PERCENT COMPOSITION 2 3 Steps for Determining Chemical


- Slides: 13

PERCENT COMPOSITION

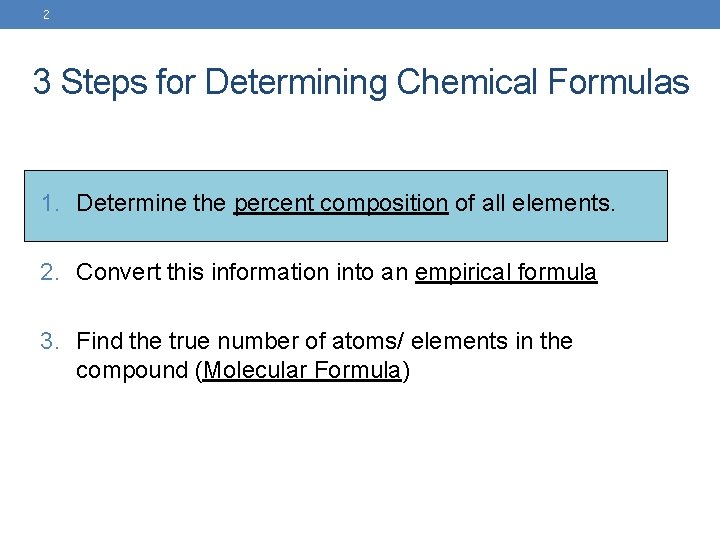
2 3 Steps for Determining Chemical Formulas 1. Determine the percent composition of all elements. 2. Convert this information into an empirical formula 3. Find the true number of atoms/ elements in the compound (Molecular Formula)

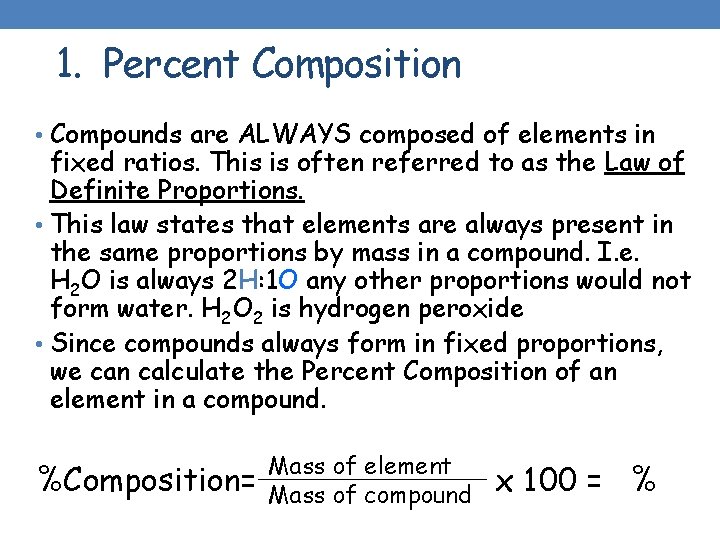
1. Percent Composition • Compounds are ALWAYS composed of elements in fixed ratios. This is often referred to as the Law of Definite Proportions. • This law states that elements are always present in the same proportions by mass in a compound. I. e. H 2 O is always 2 H: 1 O any other proportions would not form water. H 2 O 2 is hydrogen peroxide • Since compounds always form in fixed proportions, we can calculate the Percent Composition of an element in a compound. %Composition= Mass of element Mass of compound x 100 = %

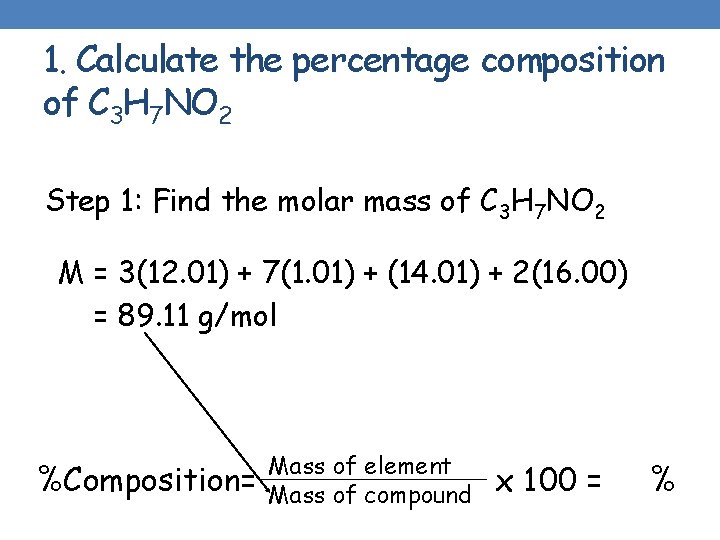
1. Calculate the percentage composition of C 3 H 7 NO 2 Step 1: Find the molar mass of C 3 H 7 NO 2 M = 3(12. 01) + 7(1. 01) + (14. 01) + 2(16. 00) = 89. 11 g/mol %Composition= Mass of element Mass of compound x 100 = %

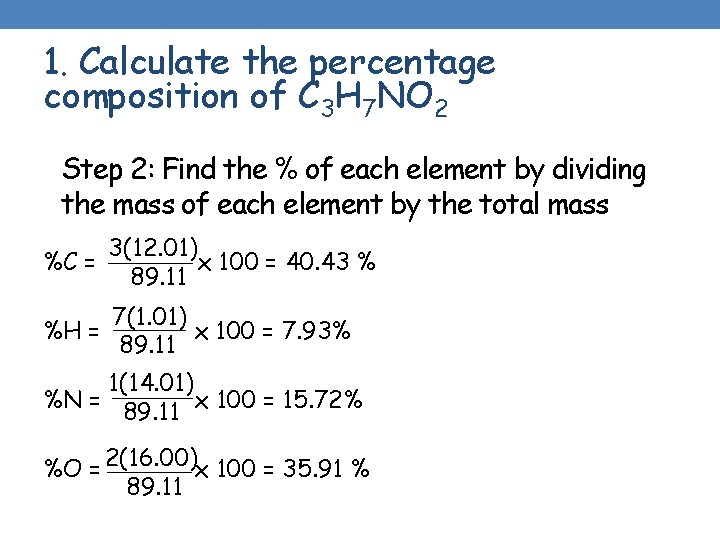
1. Calculate the percentage composition of C 3 H 7 NO 2 Step 2: Find the % of each element by dividing the mass of each element by the total mass %C = 3(12. 01) x 100 = 40. 43 % 89. 11 %H = 7(1. 01) x 100 = 7. 93% 89. 11 1(14. 01) %N = 89. 11 x 100 = 15. 72% %O = 2(16. 00)x 100 = 35. 91 % 89. 11

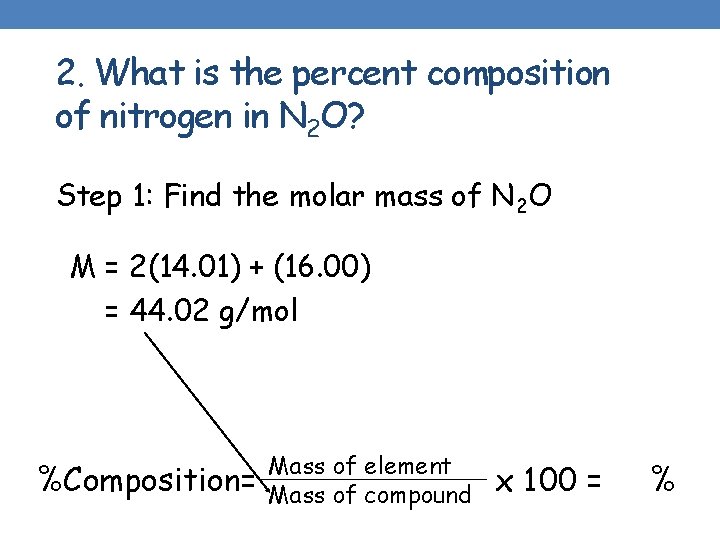
2. What is the percent composition of nitrogen in N 2 O? Step 1: Find the molar mass of N 2 O M = 2(14. 01) + (16. 00) = 44. 02 g/mol %Composition= Mass of element Mass of compound x 100 = %

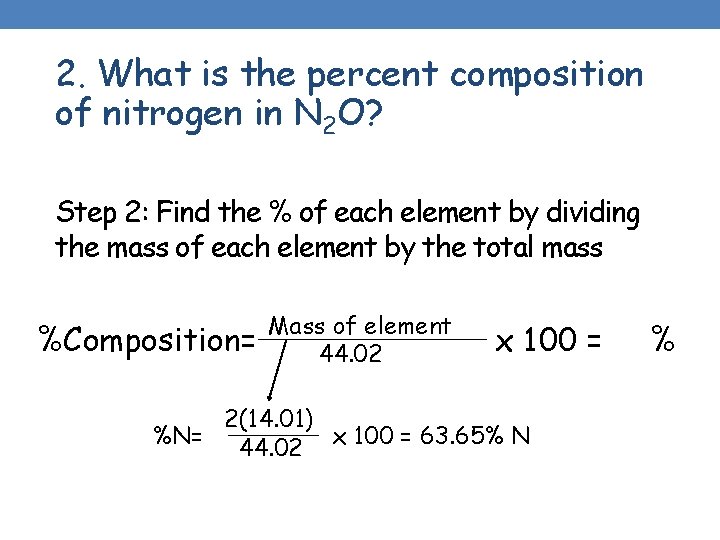
2. What is the percent composition of nitrogen in N 2 O? Step 2: Find the % of each element by dividing the mass of each element by the total mass %Composition= Mass of element 44. 02 x 100 = 2(14. 01) %N= 44. 02 x 100 = 63. 65% N %

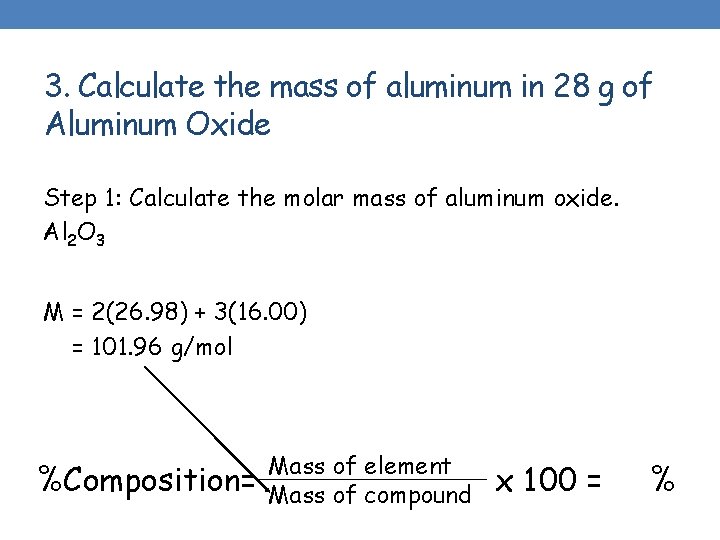
3. Calculate the mass of aluminum in 28 g of Aluminum Oxide Step 1: Calculate the molar mass of aluminum oxide. Al 2 O 3 M = 2(26. 98) + 3(16. 00) = 101. 96 g/mol %Composition= Mass of element Mass of compound x 100 = %

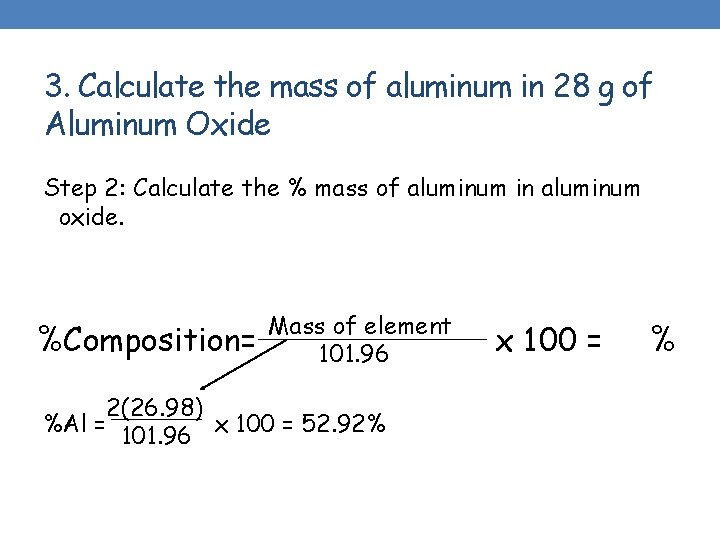
3. Calculate the mass of aluminum in 28 g of Aluminum Oxide Step 2: Calculate the % mass of aluminum in aluminum oxide. %Composition= Mass of element 101. 96 2(26. 98) %Al = x 100 = 52. 92% 101. 96 x 100 = %

3. Calculate the mass of aluminum in 28 g of Aluminum Oxide Step 3: Find the mass of aluminum in 28 g of aluminum oxide. m. Al = 52. 92 x 28 g = 15 g 100

Assignment • Continue on with the following assignment

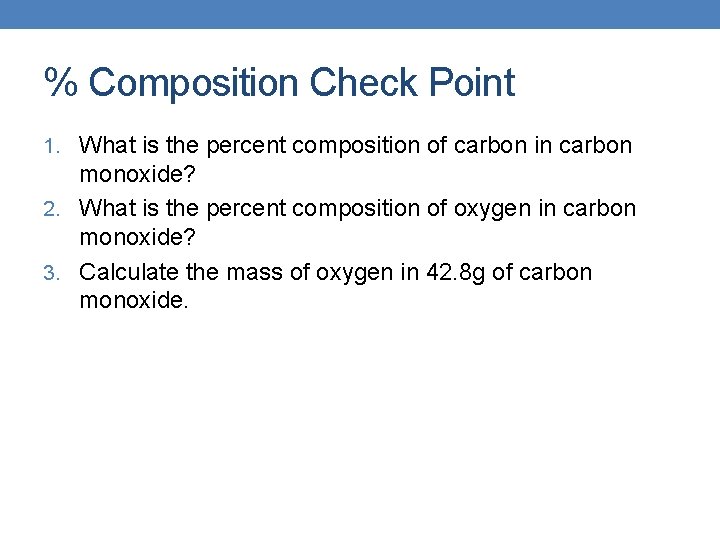
% Composition Check Point 1. What is the percent composition of carbon in carbon monoxide? 2. What is the percent composition of oxygen in carbon monoxide? 3. Calculate the mass of oxygen in 42. 8 g of carbon monoxide.

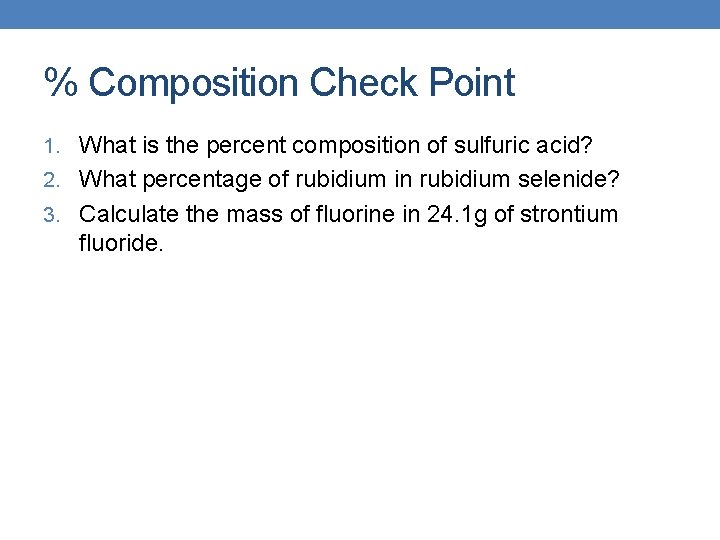
% Composition Check Point 1. What is the percent composition of sulfuric acid? 2. What percentage of rubidium in rubidium selenide? 3. Calculate the mass of fluorine in 24. 1 g of strontium fluoride.