Empirical molecular percent composition Percent Composition Percent by
Empirical, molecular, percent composition
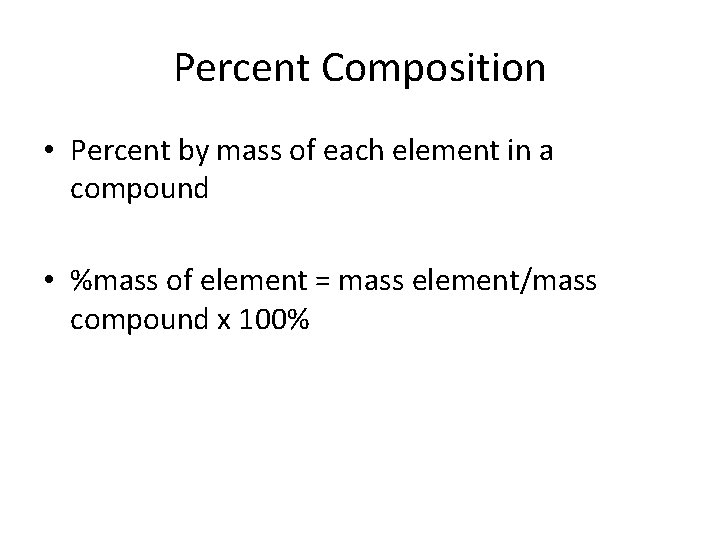
Percent Composition • Percent by mass of each element in a compound • %mass of element = mass element/mass compound x 100%
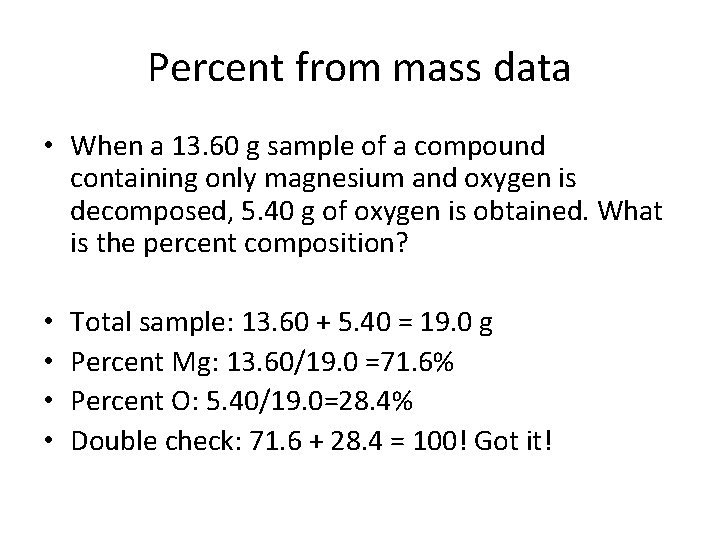
Percent from mass data • When a 13. 60 g sample of a compound containing only magnesium and oxygen is decomposed, 5. 40 g of oxygen is obtained. What is the percent composition? • • Total sample: 13. 60 + 5. 40 = 19. 0 g Percent Mg: 13. 60/19. 0 =71. 6% Percent O: 5. 40/19. 0=28. 4% Double check: 71. 6 + 28. 4 = 100! Got it!
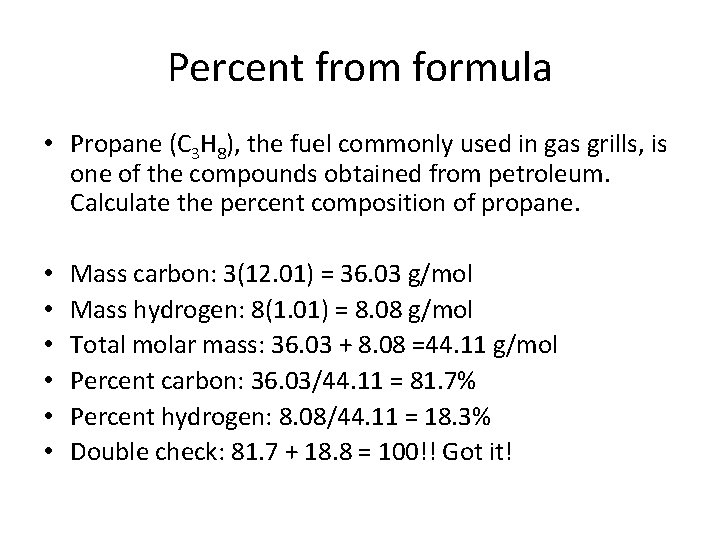
Percent from formula • Propane (C 3 H 8), the fuel commonly used in gas grills, is one of the compounds obtained from petroleum. Calculate the percent composition of propane. • • • Mass carbon: 3(12. 01) = 36. 03 g/mol Mass hydrogen: 8(1. 01) = 8. 08 g/mol Total molar mass: 36. 03 + 8. 08 =44. 11 g/mol Percent carbon: 36. 03/44. 11 = 81. 7% Percent hydrogen: 8. 08/44. 11 = 18. 3% Double check: 81. 7 + 18. 8 = 100!! Got it!
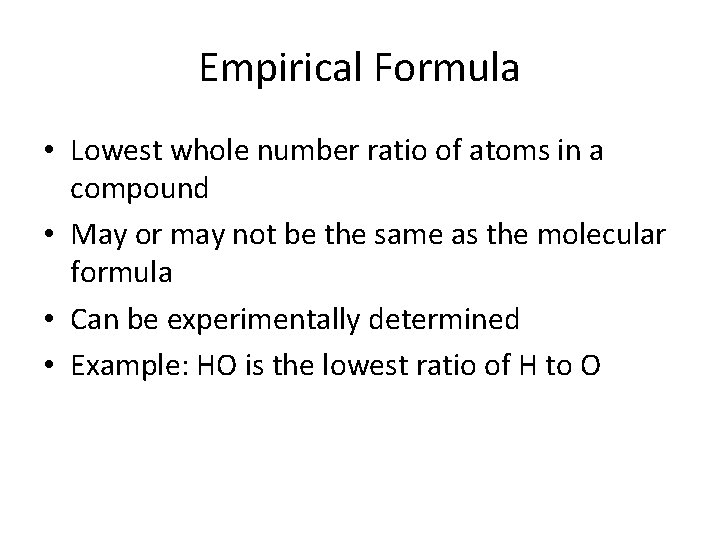
Empirical Formula • Lowest whole number ratio of atoms in a compound • May or may not be the same as the molecular formula • Can be experimentally determined • Example: HO is the lowest ratio of H to O
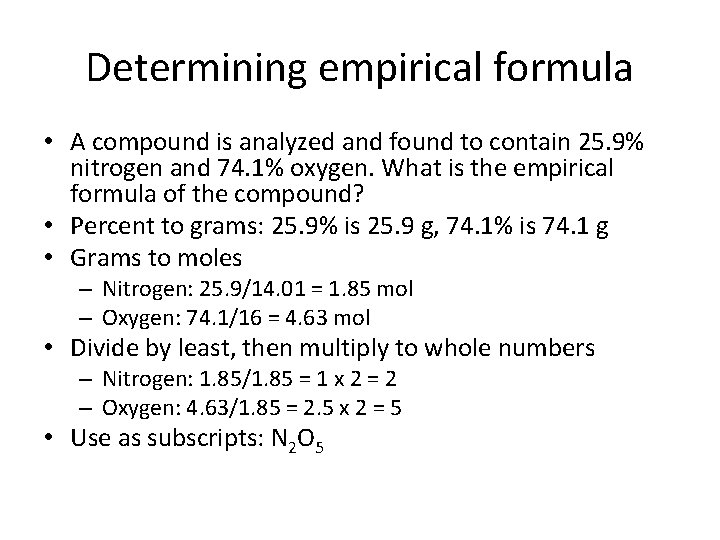
Determining empirical formula • A compound is analyzed and found to contain 25. 9% nitrogen and 74. 1% oxygen. What is the empirical formula of the compound? • Percent to grams: 25. 9% is 25. 9 g, 74. 1% is 74. 1 g • Grams to moles – Nitrogen: 25. 9/14. 01 = 1. 85 mol – Oxygen: 74. 1/16 = 4. 63 mol • Divide by least, then multiply to whole numbers – Nitrogen: 1. 85/1. 85 = 1 x 2 = 2 – Oxygen: 4. 63/1. 85 = 2. 5 x 2 = 5 • Use as subscripts: N 2 O 5
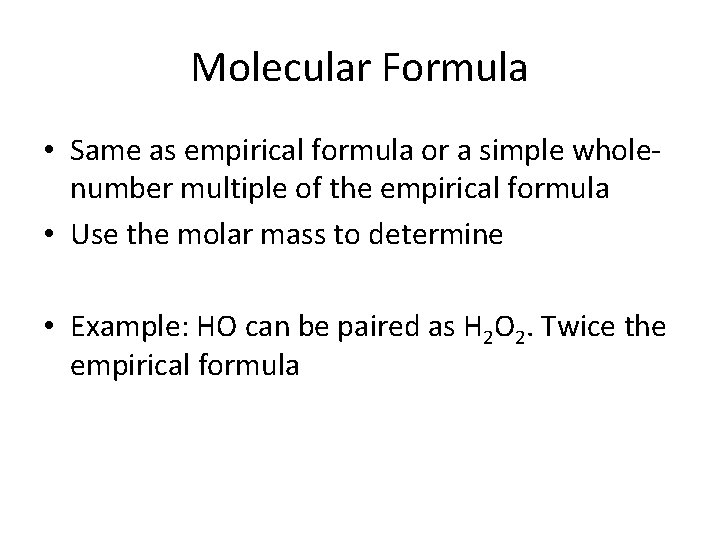
Molecular Formula • Same as empirical formula or a simple wholenumber multiple of the empirical formula • Use the molar mass to determine • Example: HO can be paired as H 2 O 2. Twice the empirical formula
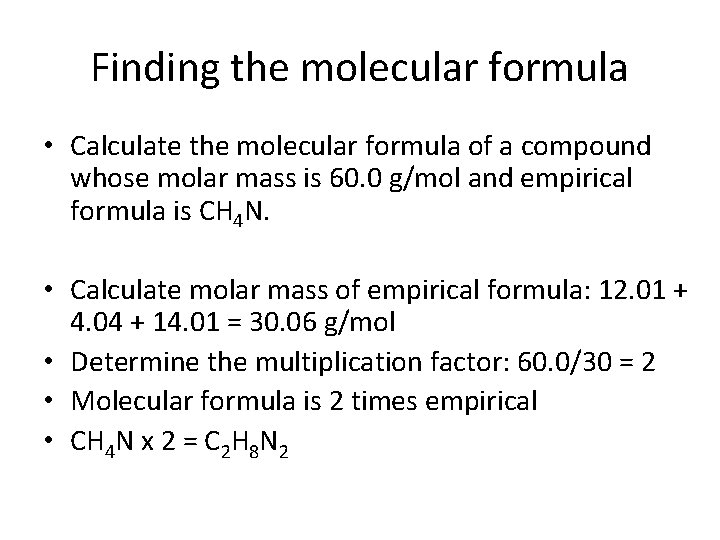
Finding the molecular formula • Calculate the molecular formula of a compound whose molar mass is 60. 0 g/mol and empirical formula is CH 4 N. • Calculate molar mass of empirical formula: 12. 01 + 4. 04 + 14. 01 = 30. 06 g/mol • Determine the multiplication factor: 60. 0/30 = 2 • Molecular formula is 2 times empirical • CH 4 N x 2 = C 2 H 8 N 2
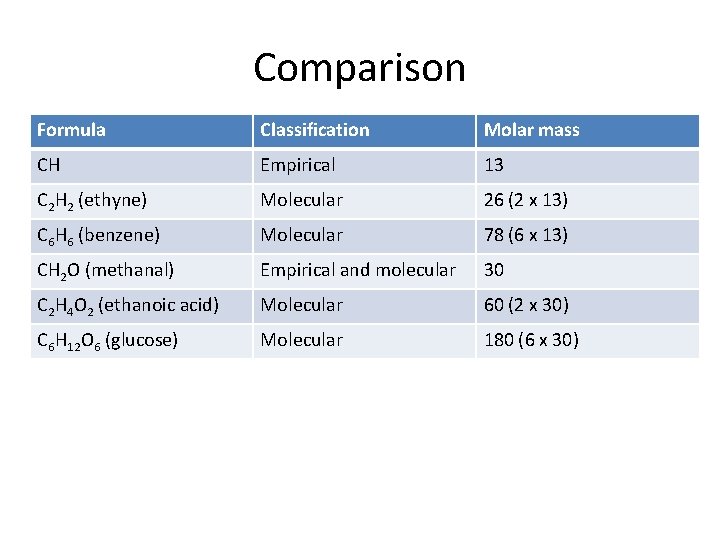
Comparison Formula Classification Molar mass CH Empirical 13 C 2 H 2 (ethyne) Molecular 26 (2 x 13) C 6 H 6 (benzene) Molecular 78 (6 x 13) CH 2 O (methanal) Empirical and molecular 30 C 2 H 4 O 2 (ethanoic acid) Molecular 60 (2 x 30) C 6 H 12 O 6 (glucose) Molecular 180 (6 x 30)

More • Let Mr. Bozeman take it away! https: //www. youtube. com/watch? v=Qc. C 4 Os. S x. WYU

Now you try: • 1. A compound is formed when 9. 03 g Mg combines completely with 3. 48 g N. What is the percent composition of this compound? • 2. Calculate the percent composition of these compounds: • A. ethane (C 2 H 6) • B. sodium hydrogen sulfate (Na. HSO 4). • 3. Calculate the empirical formula of each compound: • A. 94. 1% O, 5. 9% H • B. 67. 6% Hg, 10. 8% S, 21. 6% O • 4. Find the molecular formula of ethylene glycol, which is used as antifreeze. The molar mass is 62 g/mol and the empirical formula is CH 3 O.
- Slides: 11