PERCENT COMPOSITION mass of element mass of element
- Slides: 6

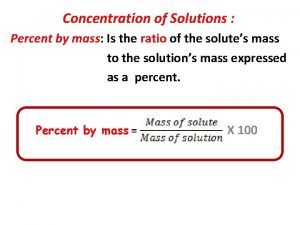
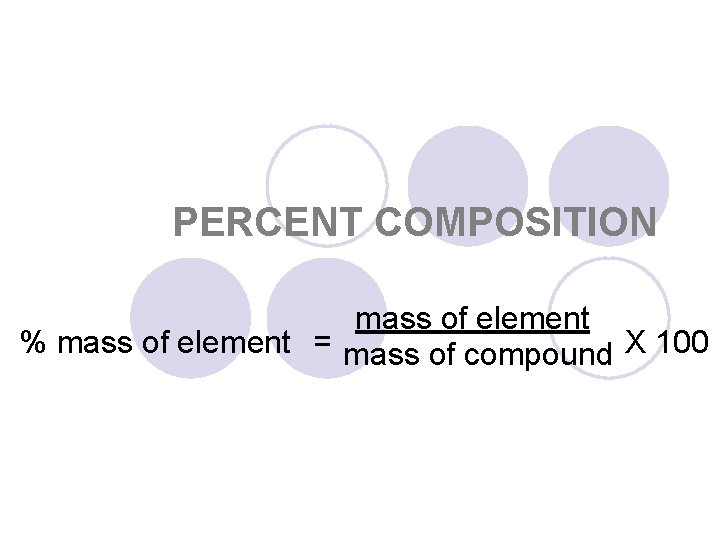
PERCENT COMPOSITION mass of element % mass of element = mass of compound X 100

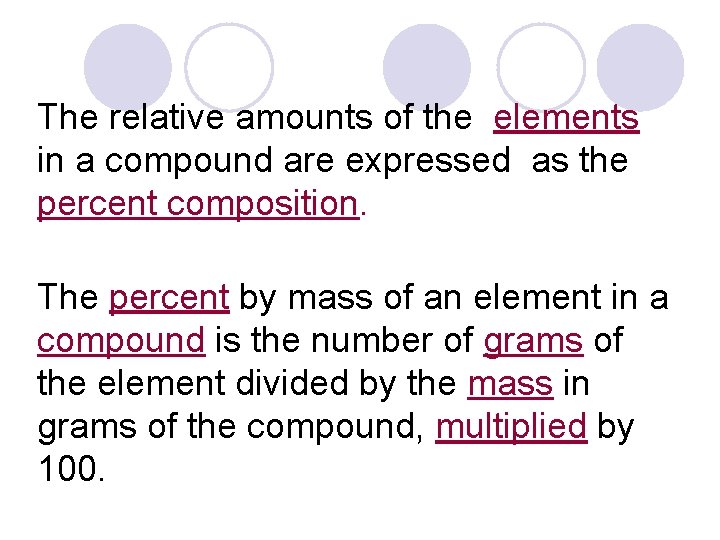
The relative amounts of the elements in a compound are expressed as the percent composition. The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100.

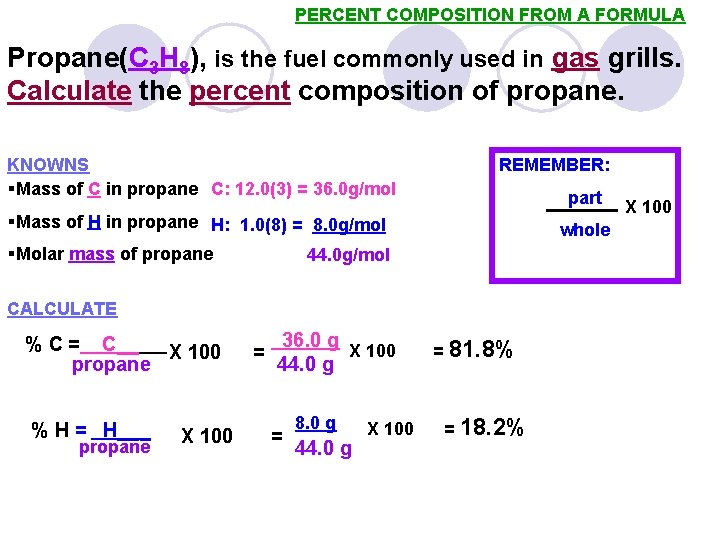
PERCENT COMPOSITION FROM A FORMULA Propane(C 3 H 8), is the fuel commonly used in gas grills. Calculate the percent composition of propane. KNOWNS §Mass of C in propane C: 12. 0(3) = 36. 0 g/mol REMEMBER: part §Mass of H in propane H: 1. 0(8) = 8. 0 g/mol §Molar mass of propane whole 44. 0 g/mol CALCULATE % C = C__ X 100 propane % H = H___ propane X 100 = 36. 0 g X 100 44. 0 g = 8. 0 g 44. 0 g X 100 = 81. 8% = 18. 2% X 100

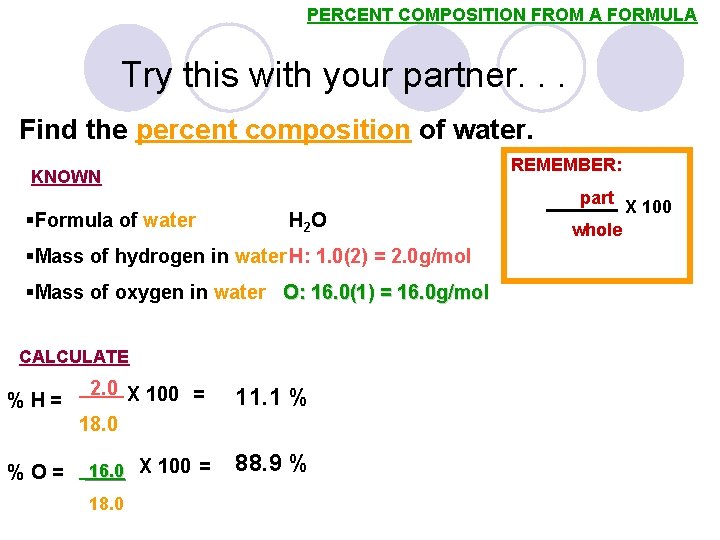
PERCENT COMPOSITION FROM A FORMULA Try this with your partner. . . Find the percent composition of water. REMEMBER: KNOWN part §Formula of water H 2 O §Mass of hydrogen in water H: 1. 0(2) = 2. 0 g/mol §Mass of oxygen in water O: 16. 0(1) = 16. 0 g/mol CALCULATE %H= %O= 2. 0 X 100 = 18. 0 16. 0 X 100 = 18. 0 11. 1 % 88. 9 % whole X 100

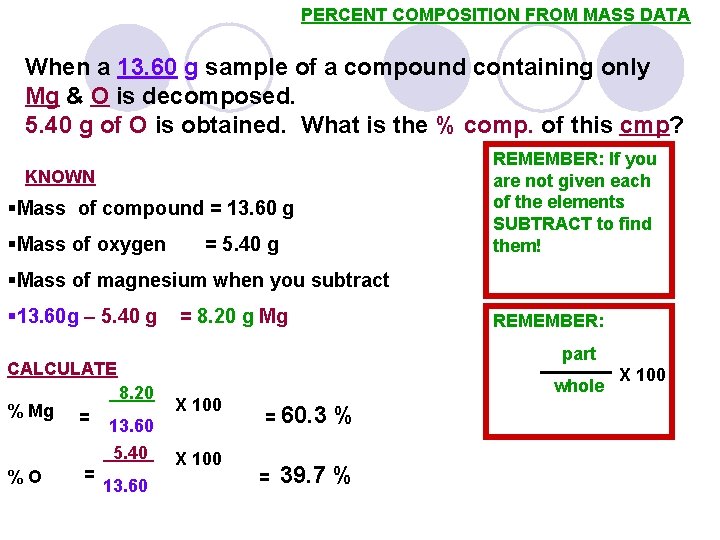
PERCENT COMPOSITION FROM MASS DATA When a 13. 60 g sample of a compound containing only Mg & O is decomposed. 5. 40 g of O is obtained. What is the % comp. of this cmp? REMEMBER: If you are not given each of the elements SUBTRACT to find them! KNOWN §Mass of compound = 13. 60 g §Mass of oxygen = 5. 40 g §Mass of magnesium when you subtract § 13. 60 g – 5. 40 g = 8. 20 g Mg REMEMBER: part CALCULATE % Mg 8. 20 = 13. 60 5. 40 %O = X 100 13. 60 X 100 whole = 60. 3 = % 39. 7 % X 100

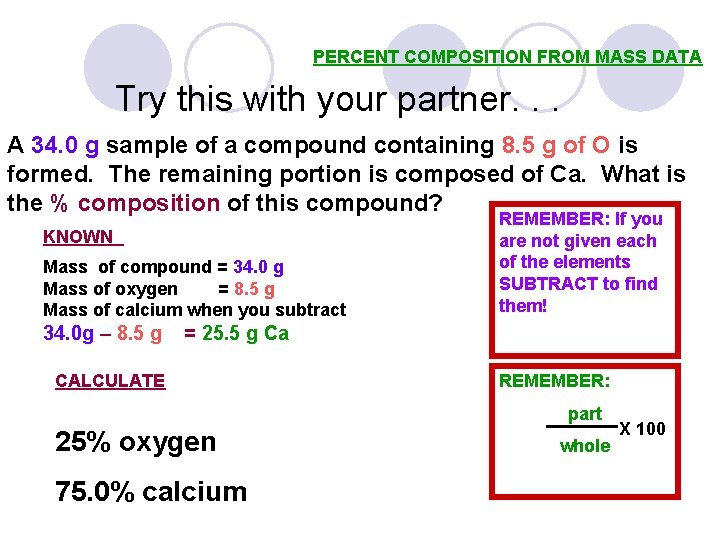
PERCENT COMPOSITION FROM MASS DATA Try this with your partner. . . A 34. 0 g sample of a compound containing 8. 5 g of O is formed. The remaining portion is composed of Ca. What is the % composition of this compound? KNOWN Mass of compound = 34. 0 g Mass of oxygen = 8. 5 g Mass of calcium when you subtract 34. 0 g – 8. 5 g REMEMBER: If you are not given each of the elements SUBTRACT to find them! = 25. 5 g Ca CALCULATE REMEMBER: part 25% oxygen 75. 0% calcium whole X 100