Percentage Composition Percent Part Whole X 100 Percent








- Slides: 11

Percentage Composition

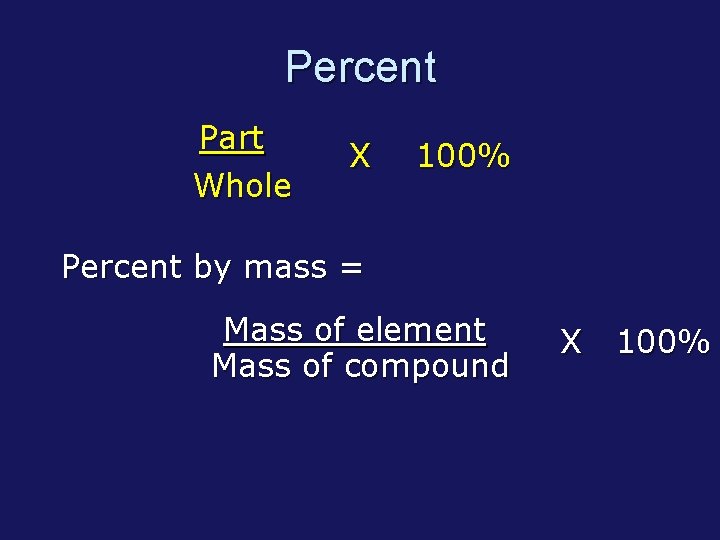
Percent Part Whole X 100% Percent by mass = Mass of element Mass of compound X 100%

Percent Composition use chemical formula & assume 1 mole divide mass each element by formula mass (FM) of compound

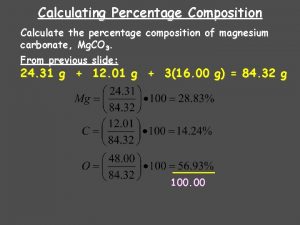
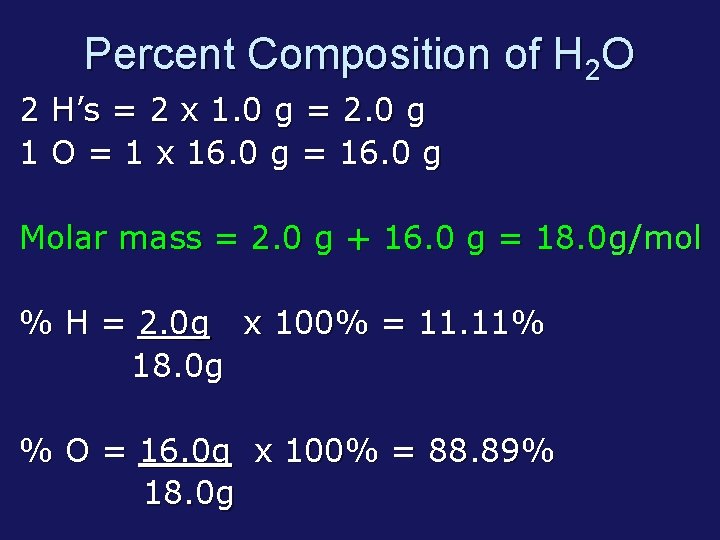
Percent Composition of H 2 O 2 H’s = 2 x 1. 0 g = 2. 0 g 1 O = 1 x 16. 0 g = 16. 0 g Molar mass = 2. 0 g + 16. 0 g = 18. 0 g/mol % H = 2. 0 g x 100% = 11. 11% 18. 0 g % O = 16. 0 g x 100% = 88. 89% 18. 0 g

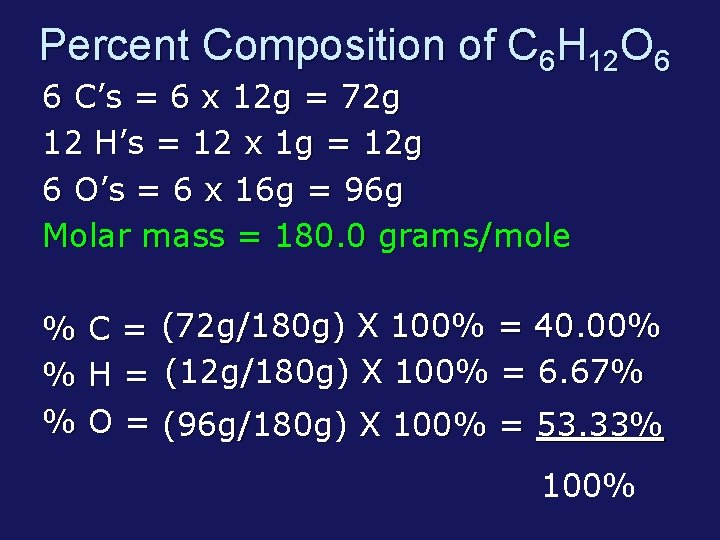
Percent Composition of C 6 H 12 O 6 6 C’s = 6 x 12 g = 72 g 12 H’s = 12 x 1 g = 12 g 6 O’s = 6 x 16 g = 96 g Molar mass = 180. 0 grams/mole % % % C = (72 g/180 g) X 100% = 40. 00% H = (12 g/180 g) X 100% = 6. 67% O = (96 g/180 g) X 100% = 53. 33% 100%

Percentage Composition Remember to calculate FM! • Nowhere in word problem will it tell you that! • Sum of individual element %’s must add up to exactly 100%

Hydrates group salts that have water molecules stuffed in their empty spaces Formulas are distinctive Ex: Cu. SO 4 5 H 2 O means “is associated with” or “included” Does NOT refer to multiplication Not true chemical bond:

What can say about Cu. SO 4 5 H 2 O? It’s hydrated salt – hydrate = water • 1 mole Cu. SO 4 contains 5 moles water • 1 molecule of Cu. SO 4 contains 5 molecules of water When heated, water is driven off & anhydrous salt is left: Cu. SO 4 – anhydrous = without water If had 2 moles of Cu. SO 4 5 H 2 O, how much water would you lose on heating?

Cu. SO 4 5 H 2 O Count up the atoms! 1 Cu 1 S 4 O + 5 x 1 O = 9 O 5 x 2 H =10 H total = 21 atoms

5 Cu. SO 4 5 H 2 O Count up the atoms! 5 = Cu 5=S 5 x 4 O + 5 x 5 x 1 O = 45 O 5 x 5 x 2 H = 50 H total = 105 atoms

FIND THE PERCENT WATER Cu. SO 4 5 H 2 O 1 Cu = 1 x 64 = 64 1 S = 1 x 32 = 32 9 O = 9 x 16 = 144 10 H = 10 x 1 = 10 total = 240 Just water 5 O = 5 x 16 = 80 10 H = 10 x 1 = 10 total = 90 90/240 x 100 = 37. 5% water
 100 100 100 100 100
100 100 100 100 100 Whole school whole community whole child model
Whole school whole community whole child model Massed practice
Massed practice Percent composition
Percent composition Waitress gets $4 000 tip
Waitress gets $4 000 tip Addition symbol
Addition symbol Part part whole
Part part whole What is the percentage composition of oxygen
What is the percentage composition of oxygen How to find mass percent composition
How to find mass percent composition Empirical formula of lactic acid
Empirical formula of lactic acid How to find empirical formula with percentages
How to find empirical formula with percentages Empirical formula from percent composition
Empirical formula from percent composition