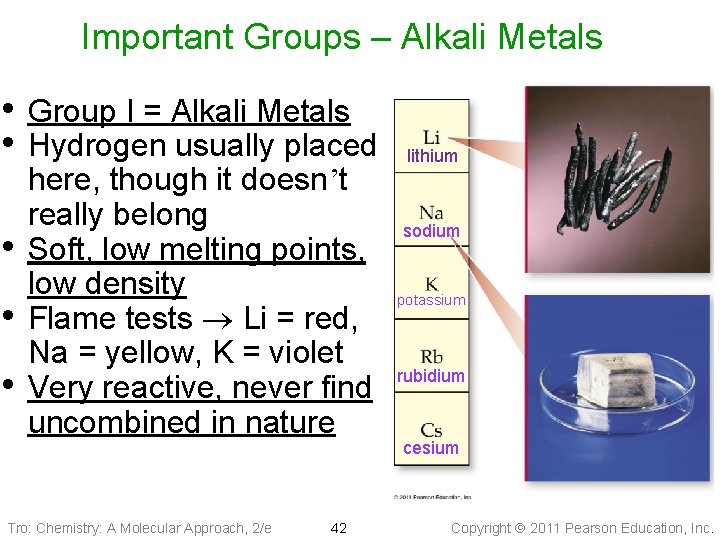
Important Groups – Alkali Metals • Group I = Alkali Metals • Hydrogen usually placed • • • here, though it doesn’t really belong Soft, low melting points, low density Flame tests ® Li = red, Na = yellow, K = violet Very reactive, never find uncombined in nature Tro: Chemistry: A Molecular Approach, 2/e 42 lithium sodium potassium rubidium cesium Copyright 2011 Pearson Education, Inc.

Important Groups – Alkali Earth Metals • Group II = Alkali earth metals • Harder, higher melting, and denser than alkali metals ü Mg alloys used as structural materials • Flame tests ® Ca = red, Sr = red, Ba = • green Reactive, but less than corresponding alkali metal Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc.

Important Groups – Halogens • Group 7 = halogens • Nonmetals • F 2 and Cl 2 gases; Br 2 liquid; I 2 solid • All diatomic • Very reactive fluorine chlorine bromine iodine astatine Tro: Chemistry: A Molecular Approach, 2/e 44 Copyright 2011 Pearson Education, Inc.

Important Groups – Noble Gases • Group 8= Noble Gases • All gases at room temperature ü very low melting and boiling points • Very unreactive, practically inert • Very hard to remove electron from or give electron to Tro: Chemistry: A Molecular Approach, 2/e 45 Copyright 2011 Pearson Education, Inc.

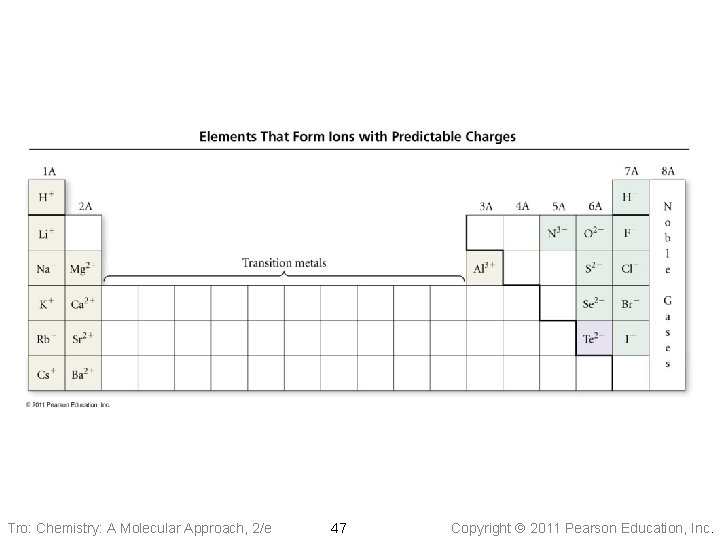
Ion Charge and the Periodic Table • The charge on an ion can often be • • determined from an element’s position on the Periodic Table Metals always form positively charged cations For many main group metals, the charge = the group number Nonmetals form negatively charged anions For nonmetals, the charge = the group number − 8 Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc.

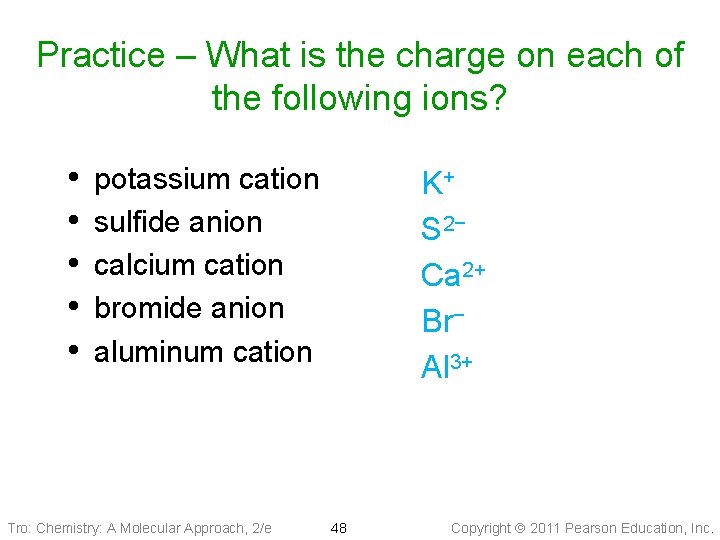
Practice – What is the charge on each of the following ions? • • • potassium cation sulfide anion calcium cation bromide anion aluminum cation Tro: Chemistry: A Molecular Approach, 2/e K+ S 2− Ca 2+ Br− Al 3+ 48 Copyright 2011 Pearson Education, Inc.

Counting Atoms by Moles • If we can find the mass of a • particular number of atoms, we can use this information to convert the mass of an element sample into the number of atoms in the sample The number of atoms we will use is 6. 022 x 1023, and we call this a mole ü 1 mole = 6. 022 x 1023 things Ølike 1 dozen = 12 things Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc.

Example 2. 6: Calculate the number of atoms in 2. 45 mol of copper Given: Find: Conceptual Plan: Relationships: 2. 45 mol Cu atoms Cu 1 mol = 6. 022 x 1023 atoms Solution: Check: because atoms are small, the large number of atoms makes sense Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc.

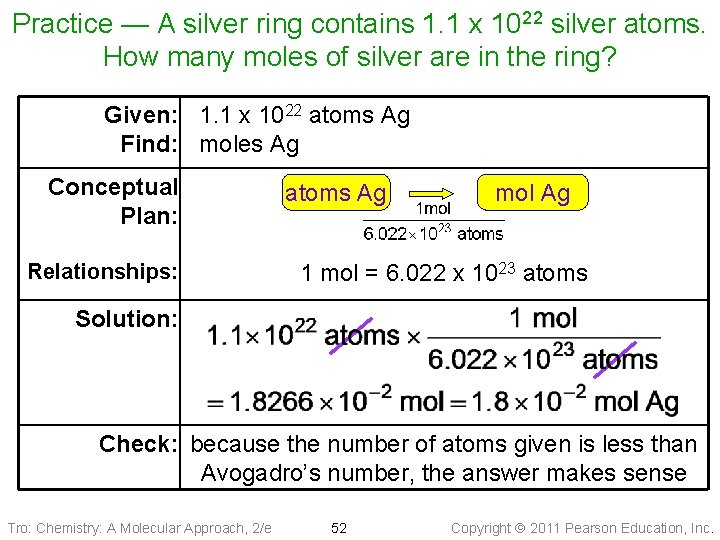
Practice — A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Tro: Chemistry: A Molecular Approach, 2/e 51 Copyright 2011 Pearson Education, Inc.

Practice — A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Given: 1. 1 x 1022 atoms Ag Find: moles Ag Conceptual Plan: Relationships: atoms Ag mol Ag 1 mol = 6. 022 x 1023 atoms Solution: Check: because the number of atoms given is less than Avogadro’s number, the answer makes sense Tro: Chemistry: A Molecular Approach, 2/e 52 Copyright 2011 Pearson Education, Inc.

Chemical Packages - Moles • The number of particles in 1 mole is called Avogadro’s Number = 6. 0221421 x 1023 ü A particle can be anything: atom, molecule, ion, electron, proton, etc. Tro: Chemistry: A Molecular Approach, 2/e 53 Copyright 2011 Pearson Education, Inc.

Relationship Between Moles and Mass • • The mass of one mole of atoms is called the molar mass The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc.

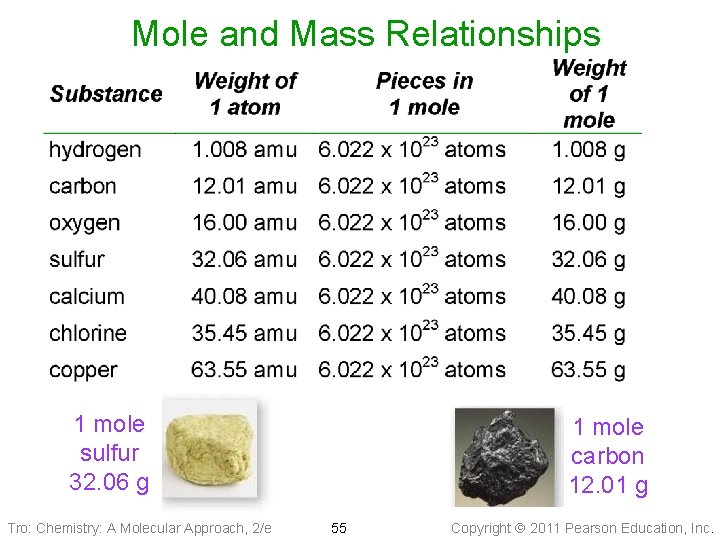
Mole and Mass Relationships 1 mole sulfur 32. 06 g Tro: Chemistry: A Molecular Approach, 2/e 1 mole carbon 12. 01 g 55 Copyright 2011 Pearson Education, Inc.

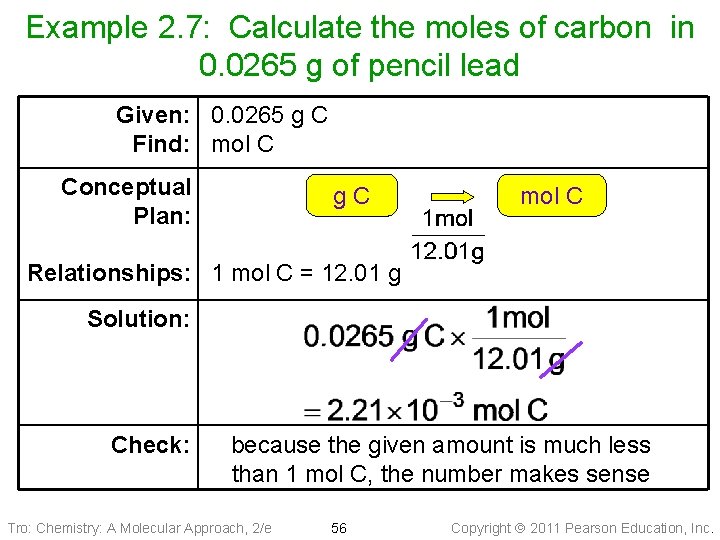
Example 2. 7: Calculate the moles of carbon in 0. 0265 g of pencil lead Given: 0. 0265 g C Find: mol C Conceptual Plan: g. C mol C Relationships: 1 mol C = 12. 01 g Solution: Check: because the given amount is much less than 1 mol C, the number makes sense Tro: Chemistry: A Molecular Approach, 2/e 56 Copyright 2011 Pearson Education, Inc.

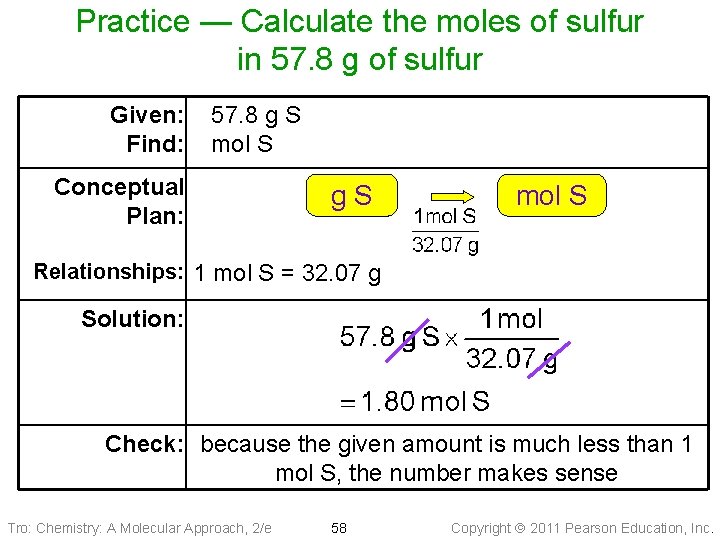
Practice — Calculate the moles of sulfur in 57. 8 g of sulfur Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc.

Practice — Calculate the moles of sulfur in 57. 8 g of sulfur Given: Find: 57. 8 g S mol S Conceptual Plan: g. S mol S Relationships: 1 mol S = 32. 07 g Solution: Check: because the given amount is much less than 1 mol S, the number makes sense Tro: Chemistry: A Molecular Approach, 2/e 58 Copyright 2011 Pearson Education, Inc.

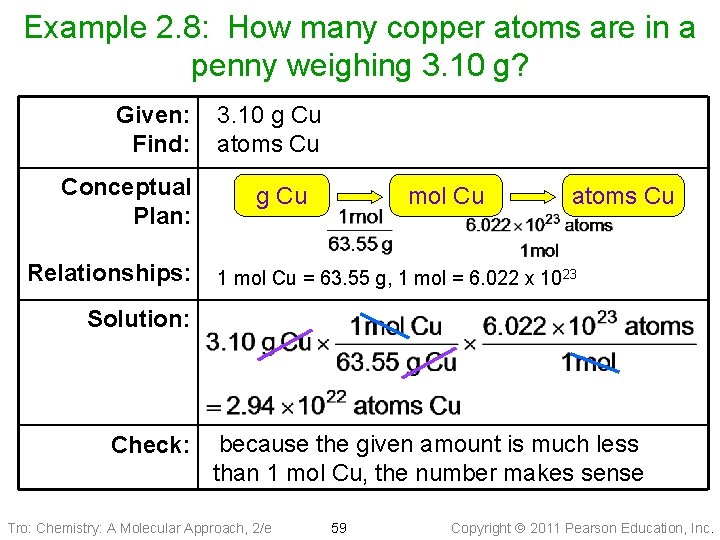
Example 2. 8: How many copper atoms are in a penny weighing 3. 10 g? Given: Find: Conceptual Plan: Relationships: 3. 10 g Cu atoms Cu g Cu mol Cu atoms Cu 1 mol Cu = 63. 55 g, 1 mol = 6. 022 x 1023 Solution: Check: because the given amount is much less than 1 mol Cu, the number makes sense Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc.

Practice — How many aluminum atoms are in a can weighing 16. 2 g? Tro: Chemistry: A Molecular Approach, 2/e 60 Copyright 2011 Pearson Education, Inc.

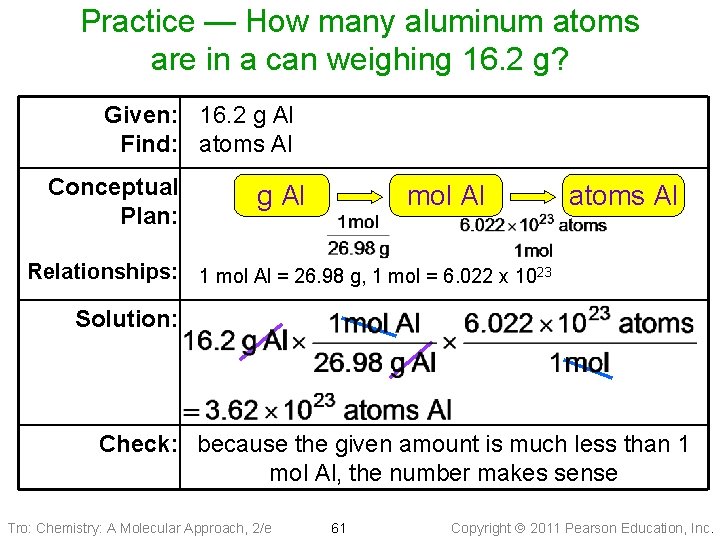
Practice — How many aluminum atoms are in a can weighing 16. 2 g? Given: 16. 2 g Al Find: atoms Al Conceptual Plan: g Al mol Al atoms Al Relationships: 1 mol Al = 26. 98 g, 1 mol = 6. 022 x 1023 Solution: Check: because the given amount is much less than 1 mol Al, the number makes sense Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc.
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Covalent bond melting point
Covalent bond melting point Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry molecular geometry
Ap chemistry molecular geometry Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Is chalk natural or manmade
Is chalk natural or manmade Datagram approach and virtual circuit approach
Datagram approach and virtual circuit approach Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Waterfall market entry strategy
Waterfall market entry strategy Multiple conflict
Multiple conflict Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach What is research approach definition
What is research approach definition Traditional approach of development
Traditional approach of development Tony wagner's seven survival skills
Tony wagner's seven survival skills Vsepr theory is a model for predicting:
Vsepr theory is a model for predicting: Molecular geometry
Molecular geometry Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Molecular biology crash course
Molecular biology crash course Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Sf5cl lewis structure
Sf5cl lewis structure Ideal gas law examples
Ideal gas law examples Covalent molecular and covalent network
Covalent molecular and covalent network Kinetic molecular theory of solids
Kinetic molecular theory of solids What is the significance of a chemical formula?
What is the significance of a chemical formula? Clasificacion molecular cancer de endometrio
Clasificacion molecular cancer de endometrio Molecular geometry chart
Molecular geometry chart Restriction enzymes
Restriction enzymes Molecular rebar
Molecular rebar Covalent molecular substances
Covalent molecular substances Empirical formula vs molecular formula
Empirical formula vs molecular formula Average molecular weight
Average molecular weight Dicots
Dicots Empirical formula to percent composition
Empirical formula to percent composition Empirical formula
Empirical formula Water percentage composition
Water percentage composition How to find empirical formula from percentages
How to find empirical formula from percentages Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Shortened structural formula
Shortened structural formula Naming and writing formulas for molecular compounds
Naming and writing formulas for molecular compounds Binary molecular
Binary molecular N srinivasan mbu iisc
N srinivasan mbu iisc Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Ch2o molecular geometry
Ch2o molecular geometry Molecular shapes quiz
Molecular shapes quiz Molecular shapes and polarity
Molecular shapes and polarity Patterson function
Patterson function Patterson
Patterson Bh3 molecular orbital diagram
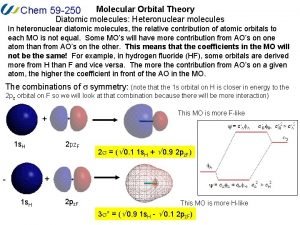
Bh3 molecular orbital diagram Heteronuclear molecular orbital diagram
Heteronuclear molecular orbital diagram B2 molecular orbital diagram
B2 molecular orbital diagram Cml treatment milestones
Cml treatment milestones Hydrogen monochloride
Hydrogen monochloride Define microbiology
Define microbiology Axe vsepr
Axe vsepr