Percent Composition Empirical Formulas and Molecular Formulas SC

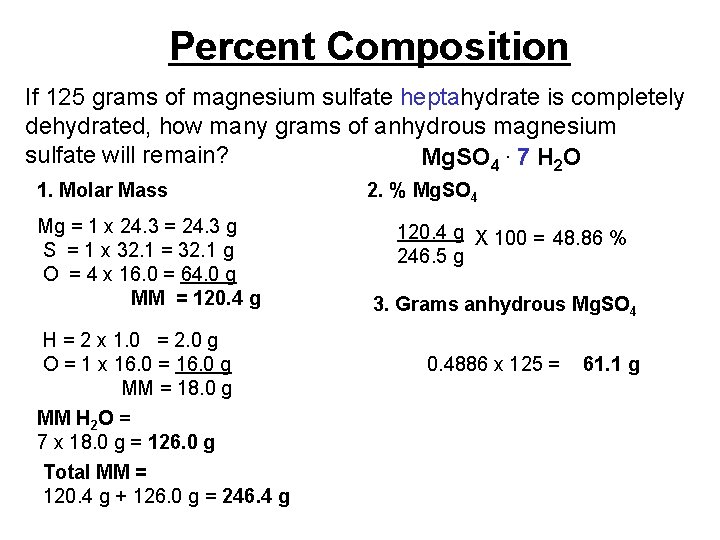


- Slides: 28

Percent Composition, Empirical Formulas, and Molecular Formulas SC 3. Obtain, evaluate, and communicate information about how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. c. Use mathematics and computational thinking to apply concepts of the mole and Avogadro’s number to conceptualize and calculate • percent composition • empirical/molecular formulas

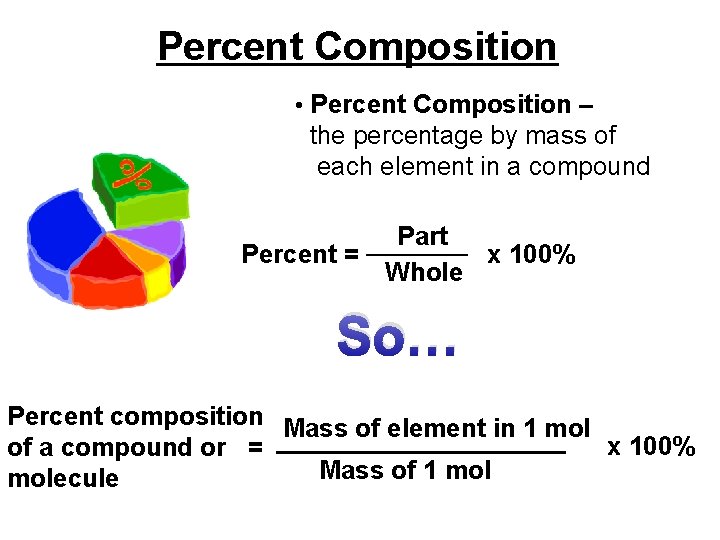
Percent Composition • Percent Composition – the percentage by mass of each element in a compound Part _______ Percent = x 100% Whole So… Percent composition Mass of element in 1 mol x 100% of a compound or = __________ Mass of 1 molecule

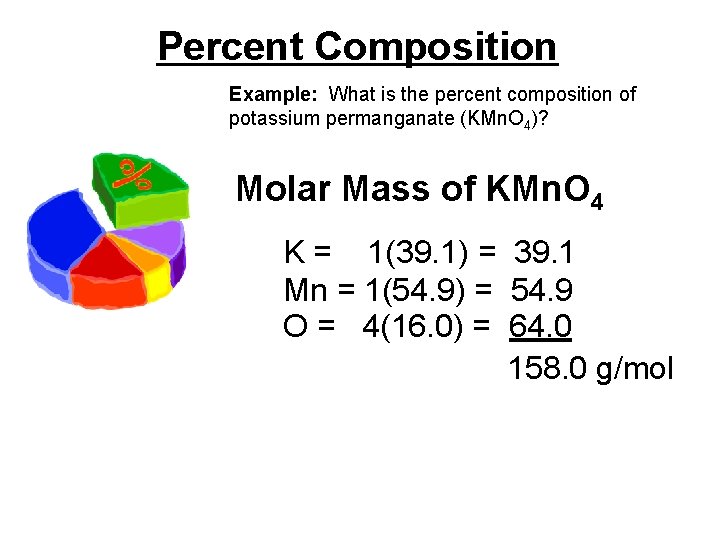
Percent Composition Example: What is the percent composition of potassium permanganate (KMn. O 4)? Molar Mass of KMn. O 4 K = 1(39. 1) = 39. 1 Mn = 1(54. 9) = 54. 9 O = 4(16. 0) = 64. 0 158. 0 g/mol

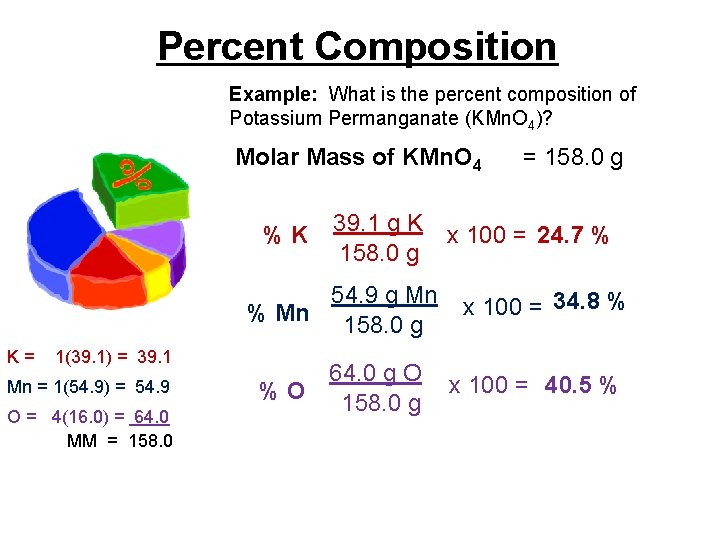
Percent Composition Example: What is the percent composition of Potassium Permanganate (KMn. O 4)? Molar Mass of KMn. O 4 %K 39. 1 g K 158. 0 g 54. 9 g Mn % Mn 158. 0 g K= 1(39. 1) = 39. 1 Mn = 1(54. 9) = 54. 9 O = 4(16. 0) = 64. 0 MM = 158. 0 64. 0 g O %O 158. 0 g = 158. 0 g x 100 = 24. 7 % x 100 = 34. 8 % x 100 = 40. 5 %

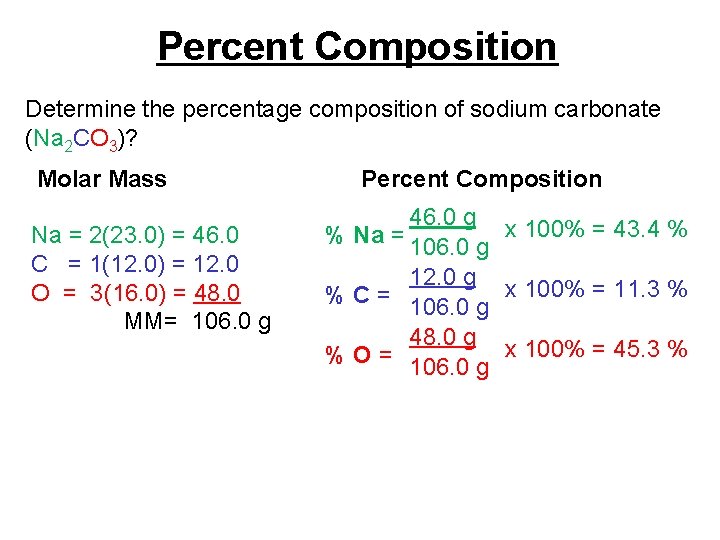
Percent Composition Determine the percentage composition of sodium carbonate (Na 2 CO 3)? Molar Mass Na = 2(23. 0) = 46. 0 C = 1(12. 0) = 12. 0 O = 3(16. 0) = 48. 0 MM= 106. 0 g Percent Composition 46. 0 g x 100% = 43. 4 % % Na = 106. 0 g 12. 0 g x 100% = 11. 3 % % C = 106. 0 g 48. 0 g x 100% = 45. 3 % % O = 106. 0 g

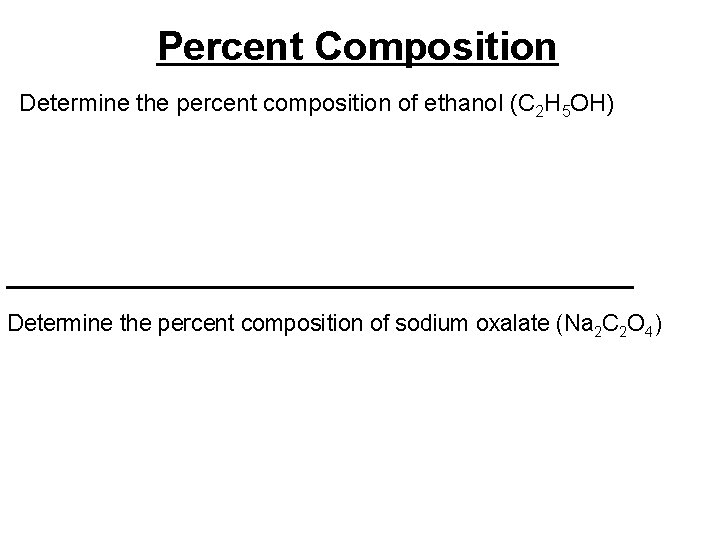
Percent Composition Determine the percent composition of ethanol (C 2 H 5 OH) ________________________ Determine the percent composition of sodium oxalate (Na 2 C 2 O 4)

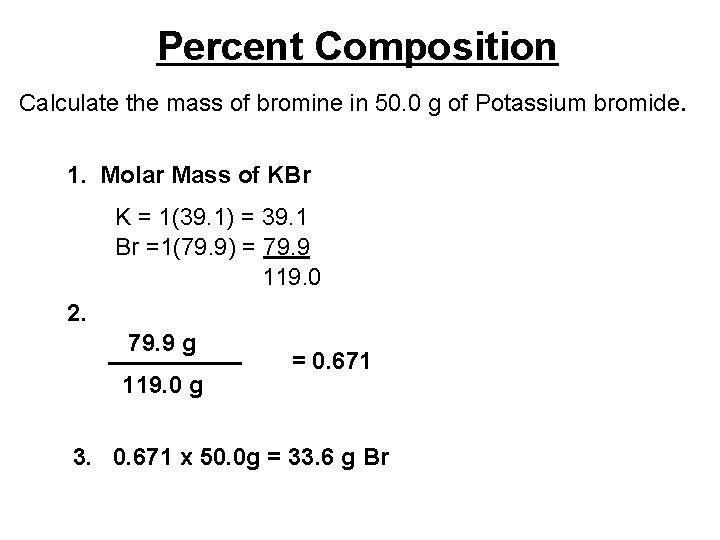
Percent Composition Calculate the mass of bromine in 50. 0 g of Potassium bromide. 1. Molar Mass of KBr K = 1(39. 1) = 39. 1 Br =1(79. 9) = 79. 9 119. 0 2. 79. 9 g _____ 119. 0 g = 0. 671 3. 0. 671 x 50. 0 g = 33. 6 g Br

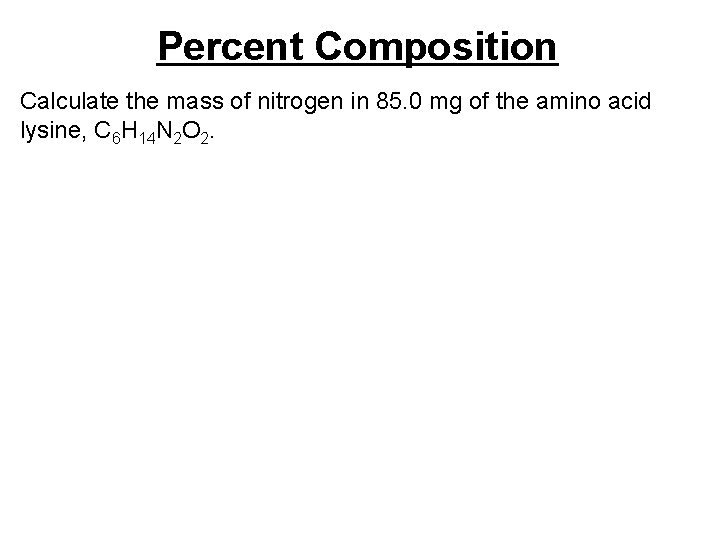
Percent Composition Calculate the mass of nitrogen in 85. 0 mg of the amino acid lysine, C 6 H 14 N 2 O 2.

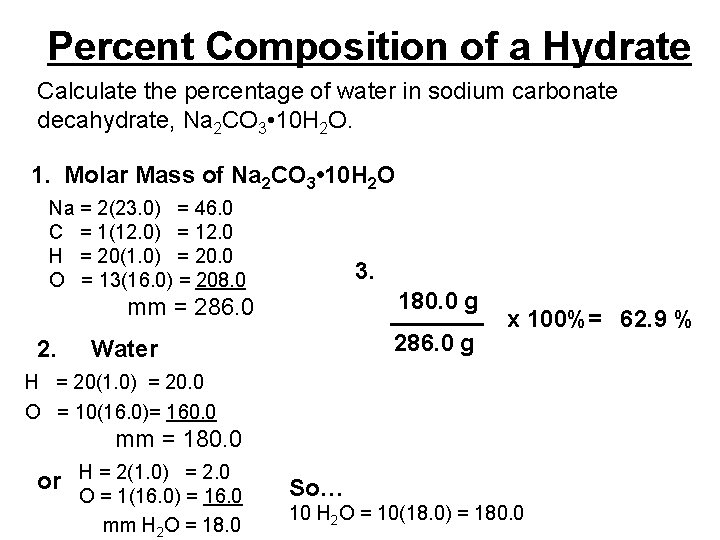
Percent Composition of a Hydrate Calculate the percentage of water in sodium carbonate decahydrate, Na 2 CO 3 • 10 H 2 O. 1. Molar Mass of Na 2 CO 3 • 10 H 2 O Na = 2(23. 0) = 46. 0 C = 1(12. 0) = 12. 0 H = 20(1. 0) = 20. 0 O = 13(16. 0) = 208. 0 3. 180. 0 g _______ x 100%= 62. 9 % 286. 0 g mm = 286. 0 2. Water H = 20(1. 0) = 20. 0 O = 10(16. 0)= 160. 0 mm = 180. 0 or H = 2(1. 0) = 2. 0 O = 1(16. 0) = 16. 0 mm H 2 O = 18. 0 So… 10 H 2 O = 10(18. 0) = 180. 0

Percent Composition Calculate the percentage of water in aluminum bromide hexahydrate, Al. Br 3 • 6 H 2 O.
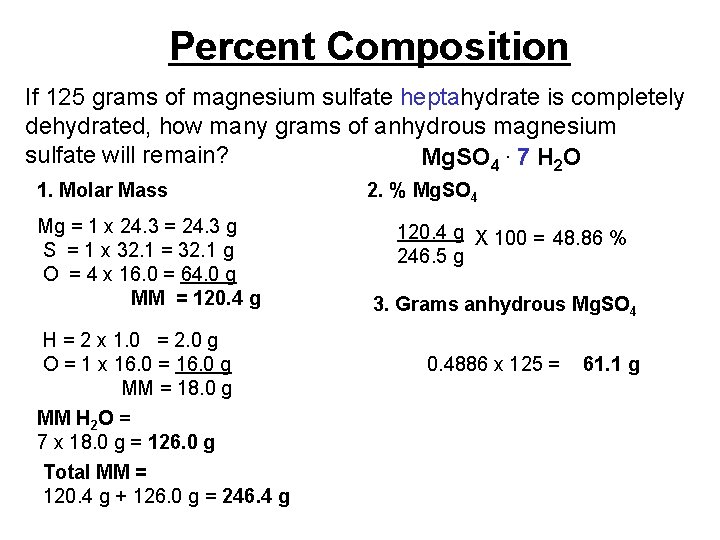
Percent Composition If 125 grams of magnesium sulfate heptahydrate is completely dehydrated, how many grams of anhydrous magnesium sulfate will remain? Mg. SO 4. 7 H 2 O 1. Molar Mass Mg = 1 x 24. 3 = 24. 3 g S = 1 x 32. 1 = 32. 1 g O = 4 x 16. 0 = 64. 0 g MM = 120. 4 g H = 2 x 1. 0 = 2. 0 g O = 1 x 16. 0 = 16. 0 g MM = 18. 0 g MM H 2 O = 7 x 18. 0 g = 126. 0 g Total MM = 120. 4 g + 126. 0 g = 246. 4 g 2. % Mg. SO 4 120. 4 g X 100 = 48. 86 % 246. 5 g 3. Grams anhydrous Mg. SO 4 0. 4886 x 125 = 61. 1 g

Percent Composition If 145 grams of copper(II) sulfate pentahydrate is completely dehydrated, how many grams of anhydrous copper sulfate will remain?

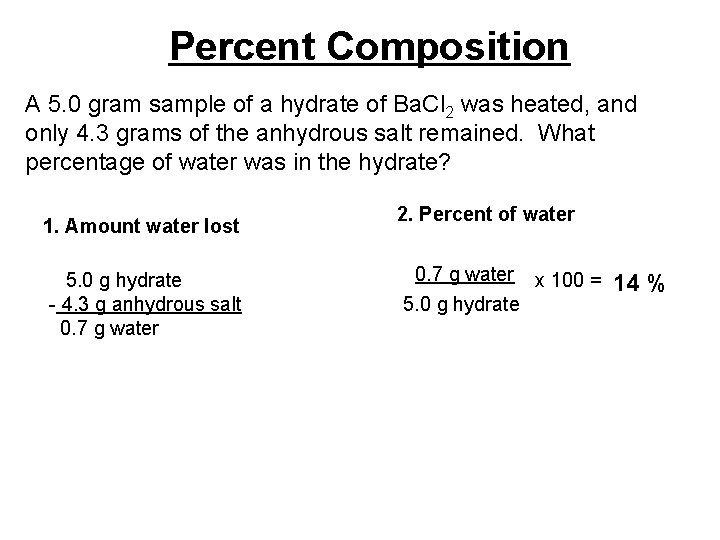
Percent Composition A 5. 0 gram sample of a hydrate of Ba. Cl 2 was heated, and only 4. 3 grams of the anhydrous salt remained. What percentage of water was in the hydrate? 1. Amount water lost 5. 0 g hydrate - 4. 3 g anhydrous salt 0. 7 g water 2. Percent of water 0. 7 g water x 100 = 5. 0 g hydrate 14 %

Percent Composition A 7. 5 gram sample of a hydrate of Cu. Cl 2 was heated, and only 5. 3 grams of the anhydrous salt remained. What percentage of water was in the hydrate?

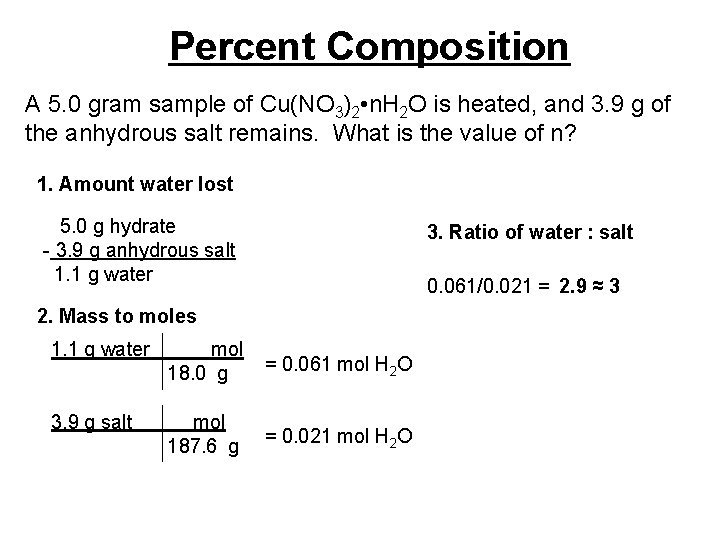
Percent Composition A 5. 0 gram sample of Cu(NO 3)2 • n. H 2 O is heated, and 3. 9 g of the anhydrous salt remains. What is the value of n? 1. Amount water lost 5. 0 g hydrate - 3. 9 g anhydrous salt 1. 1 g water 3. Ratio of water : salt 0. 061/0. 021 = 2. 9 ≈ 3 2. Mass to moles 1. 1 g water mol 18. 0 g = 0. 061 mol H 2 O 3. 9 g salt mol 187. 6 g = 0. 021 mol H 2 O

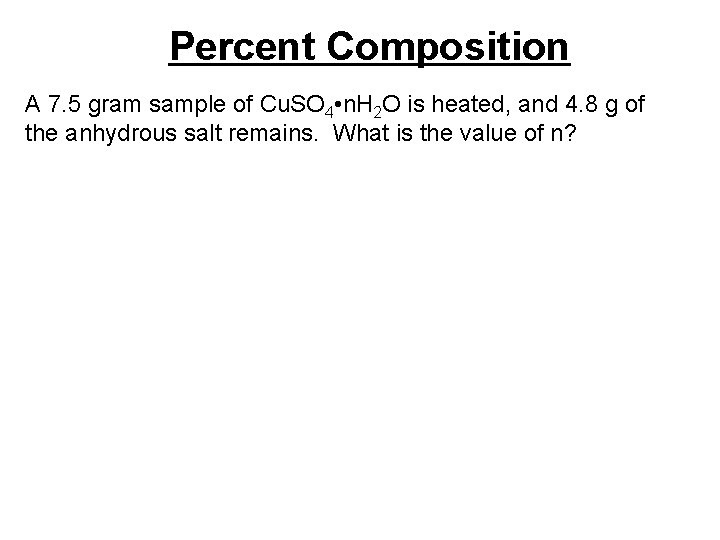
Percent Composition A 7. 5 gram sample of Cu. SO 4 • n. H 2 O is heated, and 4. 8 g of the anhydrous salt remains. What is the value of n?

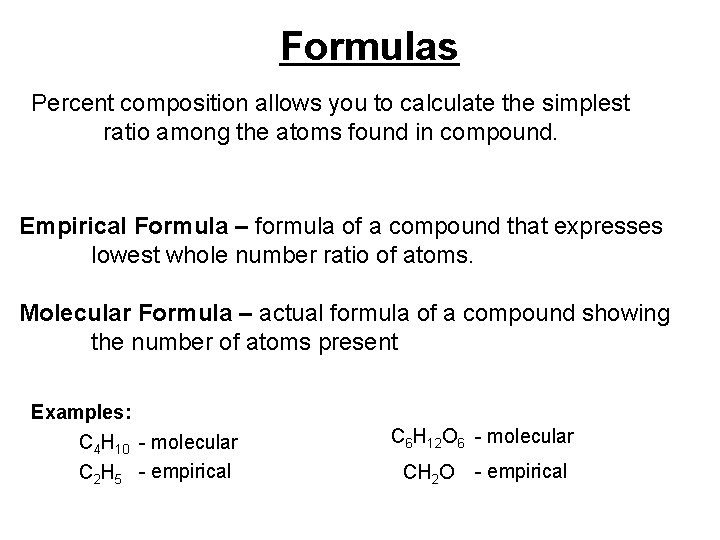
Formulas Percent composition allows you to calculate the simplest ratio among the atoms found in compound. Empirical Formula – formula of a compound that expresses lowest whole number ratio of atoms. Molecular Formula – actual formula of a compound showing the number of atoms present Examples: C 4 H 10 - molecular C 2 H 5 - empirical C 6 H 12 O 6 - molecular CH 2 O - empirical

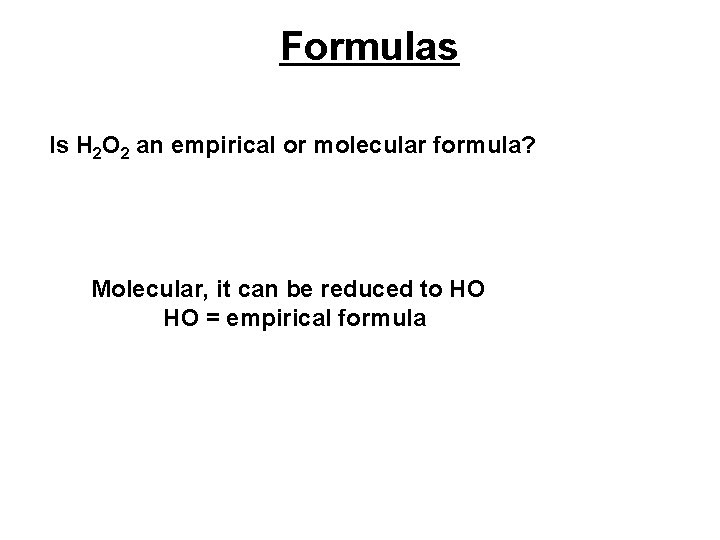
Formulas Is H 2 O 2 an empirical or molecular formula? Molecular, it can be reduced to HO HO = empirical formula

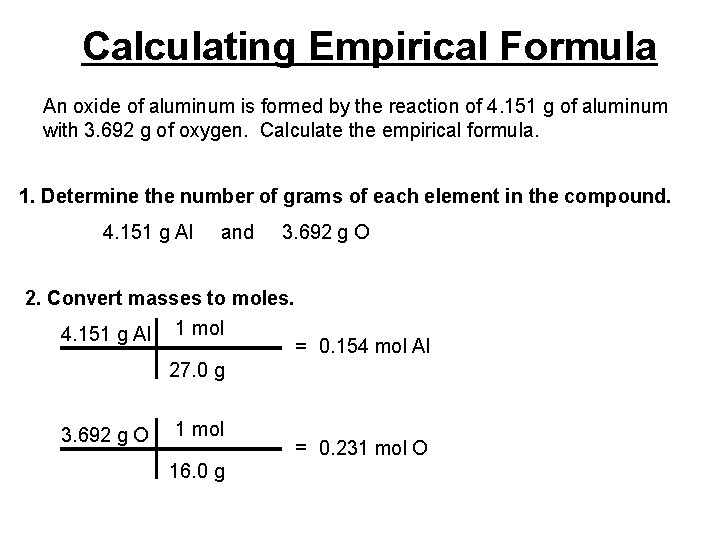
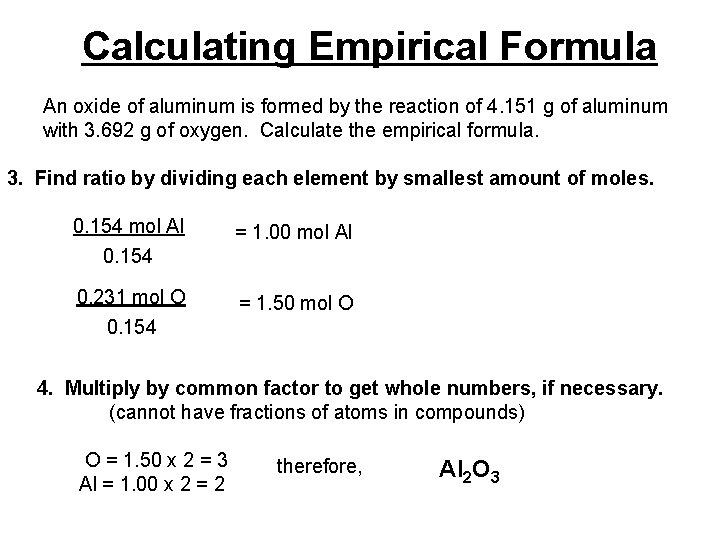
Calculating Empirical Formula An oxide of aluminum is formed by the reaction of 4. 151 g of aluminum with 3. 692 g of oxygen. Calculate the empirical formula. 1. Determine the number of grams of each element in the compound. 4. 151 g Al and 3. 692 g O 2. Convert masses to moles. 4. 151 g Al 1 mol = 0. 154 mol Al 27. 0 g 3. 692 g O 1 mol 16. 0 g = 0. 231 mol O

Calculating Empirical Formula An oxide of aluminum is formed by the reaction of 4. 151 g of aluminum with 3. 692 g of oxygen. Calculate the empirical formula. 3. Find ratio by dividing each element by smallest amount of moles. 0. 154 mol Al 0. 154 = 1. 00 mol Al 0. 231 mol O 0. 154 = 1. 50 mol O 4. Multiply by common factor to get whole numbers, if necessary. (cannot have fractions of atoms in compounds) O = 1. 50 x 2 = 3 Al = 1. 00 x 2 = 2 therefore, Al 2 O 3

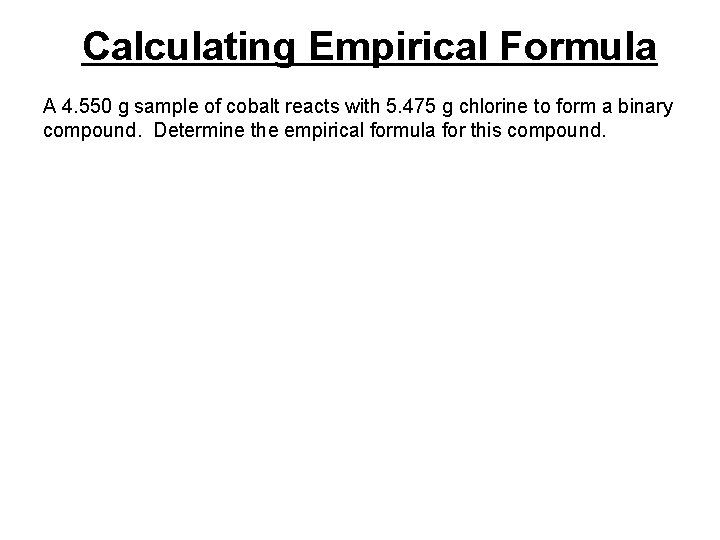
Calculating Empirical Formula A 4. 550 g sample of cobalt reacts with 5. 475 g chlorine to form a binary compound. Determine the empirical formula for this compound.

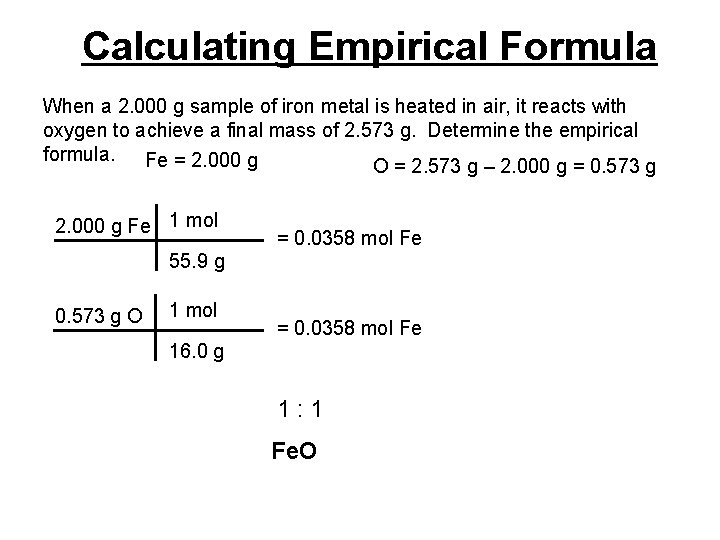
Calculating Empirical Formula When a 2. 000 g sample of iron metal is heated in air, it reacts with oxygen to achieve a final mass of 2. 573 g. Determine the empirical formula. Fe = 2. 000 g O = 2. 573 g – 2. 000 g = 0. 573 g 2. 000 g Fe 1 mol = 0. 0358 mol Fe 55. 9 g 0. 573 g O 1 mol = 0. 0358 mol Fe 16. 0 g 1: 1 Fe. O

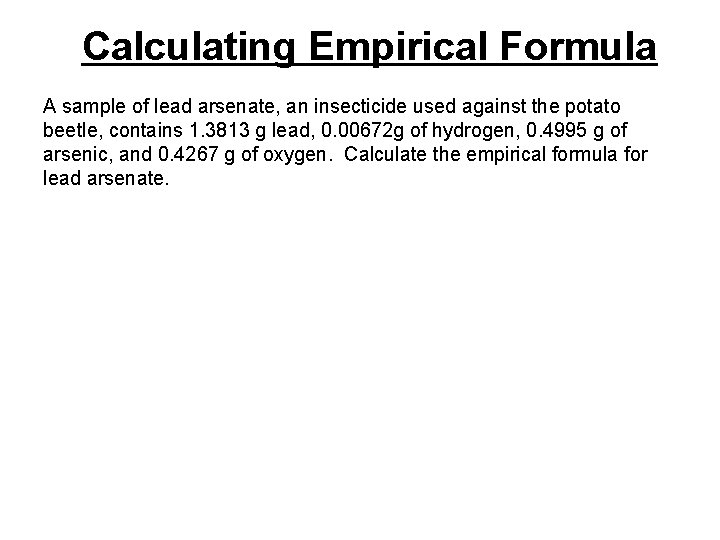
Calculating Empirical Formula A sample of lead arsenate, an insecticide used against the potato beetle, contains 1. 3813 g lead, 0. 00672 g of hydrogen, 0. 4995 g of arsenic, and 0. 4267 g of oxygen. Calculate the empirical formula for lead arsenate.

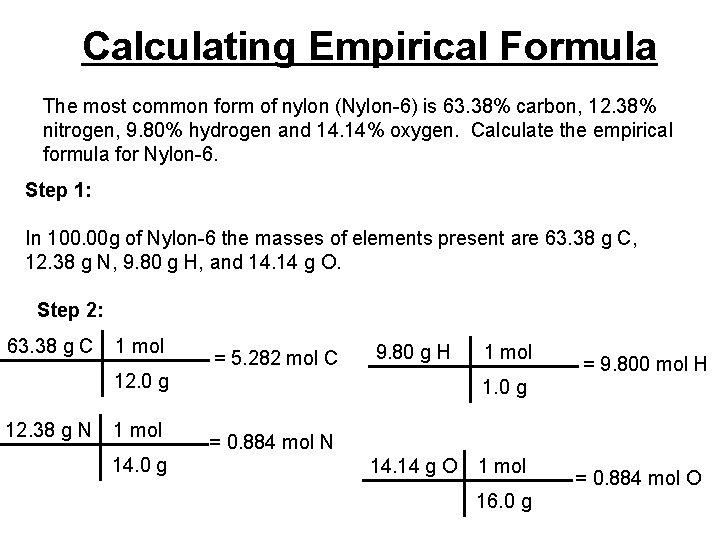
Calculating Empirical Formula The most common form of nylon (Nylon-6) is 63. 38% carbon, 12. 38% nitrogen, 9. 80% hydrogen and 14. 14% oxygen. Calculate the empirical formula for Nylon-6. Step 1: In 100. 00 g of Nylon-6 the masses of elements present are 63. 38 g C, 12. 38 g N, 9. 80 g H, and 14. 14 g O. Step 2: 63. 38 g C 1 mol = 5. 282 mol C 9. 80 g H 12. 0 g 12. 38 g N 1 mol 14. 0 g 1 mol = 9. 800 mol H 1. 0 g = 0. 884 mol N 14. 14 g O 1 mol 16. 0 g = 0. 884 mol O

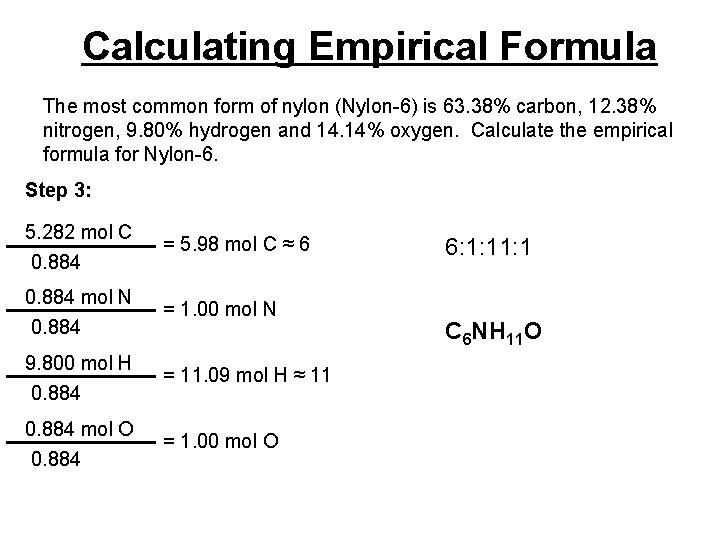
Calculating Empirical Formula The most common form of nylon (Nylon-6) is 63. 38% carbon, 12. 38% nitrogen, 9. 80% hydrogen and 14. 14% oxygen. Calculate the empirical formula for Nylon-6. Step 3: 5. 282 mol C 0. 884 = 5. 98 mol C ≈ 6 0. 884 mol N 0. 884 = 1. 00 mol N 9. 800 mol H 0. 884 = 11. 09 mol H ≈ 11 0. 884 mol O 0. 884 = 1. 00 mol O 6: 1: 1 C 6 NH 11 O

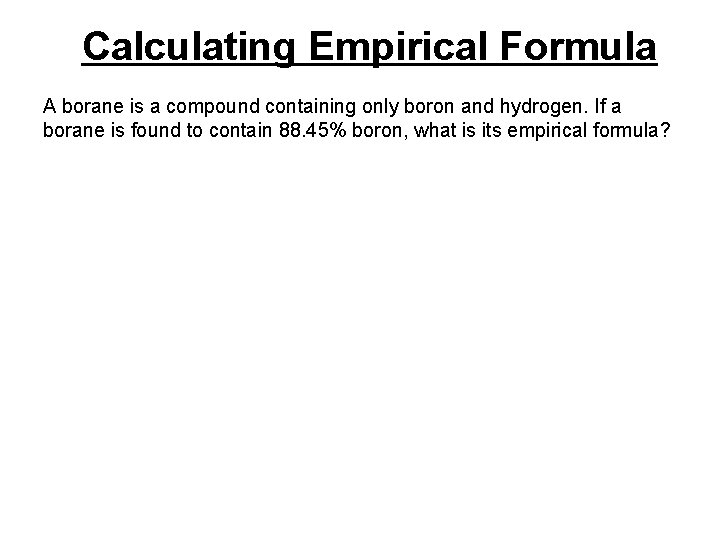
Calculating Empirical Formula A borane is a compound containing only boron and hydrogen. If a borane is found to contain 88. 45% boron, what is its empirical formula?

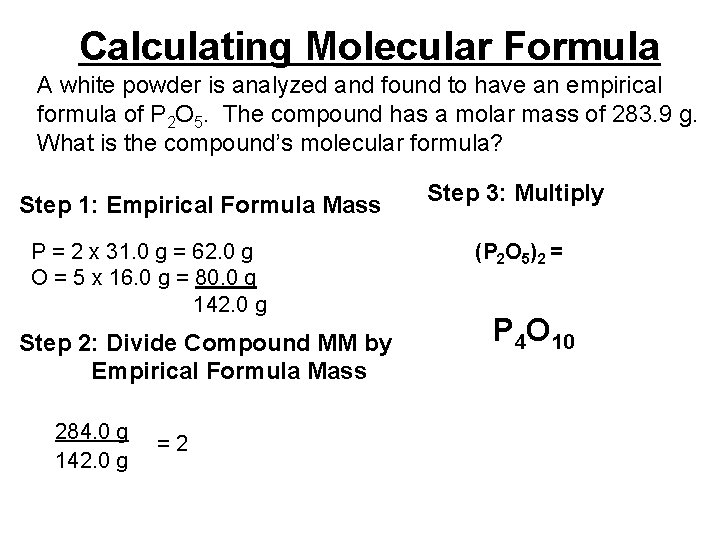
Calculating Molecular Formula A white powder is analyzed and found to have an empirical formula of P 2 O 5. The compound has a molar mass of 283. 9 g. What is the compound’s molecular formula? Step 1: Empirical Formula Mass P = 2 x 31. 0 g = 62. 0 g O = 5 x 16. 0 g = 80. 0 g 142. 0 g Step 2: Divide Compound MM by Empirical Formula Mass 284. 0 g 142. 0 g =2 Step 3: Multiply (P 2 O 5)2 = P 4 O 10

Calculating Molecular Formula A compound has an experimental molar mass of 78 g/mol. Its empirical formula is CH. What is its molecular formula?