Foundation Year Molecular mass and formula mass 2


- Slides: 28

Foundation Year Molecular mass and formula mass 2 Dr. Marwa Eid

Molecular Mass Objectives -After this Lecture the students will be able to convert between: -Moles and grams -Moles and particles

Molecular Mass “MM” Molecular Mass is conversion factor between grams and moles. Number of grams in 1 mole (n= mass/MM MM=mass/n) MM= # Protons + # Neutrons in a nucleus. = atomic mass

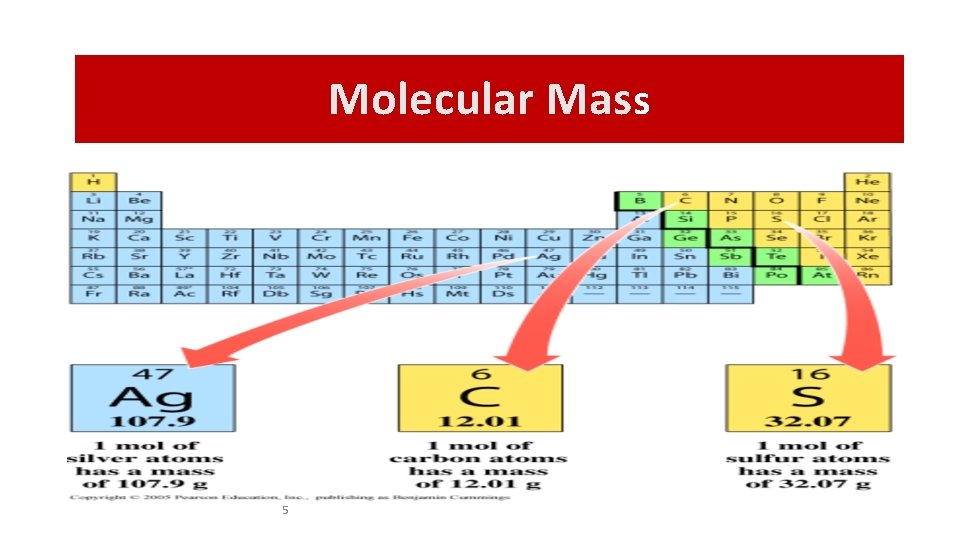
Molecular Mass 1 mole of C atoms = 12. 0 g 1 mole of Mg atoms = 24. 3 g 1 mole of Cu atoms = 63. 5 g 4

Molecular Mass 5

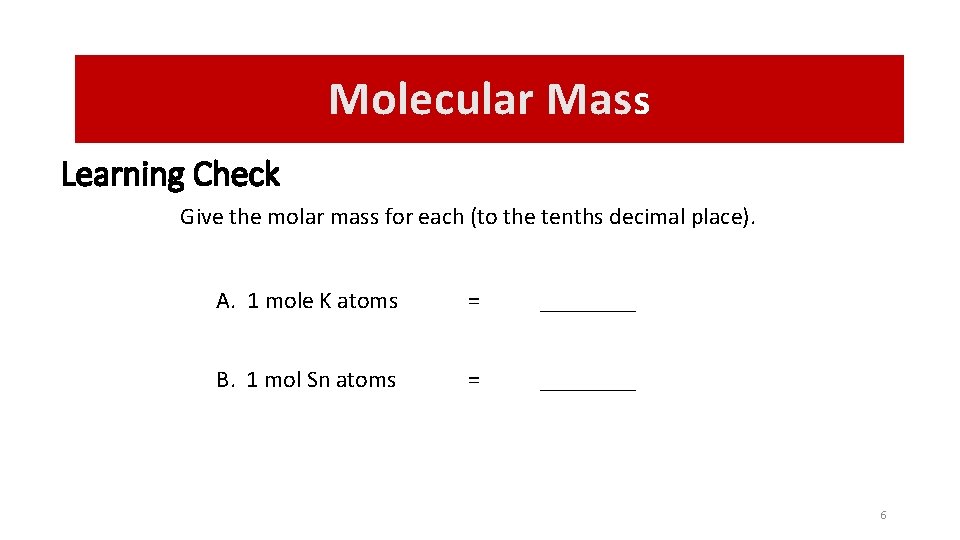
Molecular Mass Learning Check Give the molar mass for each (to the tenths decimal place). A. 1 mole K atoms = ____ B. 1 mol Sn atoms = ____ 6

7

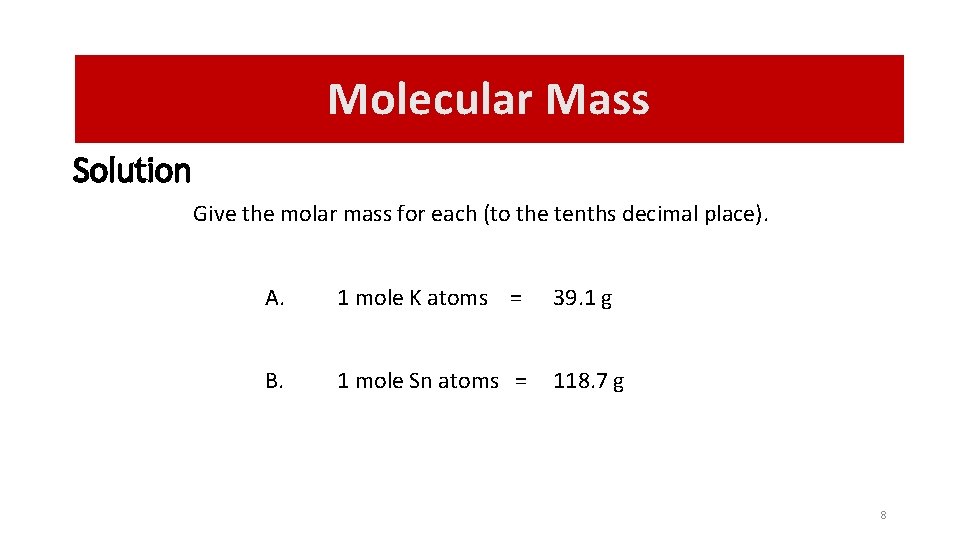
Molecular Mass Solution Give the molar mass for each (to the tenths decimal place). A. 1 mole K atoms = 39. 1 g B. 1 mole Sn atoms = 118. 7 g 8

Moles and Grams What is the mass of 3. 5 moles of sodium (Na)? I mole of Na = 22. 99 g 3. 5 mole (Na)= ? ? ? g Mass = (3. 5* 22. 99)/1 Mass = 80. 5 g 9

Moles and Grams How many moles of iron (Fe) does 25 g of Fe represents? I mole of Fe = 55. 85 g ? ? ? mole (Na)= 25 g # moles = (25*1 )/ 55. 85 # moles (n) = 0. 448 10

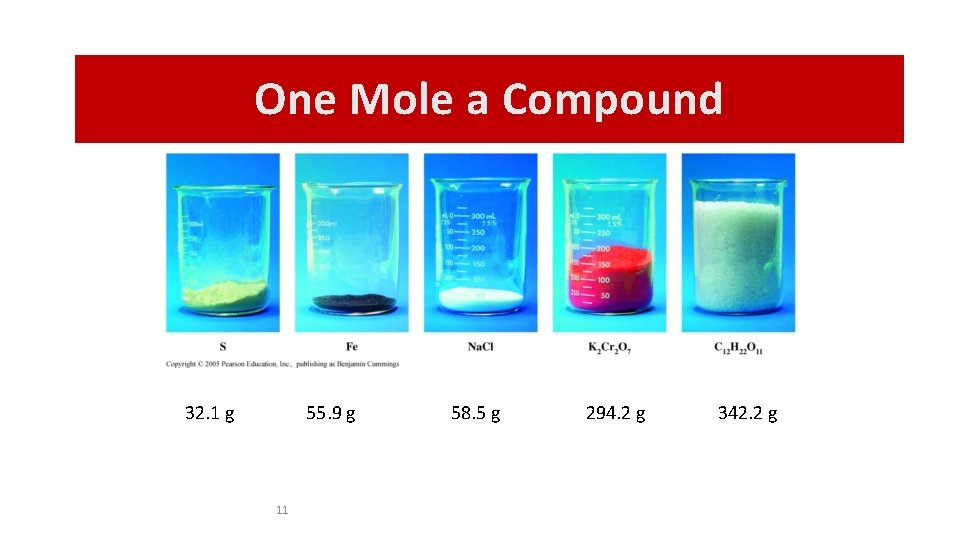
One Mole a Compound One-Mole Quantities 32. 1 g 55. 9 g 11 58. 5 g 294. 2 g 342. 2 g

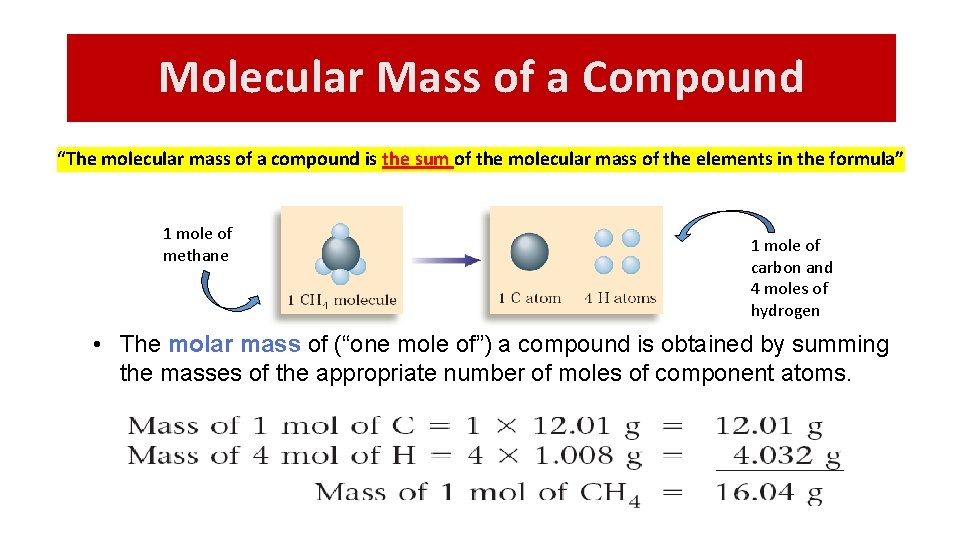
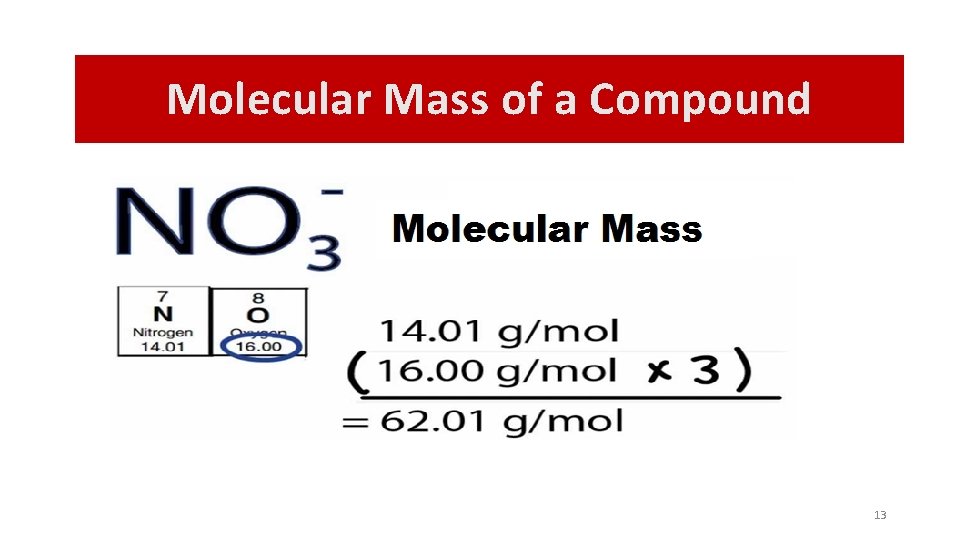
Molecular Mass of a Compound “The molecular mass of a compound is the sum of the molecular mass of the elements in the formula” 1 mole of methane 1 mole of carbon and 4 moles of hydrogen • The molar mass of (“one mole of”) a compound is obtained by summing the masses of the appropriate number of moles of component atoms.

Molecular Mass of a Compound 13

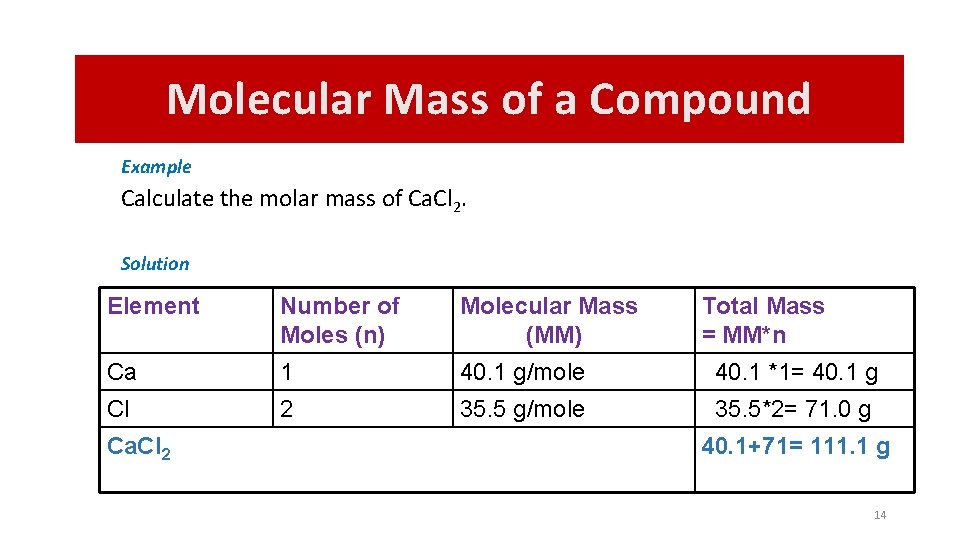
Molecular Mass of a Compound Example Calculate the molar mass of Ca. Cl 2. Solution Element Number of Moles (n) Molecular Mass (MM) Ca 1 40. 1 g/mole 40. 1 *1= 40. 1 g Cl 2 35. 5 g/mole 35. 5*2= 71. 0 g Ca. Cl 2 Total Mass = MM*n 40. 1+71= 111. 1 g 14

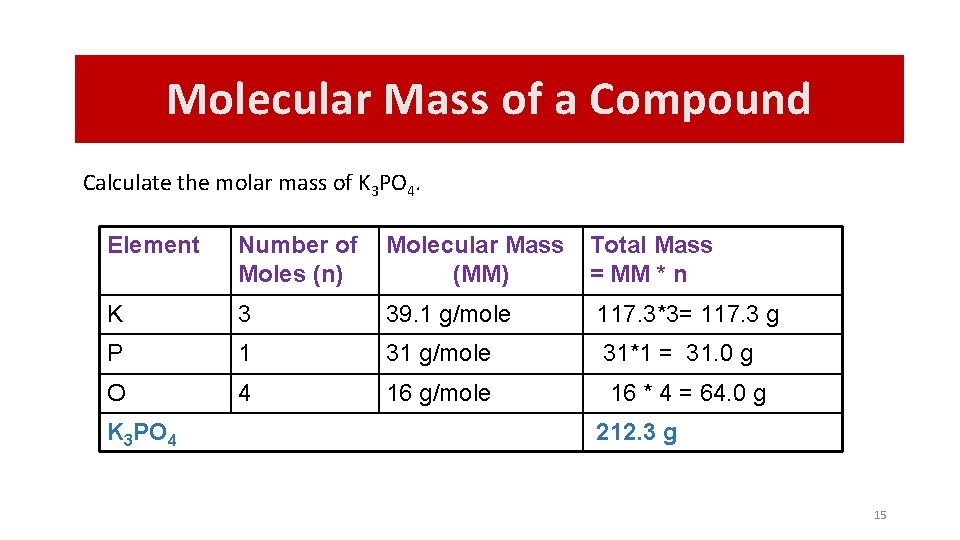
Molecular Mass of a Compound Calculate the molar mass of K 3 PO 4. Element Number of Moles (n) Molecular Mass (MM) Total Mass = MM * n K 3 39. 1 g/mole 117. 3*3= 117. 3 g P 1 31 g/mole 31*1 = 31. 0 g O 4 16 g/mole 16 * 4 = 64. 0 g K 3 PO 4 212. 3 g 15

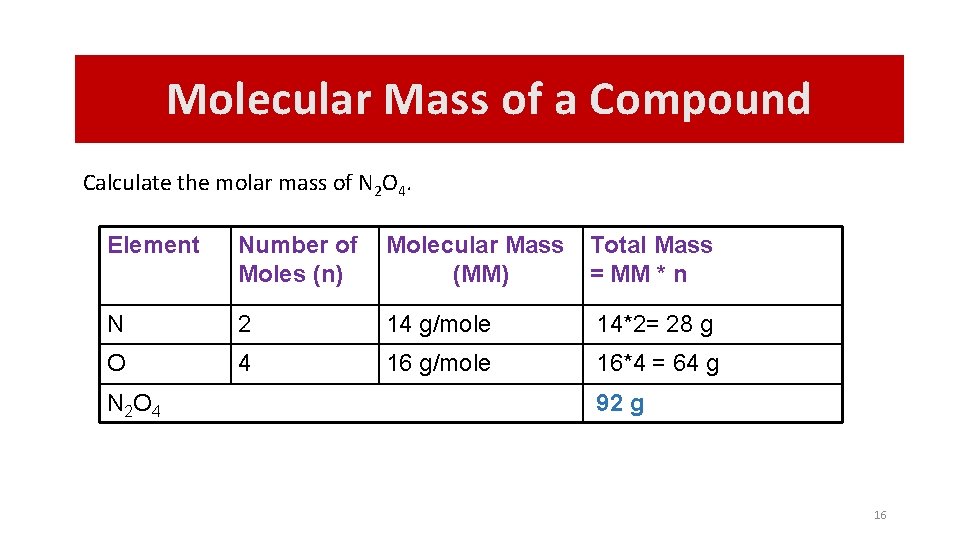
Molecular Mass of a Compound Calculate the molar mass of N 2 O 4. Element Number of Moles (n) Molecular Mass (MM) Total Mass = MM * n N 2 14 g/mole 14*2= 28 g O 4 16 g/mole 16*4 = 64 g N 2 O 4 92 g 16

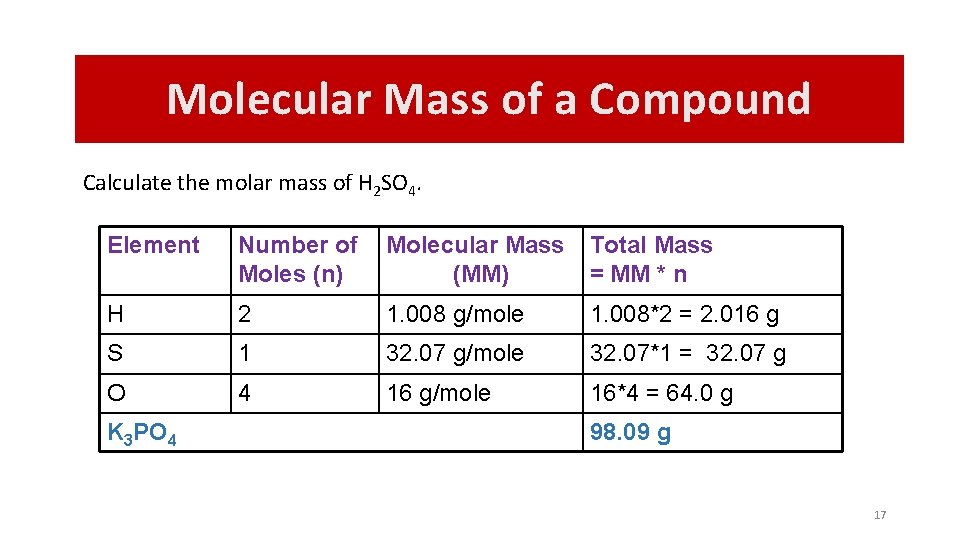
Molecular Mass of a Compound Calculate the molar mass of H 2 SO 4. Element Number of Moles (n) Molecular Mass (MM) Total Mass = MM * n H 2 1. 008 g/mole 1. 008*2 = 2. 016 g S 1 32. 07 g/mole 32. 07*1 = 32. 07 g O 4 16 g/mole 16*4 = 64. 0 g K 3 PO 4 98. 09 g 17

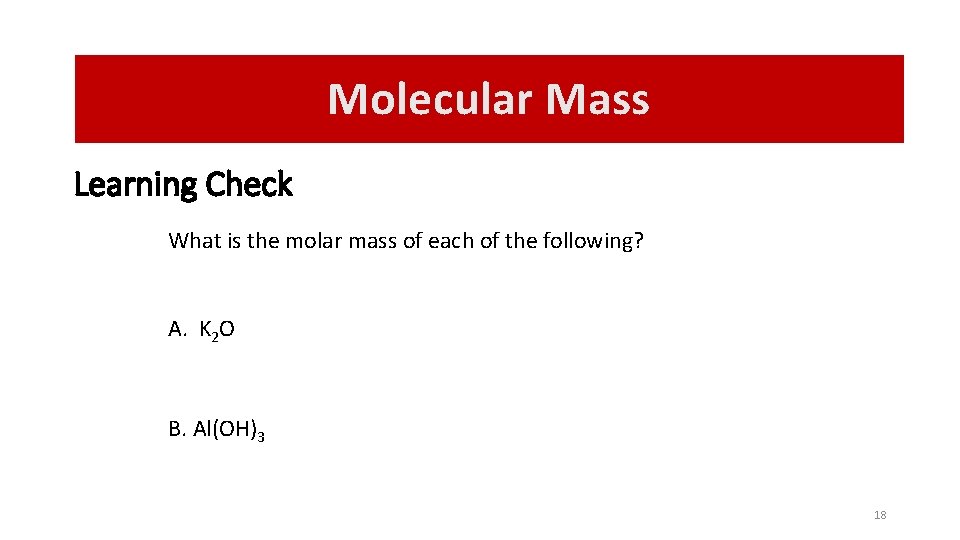
Molecular Mass Learning Check What is the molar mass of each of the following? A. K 2 O B. Al(OH)3 18

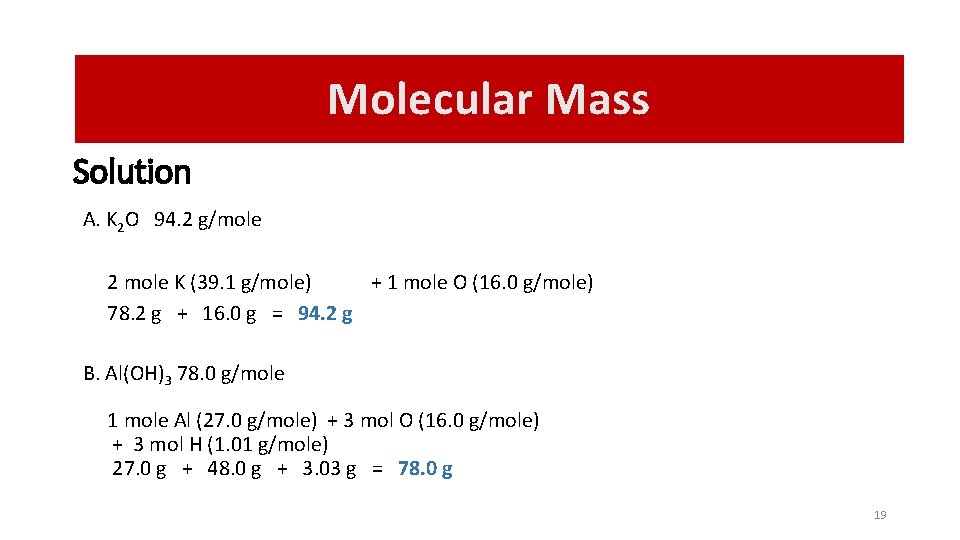
Molecular Mass Solution A. K 2 O 94. 2 g/mole 2 mole K (39. 1 g/mole) + 1 mole O (16. 0 g/mole) 78. 2 g + 16. 0 g = 94. 2 g B. Al(OH)3 78. 0 g/mole 1 mole Al (27. 0 g/mole) + 3 mol O (16. 0 g/mole) + 3 mol H (1. 01 g/mole) 27. 0 g + 48. 0 g + 3. 03 g = 78. 0 g 19

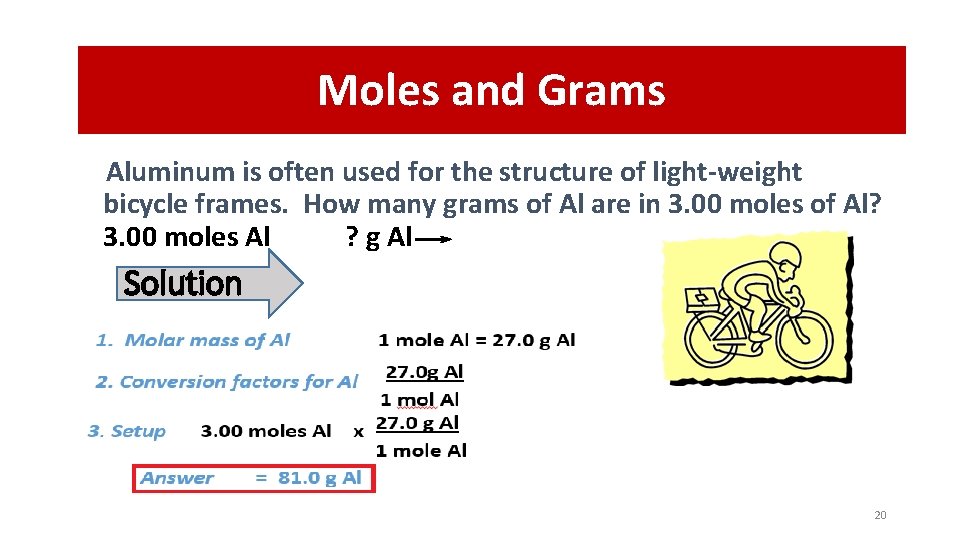
Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al ? g Al Solution 20

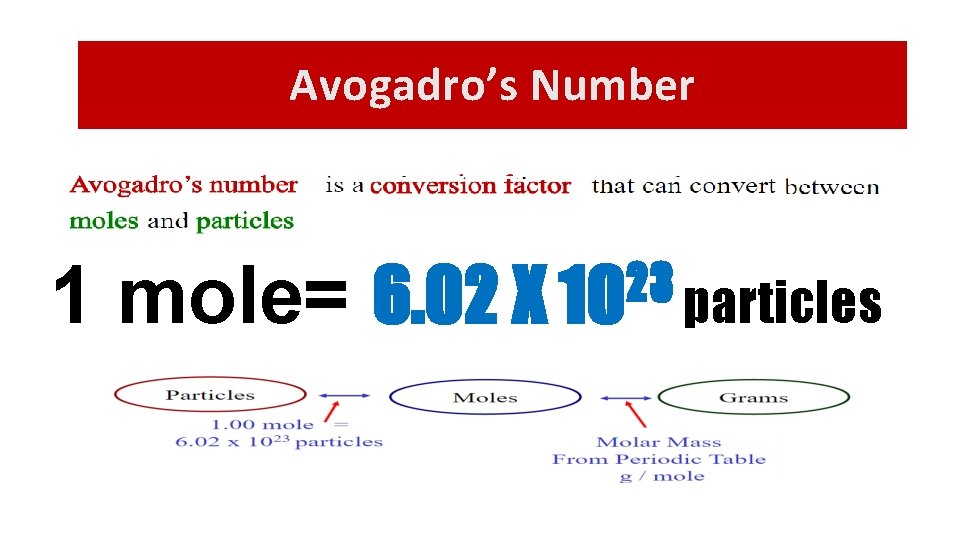
Avogadro’s Number 1 mole= 23 6. 02 X 10 particles

Moles and Particles • 1 dozen cookies = 12 cookies • 1 mole of cookies = 6. 02 X 1023 cookies • 1 dozen cars = 12 cars • 1 mole of cars = 6. 02 X 1023 cars • 1 dozen Al atoms = 12 Al atoms • 1 mole of Al atoms = 6. 02 X 1023 atoms Note that the NUMBER is always the same, but the MASS is very different! Mole is abbreviated mol (gee, that’s a lot quicker to write, huh? )

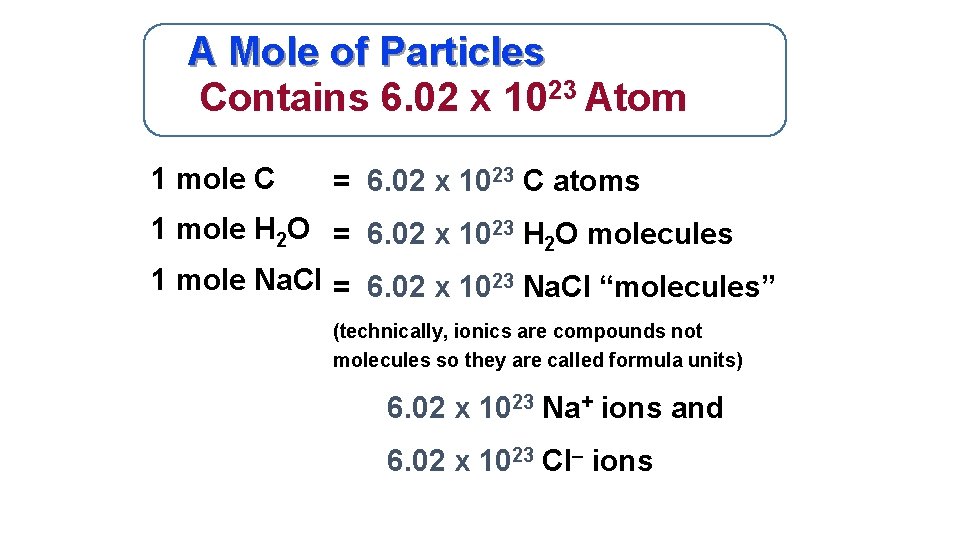
A Mole of Particles Contains 6. 02 x 1023 Atom 1 mole C = 6. 02 x 1023 C atoms 1 mole H 2 O = 6. 02 x 1023 H 2 O molecules 1 mole Na. Cl = 6. 02 x 1023 Na. Cl “molecules” (technically, ionics are compounds not molecules so they are called formula units) 6. 02 x 1023 Na+ ions and 6. 02 x 1023 Cl– ions

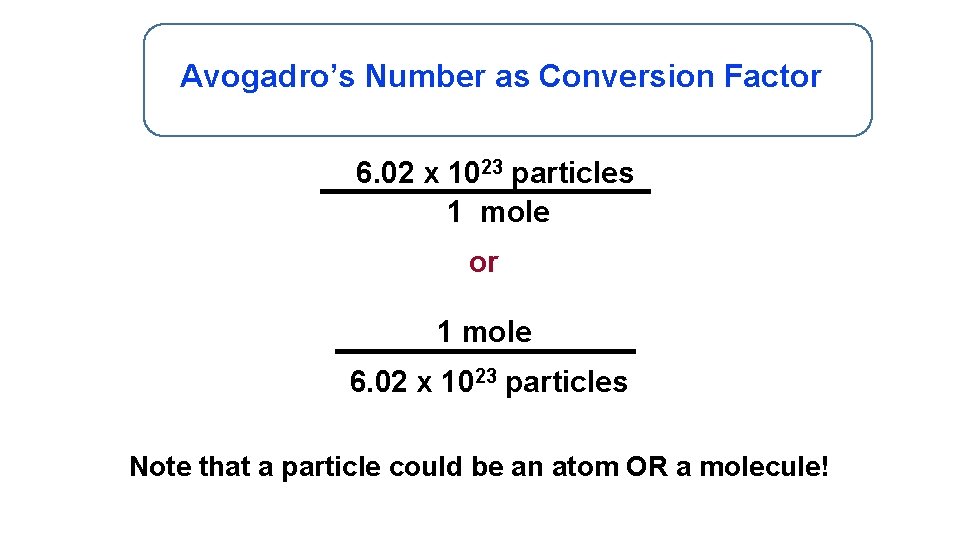
Avogadro’s Number as Conversion Factor 6. 02 x 1023 particles 1 mole or 1 mole 6. 02 x 1023 particles Note that a particle could be an atom OR a molecule!

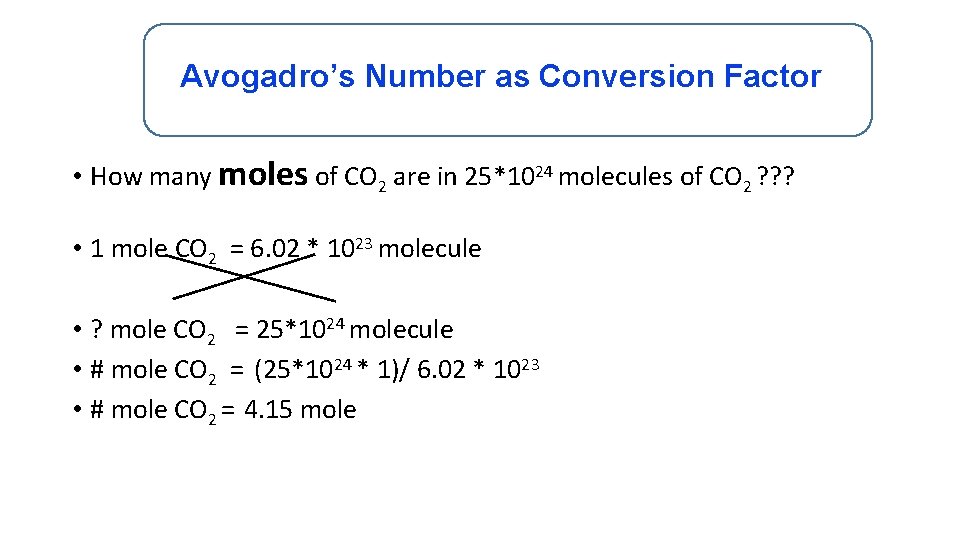
Avogadro’s Number as Conversion Factor • How many moles of CO 2 are in 25*1024 molecules of CO 2 ? ? ? • 1 mole CO 2 = 6. 02 * 1023 molecule • ? mole CO 2 = 25*1024 molecule • # mole CO 2 = (25*1024 * 1)/ 6. 02 * 1023 • # mole CO 2 = 4. 15 mole

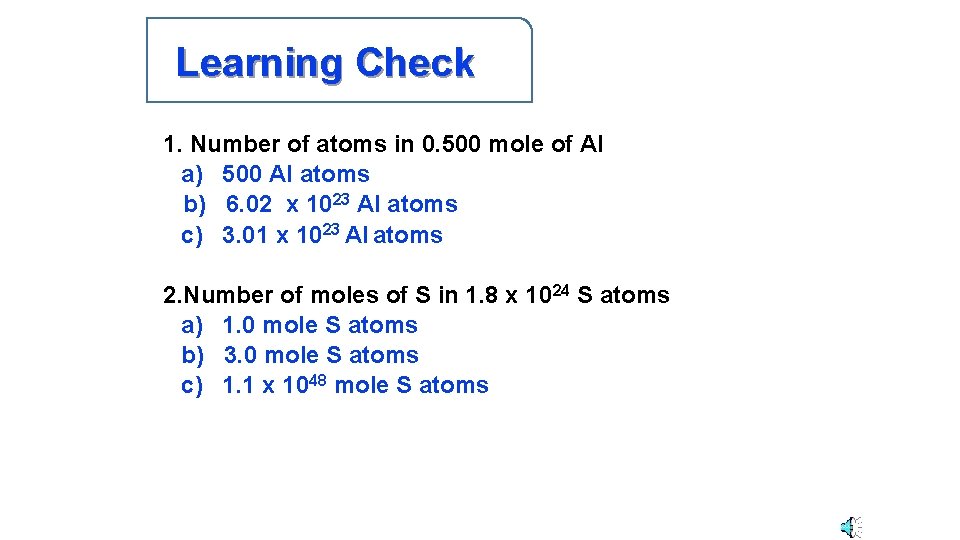
Learning Check 1. Number of atoms in 0. 500 mole of Al a) 500 Al atoms b) 6. 02 x 1023 Al atoms c) 3. 01 x 1023 Al atoms 2. Number of moles of S in 1. 8 x 1024 S atoms a) 1. 0 mole S atoms b) 3. 0 mole S atoms c) 1. 1 x 1048 mole S atoms

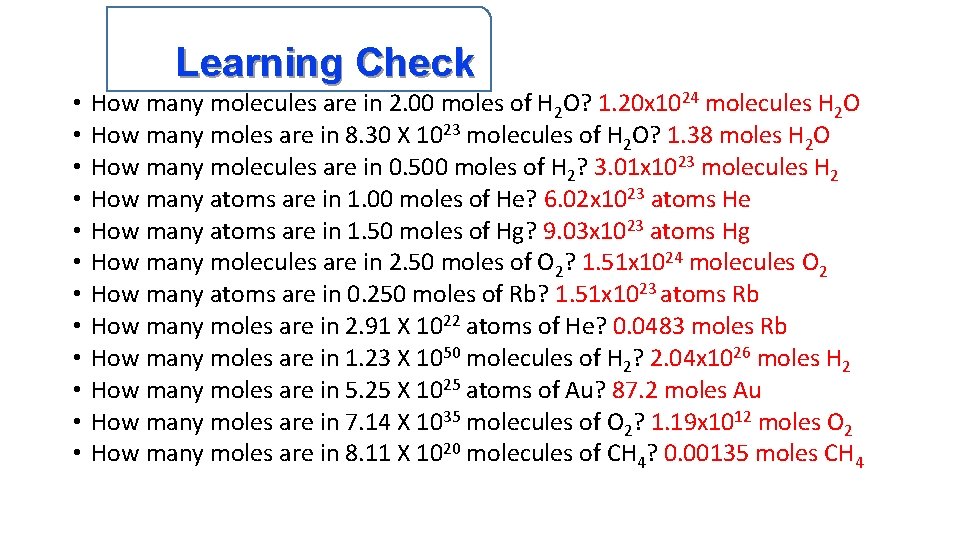
Learning Check • • • How many molecules are in 2. 00 moles of H 2 O? 1. 20 x 1024 molecules H 2 O How many moles are in 8. 30 X 1023 molecules of H 2 O? 1. 38 moles H 2 O How many molecules are in 0. 500 moles of H 2? 3. 01 x 1023 molecules H 2 How many atoms are in 1. 00 moles of He? 6. 02 x 1023 atoms He How many atoms are in 1. 50 moles of Hg? 9. 03 x 1023 atoms Hg How many molecules are in 2. 50 moles of O 2? 1. 51 x 1024 molecules O 2 How many atoms are in 0. 250 moles of Rb? 1. 51 x 1023 atoms Rb How many moles are in 2. 91 X 1022 atoms of He? 0. 0483 moles Rb How many moles are in 1. 23 X 1050 molecules of H 2? 2. 04 x 1026 moles H 2 How many moles are in 5. 25 X 1025 atoms of Au? 87. 2 moles Au How many moles are in 7. 14 X 1035 molecules of O 2? 1. 19 x 1012 moles O 2 How many moles are in 8. 11 X 1020 molecules of CH 4? 0. 00135 moles CH 4
