COVALENT COMPOUNDS ACIDS MOLECULAR GEOMETRY INTERMOLECULAR FORCES Why


















- Slides: 65

COVALENT COMPOUNDS – ACIDS – MOLECULAR GEOMETRY – INTERMOLECULAR FORCES

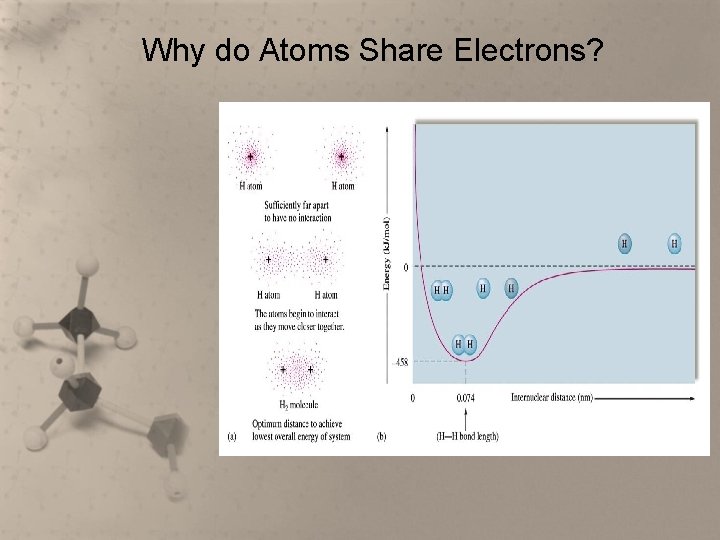
Why do Atoms Share Electrons? • In the last unit we learned that some metals and nonmetals react to form binary ionic compounds. • Electrons are transferred and the resulting ions have noble gas electron configurations. • Compounds are then formed because the ions are attracted to one another.

Why do Atoms Share Electrons? • Sometimes two atoms that both need to gain valence electrons have a similar attraction for the electrons. • Sharing electrons is one way these atoms can acquire the electron configuration of a noble gas, even though it will be on a parttime basis.

Why do Atoms Share Electrons? • In a covalent bond, atoms do not lose or gain electrons. Instead they share pairs of electrons to achieve stability by filling their outer energy levels so they can have a stable octet. • A molecule is formed when two or more atoms bond covalently. They are often called molecular compounds. • They are also called covalent compounds.

Why do Atoms Share Electrons?

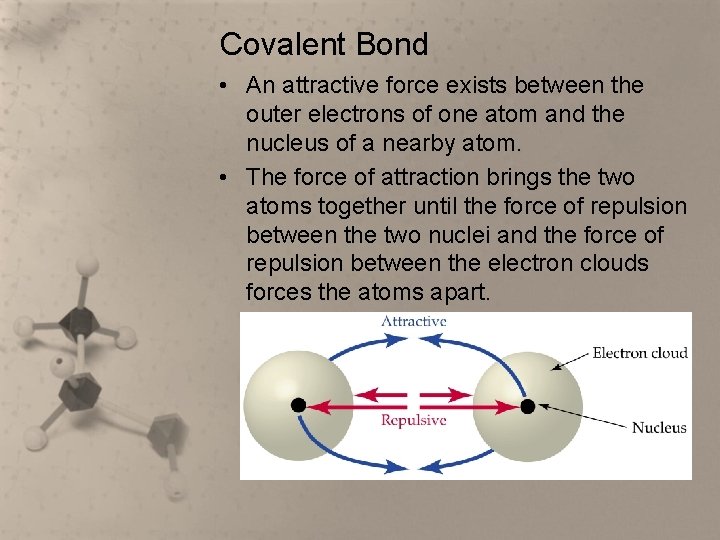
Covalent Bond • An attractive force exists between the outer electrons of one atom and the nucleus of a nearby atom. • The force of attraction brings the two atoms together until the force of repulsion between the two nuclei and the force of repulsion between the electron clouds forces the atoms apart.

Covalent Bond • If the forces of attraction are greater than the forces of repulsion, then a covalent bond forms between the atoms. • Besides the comparative strengths of the attractive and repulsive forces, another reason the attractive forces can be stronger is that a pair of electrons shared between atoms in a stable covalent bond have opposite spins and occupy less space than a pair of electrons in an orbital of only one atom.

Covalent Bond • The bond is not rigid. It is much like a spring where the atoms vibrate back and forth at some average distance where the attractive force and the repulsive force are balanced.

Covalent Bond – Sharing More Than Two Electrons • Covalent bonds between atoms can involve sharing more than two electrons. • When a single pair of electrons (2 electrons) is shared, this is known as a: • single bond • When two pairs of electrons (4 electrons) are shared, this is known as a: • double bond • When three pairs of electrons (6 electrons) are shared, this is known as a: • triple bond

Bond Length and Bond Energy • The average distance that separates the atoms in a bond is known as the bond length. • Bond lengths are never really fixed distances because the atoms vibrate. They can also vary depending on the other bonds present in a molecule. • Bond energy is the energy required to break a chemical bond to produce individual atoms, each keeping its own electrons.

Bond Length and Bond Energy • Bond length and bond energy are inversely related. • A short bond length requires higher bond energy to break it while a long bond length requires less energy to break it.

Bond Properties • Few chemical bonds are either totally molecular or totally ionic. • The bonds in most compounds have characteristics of both. • The electrons in a bond are not necessarily shared equally. To determine whether this uneven sharing will be very small or very large, one compares the ability of each atom to pull electrons toward itself.

Bond Properties • This property is called electronegativity. • The electronegativity table is used to provide numbers for comparison. • The greater the difference in electronegativity values between two atoms, the more unequal the sharing and the more ionic character the bond will have.

Bond Properties • A covalent bond formed between two atoms with equally shared bonding electrons is said to be a: • nonpolar covalent bond • Examples are: H – H, O – O, F – F • When atoms of different elements bond, the sharing of electrons can never be truly equal.

Bond Properties • A covalent bond formed between two atoms in which the bonding electrons are more strongly attracted to one atom over the other is said to be a: • polar covalent bond • Examples are: Rb – O, Al – N, C – O

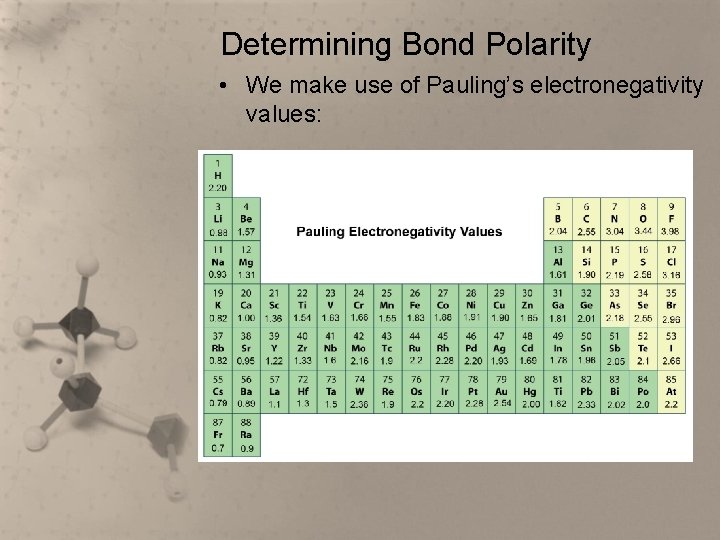
Determining Bond Polarity • We make use of Pauling’s electronegativity values:

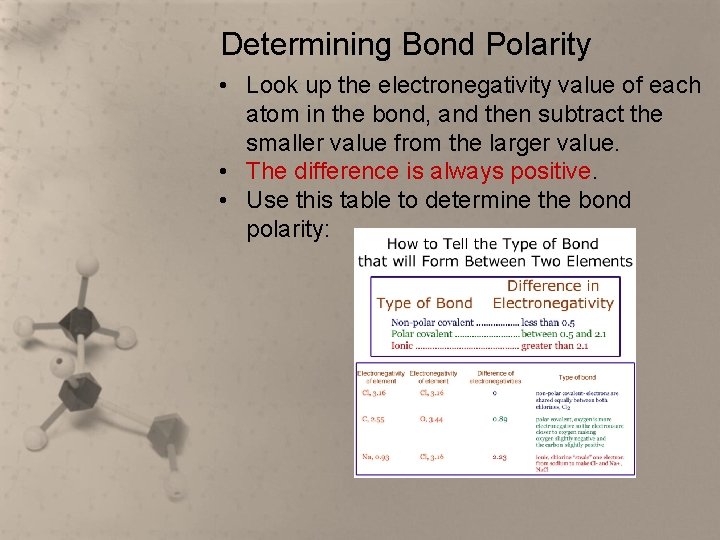
Determining Bond Polarity • Look up the electronegativity value of each atom in the bond, and then subtract the smaller value from the larger value. • The difference is always positive. • Use this table to determine the bond polarity:

Determining Bond Polarity • The uneven sharing causes the more electronegative atom to have a partial negative charge while the less electronegative atom will have a partial positive charge. • EXAMPLE: Determine the bond polarity of the following bonds: C–H 2. 55 – 2. 20 =. 35 nonpolar N–H 3. 04 – 2. 20 =. 84 polar C–O 3. 44 – 2. 55 =. 89 polar

Naming Covalent Compounds • Naming covalent compounds is similar to naming ionic compounds. • One can use either the Stock naming system or one that makes use of prefixes, roots, and suffixes. • The latter is known simply as the prefix naming system.

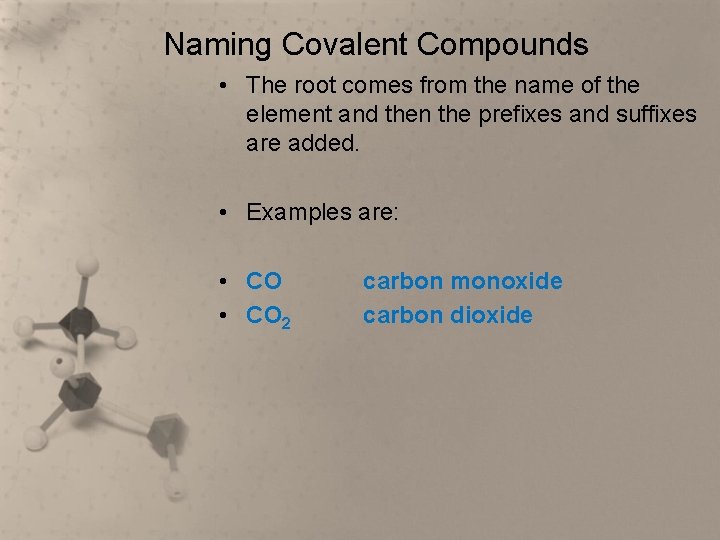
Naming Covalent Compounds • The root comes from the name of the element and then the prefixes and suffixes are added. • Examples are: • CO 2 carbon monoxide carbon dioxide

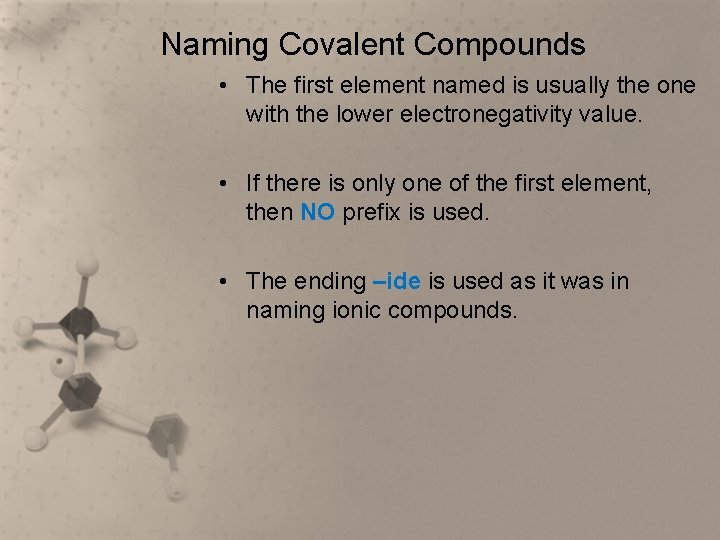
Naming Covalent Compounds • The first element named is usually the one with the lower electronegativity value. • If there is only one of the first element, then NO prefix is used. • The ending –ide is used as it was in naming ionic compounds.

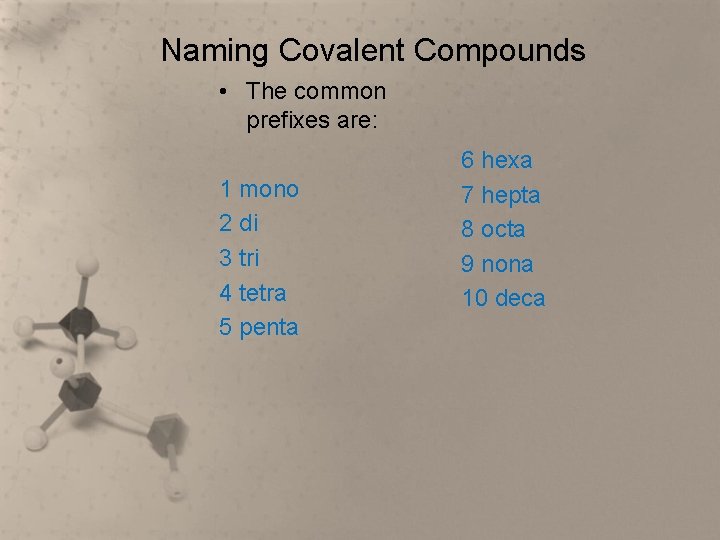
Naming Covalent Compounds • The common prefixes are: 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca

Naming Covalent Compounds • Name the following covalent compounds: P 2 O 5 diphosphorus pentoxide CCl 4 carbon tetrachloride As 2 O 3 diarsenic trioxide SO 2 sulfur dioxide NF 3 nitrogen trifluoride

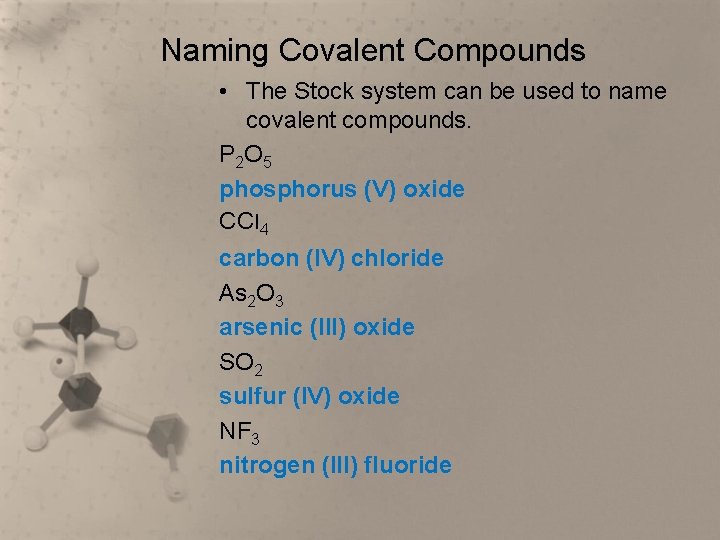
Naming Covalent Compounds • The Stock system can be used to name covalent compounds. P 2 O 5 phosphorus (V) oxide CCl 4 carbon (IV) chloride As 2 O 3 arsenic (III) oxide SO 2 sulfur (IV) oxide NF 3 nitrogen (III) fluoride

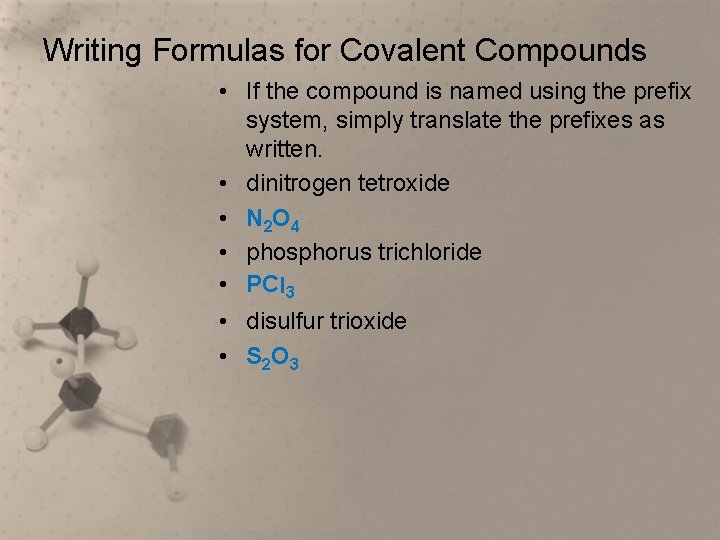
Writing Formulas for Covalent Compounds • If the compound is named using the prefix system, simply translate the prefixes as written. • dinitrogen tetroxide • N 2 O 4 • phosphorus trichloride • PCl 3 • disulfur trioxide • S 2 O 3

Naming Acids • Water solutions of some molecules are acidic and are therefore named as acids. • A binary acid contains: • hydrogen • an anion • but, NO OXYGEN

Naming Acids – Binary Acids • When naming a binary acid, use the prefix hydro to name the hydrogen part of the compound. • The rest of the name consists of a form of the root of the second element, or polyatomic ion, plus the suffix –ic. • Then add the word acid. • HBr in a water solution (aqueous) is known as: hydrobromic acid.

Naming Acids - Examples • • • Name the following acids: HF hydrofluoric acid HCN hydrocyanic acid HI hydroiodic acid H 2 S hydrosulfuric acid

Naming Acids – Oxyacids • Any acid that contains hydrogen and an oxyanion is known as an oxyacid. • To name it, first identify the anion present. • The name of the oxyacid consists of the root of the anion, a suffix, and the word acid.

Naming Acids – Oxyacids • If the anion suffix is ATE, change it to IC. • If the anion suffix is ITE, change it to OUS. • ATE – IC, ITE – OUS

Naming Acids – Oxyacids - Examples • • • Name the following oxyacids: HNO 2 nitrous acid HCl. O 3 chloric acid HCl. O 4 perchloric acid HC 2 H 3 O 2 acetic acid • Notice there is NO use of the hydro.

Naming Acids – Oxyacids - Exceptions • The following oxyacids were named before the rules went into effect, so they must be memorized: • H 2 SO 3 • sulfurous acid • H 2 SO 4 • sulfuric acid • H 3 PO 4 • phosphoric acid

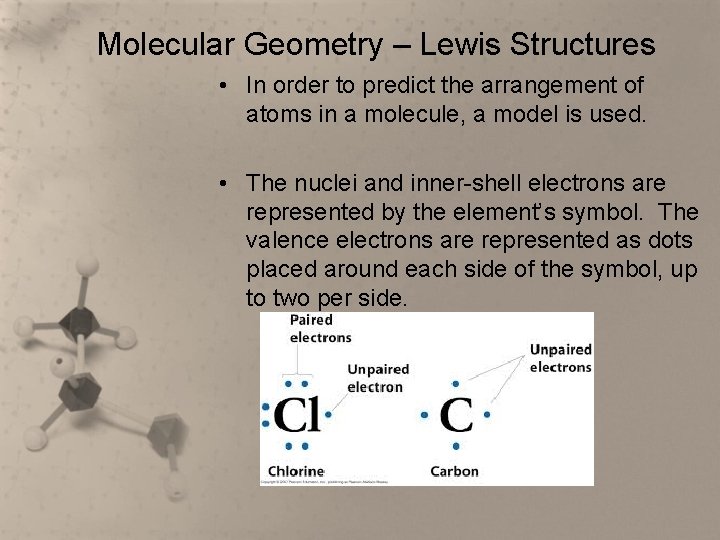
Molecular Geometry – Lewis Structures • In order to predict the arrangement of atoms in a molecule, a model is used. • The nuclei and inner-shell electrons are represented by the element’s symbol. The valence electrons are represented as dots placed around each side of the symbol, up to two per side.

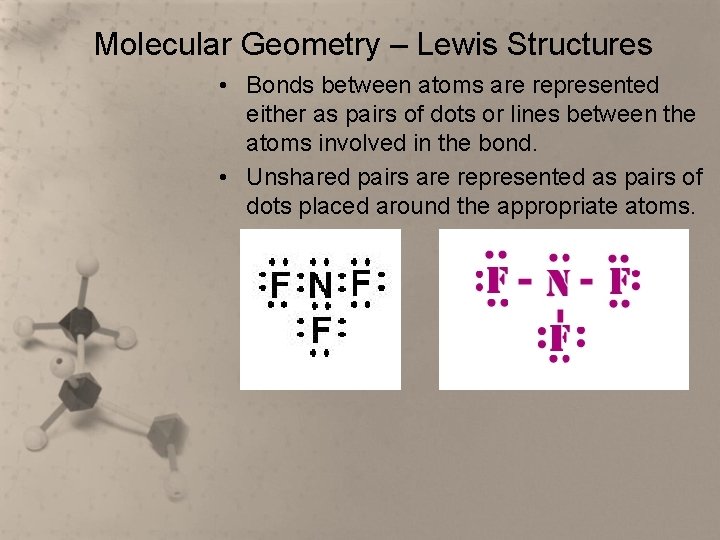
Molecular Geometry – Lewis Structures • Bonds between atoms are represented either as pairs of dots or lines between the atoms involved in the bond. • Unshared pairs are represented as pairs of dots placed around the appropriate atoms.

Molecular Geometry – Drawing Lewis Structures (a) determine the total number of valence electrons in the compound by adding up all the valence electrons of the atoms in the compound. (b) arrange all the element symbols according to which element can form more than one bond and those that can only form one bond

Molecular Geometry – Drawing Lewis Structures • Atoms that can form only one bond are: H and F. Cl, Br, and I will normally form only one bond unless outnumbered by O or F. • Atoms that “love” being in the middle of things are: B, C, N, O, Si, P, S, As, Se, Sb. (c) draw single lines between all the atoms that are bonded together.

Molecular Geometry – Drawing Lewis Structures (d) count each line, multiply by two, and subtract that number from the total number of valence electrons. (e) this gives you the electrons left to distribute to all the elements still needing electrons so each can have an octet. (f) if there are not enough electrons available to distribute, then one or more of the single bonds may have to be made double or triple bonds.

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for iodomethane (CH 3 I) C = 4 e 3 H = 3 e. I = 7 eval e- = 14 bond e- = unshared e- = used e- = remaining e- =

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for: methanol (CH 3 OH)

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for: dinitrogen difluoride (N 2 F 2)

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for: formaldehyde (H 2 CO)

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for the hydroxide ion (OH-)

Molecular Geometry – Drawing Lewis Structures - Examples • Draw the Lewis structure for the ammonium ion (NH 4+)

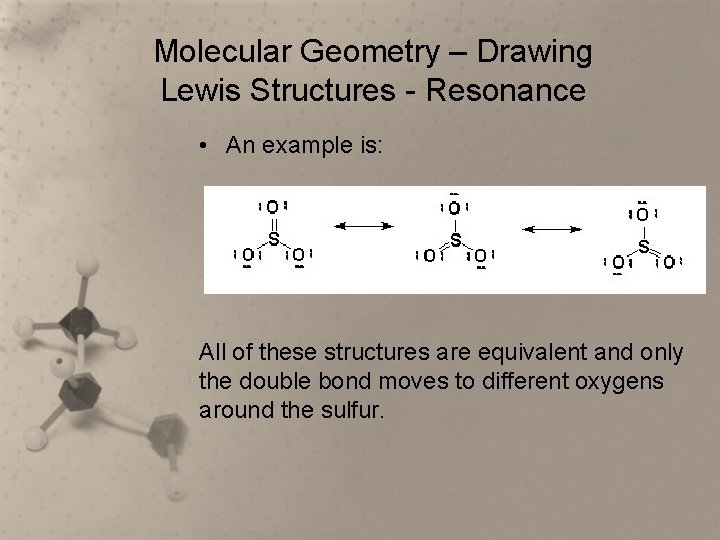
Molecular Geometry – Drawing Lewis Structures - Resonance • Sometimes a single Lewis structure is not enough to accurately depict a molecule. • When that happens, more than one equivalent structure is used to represent the molecule. • When more than one Lewis structure can be drawn for a molecule, the molecule is said to be a resonance hybrid.

Molecular Geometry – Drawing Lewis Structures - Resonance • An example is: All of these structures are equivalent and only the double bond moves to different oxygens around the sulfur.

Molecular Geometry – Drawing Lewis Structures - Resonance • Draw the three resonance structures for: dinitrogen monoxide (N 2 O).

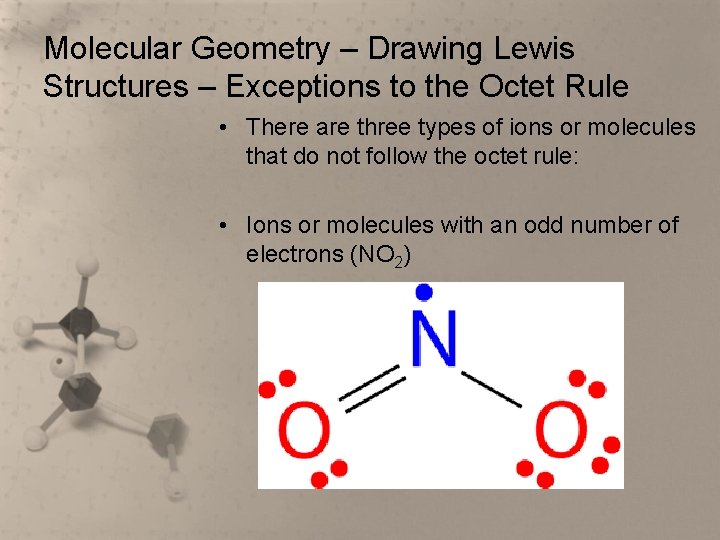
Molecular Geometry – Drawing Lewis Structures – Exceptions to the Octet Rule • There are three types of ions or molecules that do not follow the octet rule: • Ions or molecules with an odd number of electrons (NO 2)

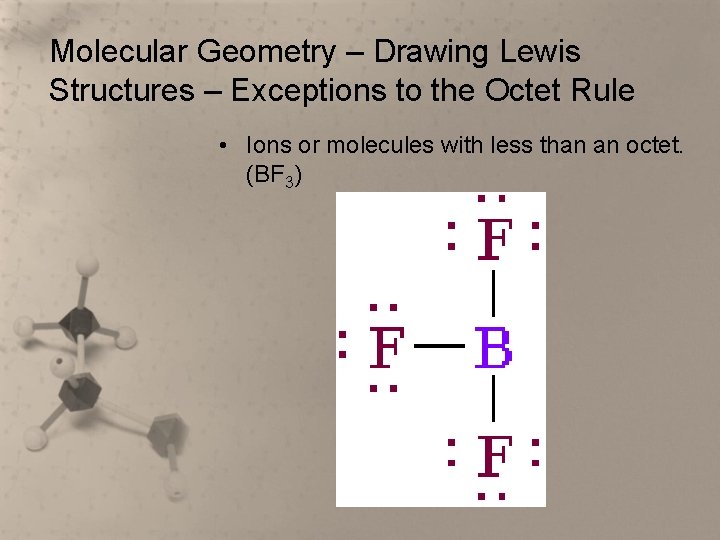
Molecular Geometry – Drawing Lewis Structures – Exceptions to the Octet Rule • Ions or molecules with less than an octet. (BF 3)

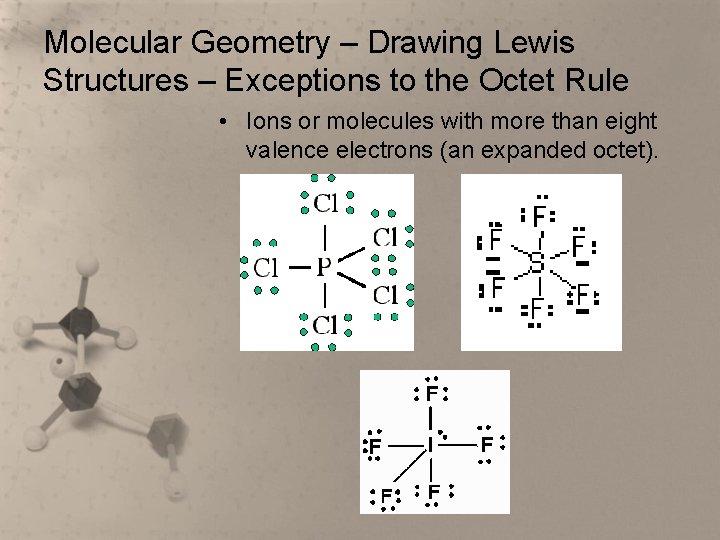
Molecular Geometry – Drawing Lewis Structures – Exceptions to the Octet Rule • Ions or molecules with more than eight valence electrons (an expanded octet).

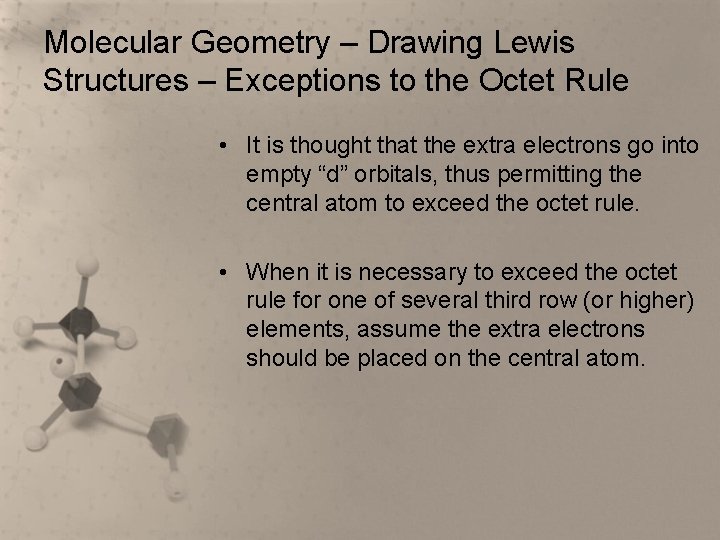
Molecular Geometry – Drawing Lewis Structures – Exceptions to the Octet Rule • It is thought that the extra electrons go into empty “d” orbitals, thus permitting the central atom to exceed the octet rule. • When it is necessary to exceed the octet rule for one of several third row (or higher) elements, assume the extra electrons should be placed on the central atom.

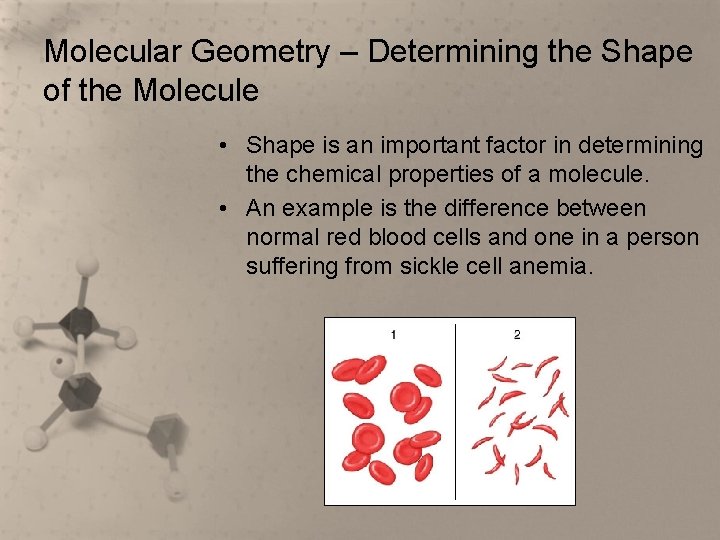
Molecular Geometry – Determining the Shape of the Molecule • Shape is an important factor in determining the chemical properties of a molecule. • An example is the difference between normal red blood cells and one in a person suffering from sickle cell anemia.

Molecular Geometry – Valence Shell Electron Pair Repulsion Theory • The theory used to predict shapes is called the Valence Shell Electron Pair Repulsion Theory or VSEPR. • It is based on the idea that electron pairs surrounding the central atom in a bond will arrange themselves to be as far apart as possible.

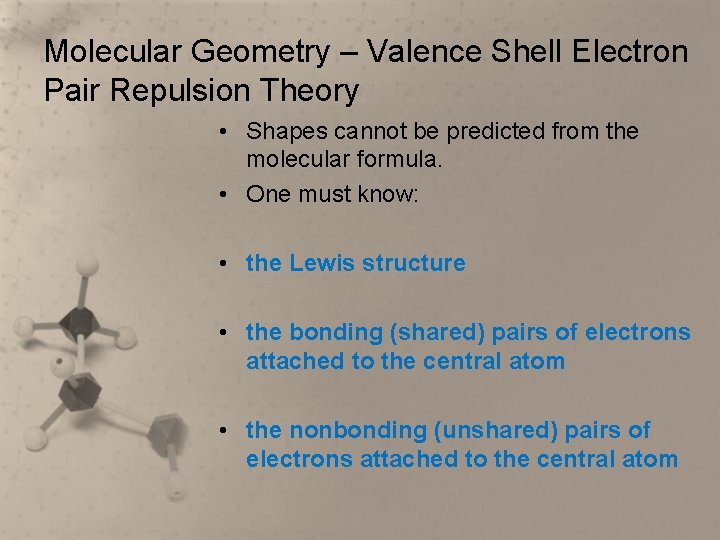
Molecular Geometry – Valence Shell Electron Pair Repulsion Theory • Shapes cannot be predicted from the molecular formula. • One must know: • the Lewis structure • the bonding (shared) pairs of electrons attached to the central atom • the nonbonding (unshared) pairs of electrons attached to the central atom

Molecular Geometry – Valence Shell Electron Pair Repulsion Theory • Once the bonding pairs and nonbonding pairs attached to the central atom have been determined, use this chart to determine the molecular geometry.

Molecular Geometry – Valence Shell Electron Pair Repulsion Theory

Molecular Geometry – Valence Shell Electron Pair Repulsion Theory - Example • Determine the shape of ammonia (NH 3) (1) draw the Lewis structure (2) number of shared (bonding) pairs = (3) number of unshared (nonbonding) pairs = (4) shape:

Molecular Geometry – Valence Shell Electron Pair Repulsion Theory – More Examples • Determine the shape of each of the following molecules: • water (H 2 O) • dichlorodifluoromethane (CF 2 Cl 2) • arsenic pentafluoride (As. F 5) • selenium hexabromide (Se. Br 6)

Molecular Dipole • Remember that each bond within a molecule can be either nonpolar or polar. • If the bond is polar, then one end of the bond appears to have a slight positive charge while the other appears to have a slight negative charge. • This creates a dipole.

Molecular Dipole • If the molecule has polar bonds and the shape of the molecule causes the polarities to cancel, then the molecule is nonpolar. • If the molecule has polar bonds and the shape of the molecule does not cause the polarities to cancel, then the molecule will be polar. • If the molecule has all nonpolar bonds, then no matter what the shape is, the molecule will be nonpolar.

Molecular Dipole • Determine the polarity of each of the following molecules: • ammonia (NH 3) • water (H 2 O) • dichlorodifluoromethane (CF 2 Cl 2) • arsenic pentafluoride (As. F 5) • selenium hexabromide (Se. Br 6)

Intermolecular Forces • Atoms and molecules are attracted to one another. But, these forces of attraction are not as strong as the forces of attraction between atoms in the bonding process (ionic and covalent bonds). • Intermolecular forces are forces that cause attractions between molecules. • Intermolecular forces of attraction between molecules, or between atoms and molecules, do not involve the transferring or sharing of electrons.

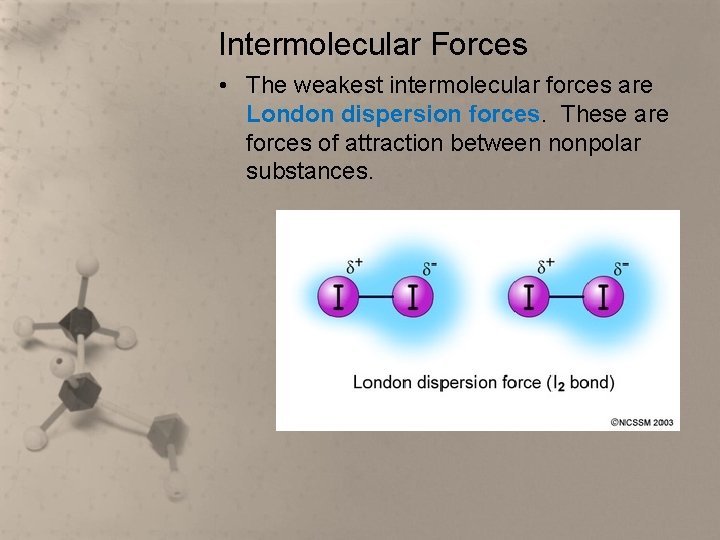
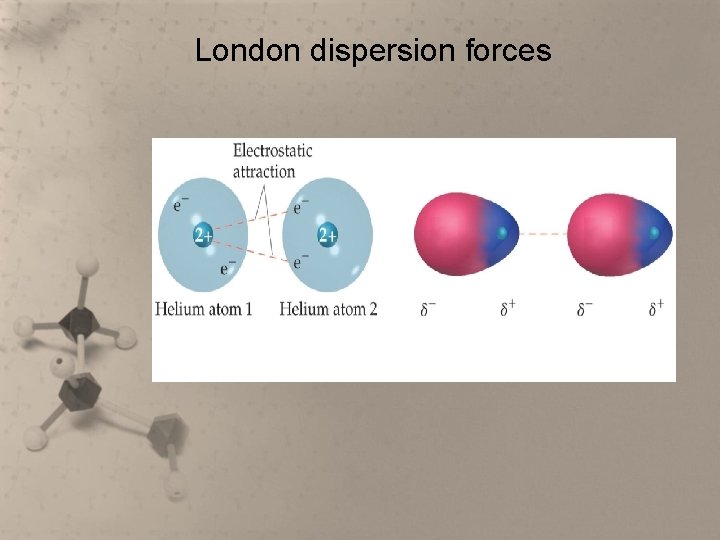
Intermolecular Forces • The weakest intermolecular forces are London dispersion forces. These are forces of attraction between nonpolar substances.

London dispersion forces

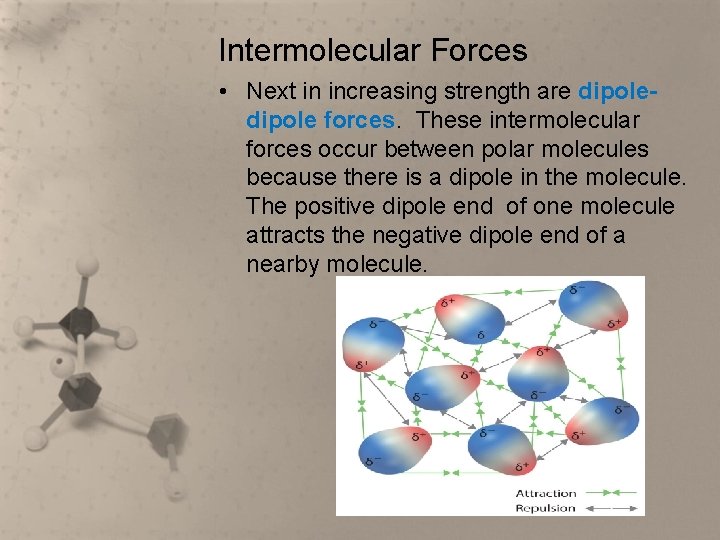
Intermolecular Forces • Next in increasing strength are dipole forces. These intermolecular forces occur between polar molecules because there is a dipole in the molecule. The positive dipole end of one molecule attracts the negative dipole end of a nearby molecule.

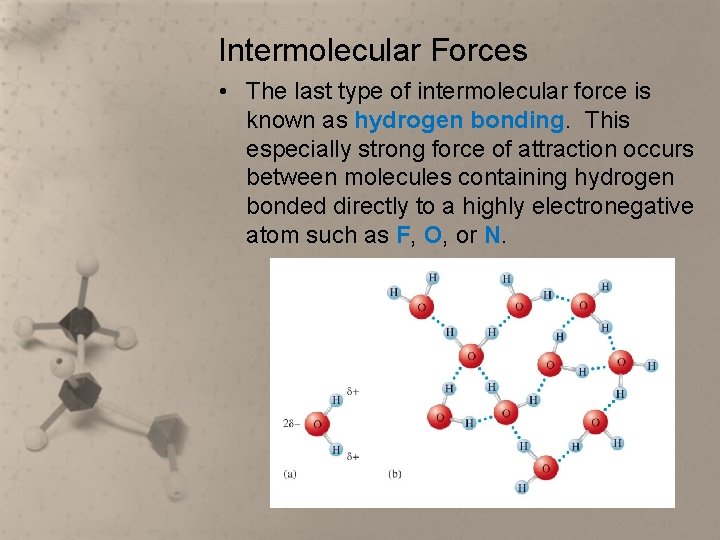
Intermolecular Forces • The last type of intermolecular force is known as hydrogen bonding. This especially strong force of attraction occurs between molecules containing hydrogen bonded directly to a highly electronegative atom such as F, O, or N.