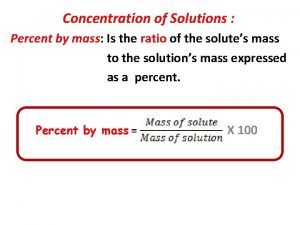
Mole Avogadros Number Molar Mass percent composition Empirical

Mole, Avogadro’s Number Molar Mass percent composition Empirical Formula

Why is Knowledge of Composition Important? • Everything in nature is either chemically or physically combined with other substances • Some Applications: üthe amount of sodium in Na. Cl for diet üthe amount of iron in iron ore for steel production üthe amount of carbon in fossil fuel in terms of green house effect 2

Counting Pennies by Weight • What if a person doesn’t have bills but pounds of pennies when he wants to buy a $10 pizza? • Seinfeld : Kramer’s attempt to buy calzone with pennies • https: //www. youtube. com/watch? v=ywidjw 9 o. QVw http: //www. youtube. com/watch? v=k. Mimyg. VTgb. U • Assuming each penny weighs exactly the same (2. 500 g), we can count pennies by weight the total weight of pennies… 3

Counting Pennies by Weight Kramer brought 2, 500. g (ca. 5. 5 lbs) of new pennies to the Italian restaurant. Do you think he can buy three calzones ($10. 0 total)? Each new penny weighs 2. 500 g. 4

Counting Coins by Weight • What if Kramer bought a different coin? üWould the mass of single dime be 2. 500 g? üWould there be $10. 00 in 2, 500. g dimes? üHow would this affect the weight-dollar conversion factors? 5
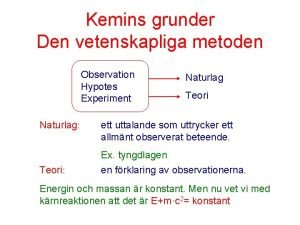
Mass of atoms • Atoms of the same element have on average the same mass. Mass of atom in amu (atomic mass unit): 1 amu = 1. 66054× 10 -27 kg • Mass of a C-12 atom = 12 (exact) amu • Mass of a gold atom on average = 197. 0 amu • Do I have to memorize these numbers? ! 6
Counting atoms by mass • Similar to how we count the number of pennies, we can count the number of atoms using their mass • The number of atoms in a sample often is an astronomical number, so we use a big unit to account for the number of atoms. Just like 1 dozen = 12 items 7
“Chemical Dozen” – the Mole • The number of particles in 1 mole: Avogadro’s Number = ________ units ü 1 mole of any atomic element has 6. 022 x 1023 atoms ü 1 mole of oxygen gas has 6. 022 x 1023 O 2 molecules ü 1 mole of Na. Cl solid has 6. 022 x 1023 Na+ ions and 6. 022 x 1023 Cl- ions 8
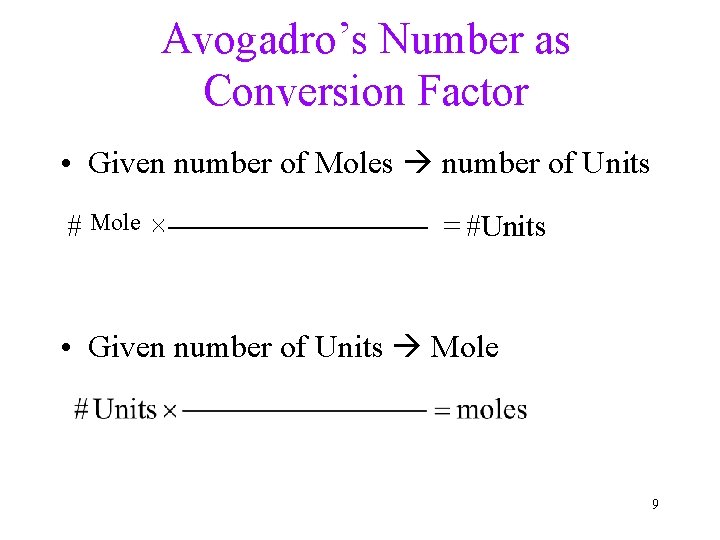
Avogadro’s Number as Conversion Factor • Given number of Moles number of Units # Mole ´ = #Units • Given number of Units Mole 9

Example: A silver ring contains 0. 041 moles of silver. How many silver atoms silver are in the ring? Information Given: 0. 041 moles of Ag atoms Find: ? number of Ag atoms 1 mole Ag atoms = 6. 022 x 1023 Ag atoms 2. 5 x 1022 Ag atoms (2 sig. figs) 10
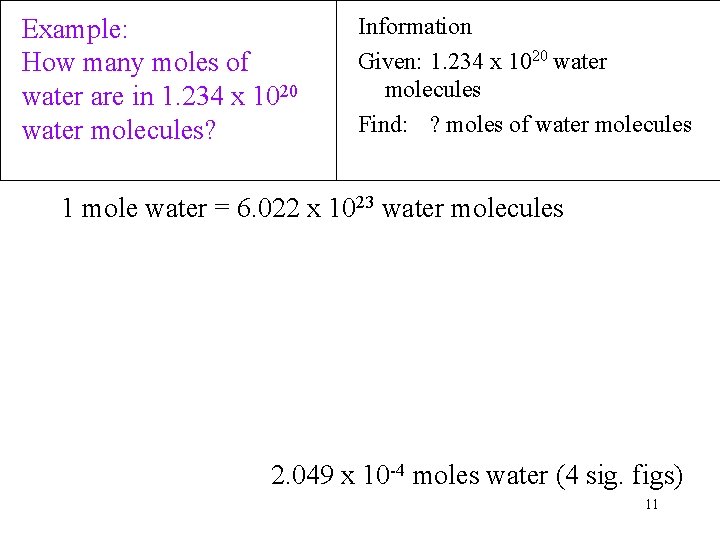
Example: How many moles of water are in 1. 234 x 1020 water molecules? Information Given: 1. 234 x 1020 water molecules Find: ? moles of water molecules 1 mole water = 6. 022 x 1023 water molecules 2. 049 x 10 -4 moles water (4 sig. figs) 11
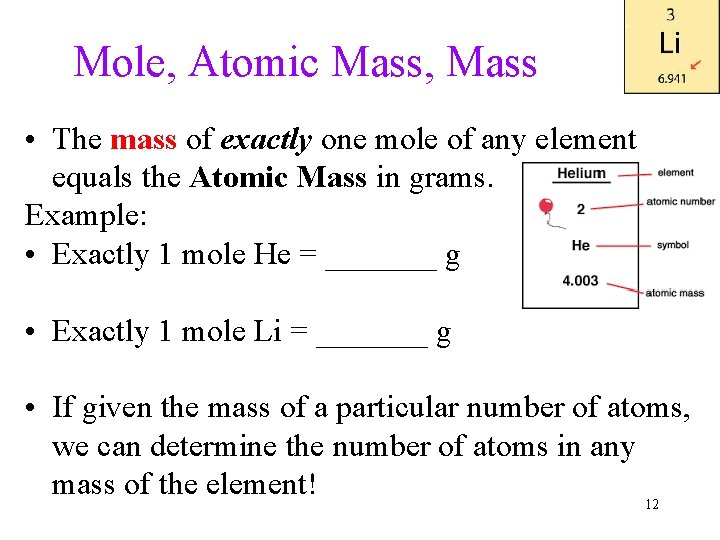
Mole, Atomic Mass, Mass • The mass of exactly one mole of any element equals the Atomic Mass in grams. Example: • Exactly 1 mole He = _______ g • Exactly 1 mole Li = _______ g • If given the mass of a particular number of atoms, we can determine the number of atoms in any mass of the element! 12
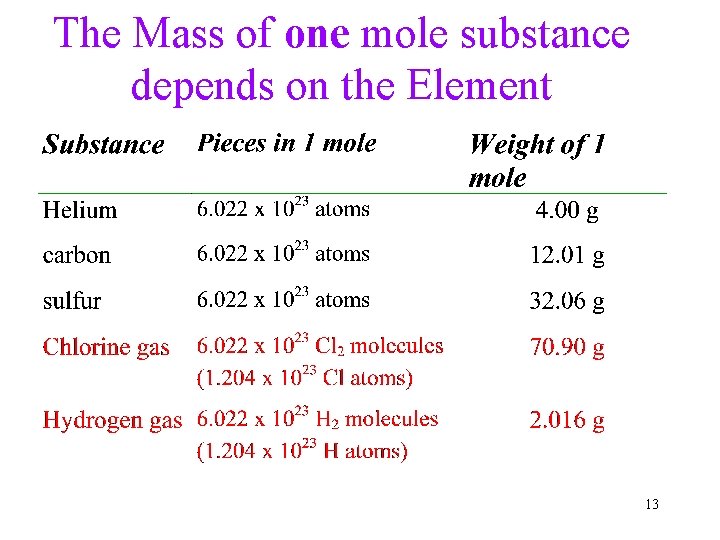
The Mass of one mole substance depends on the Element 13
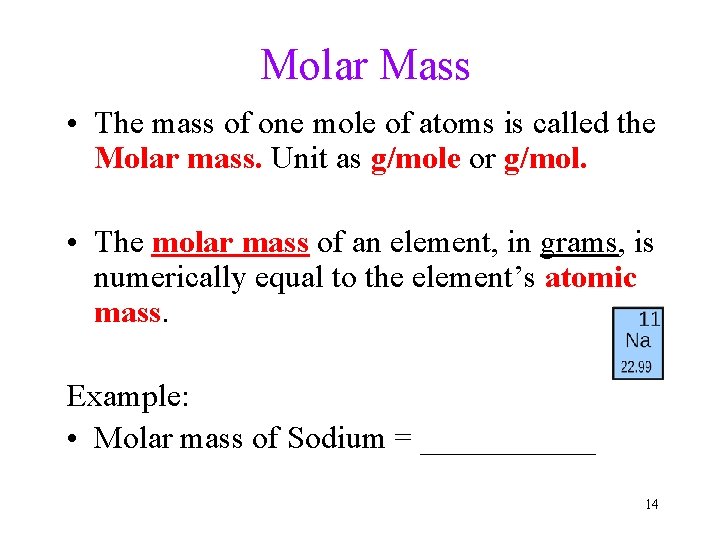
Molar Mass • The mass of one mole of atoms is called the Molar mass. Unit as g/mole or g/mol. • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass. Example: • Molar mass of Sodium = ______ 14

Molar Mass as Conversion Factor • Example: molar mass He = 4. 003 g So 1 mole He = 4. 003 gram He • Given number of Moles Mass (gram) = _________ • Given Mass Mole = __________ 15
Example: Calculate the number of moles of sulfur in 57. 8 g of sulfur Information Given: 57. 8 g S Find: ? moles S Conv. Fact. : 1 mole S = 32. 06 g 1. 80 moles S (3 sig. figs) 16
Example: Calculate the mass of 3. 34 x 10 -3 mole of helium gas. Information Given: 3. 34 x 10 -3 moles He Find: ? Grams of He Conv. Fact. : 1 mole He = 4. 00 g 0. 0133 g He 17
Practice: #atoms, moles, molar mass • The largest uncut diamond (pure carbon) weighs 755 carat (1 carat = 0. 2 grams exactly). How many moles of carbon atoms are in this diamond? 12. 6 mol C • What is mass for 1. 2 x 10 -3 mole gold? 0. 24 g mol Au • (optioinal) 70% of the mass of the Sun is hydrogen. The mass of the Sun is 1. 99 x 1030 kg. How many moles of hydrogen atoms are in the Sun? 1. 38 x 1033 mol H atom 18
Molar Mass and Avogadro’s Number • Since 1 mole contains Avogadro’s number of units whilst has a mass of molar mass • there is direct connection/conversion factor between Avogadro’s number and molar mass. Example: • 6. 022 x 1023 Al atoms = _______ g Al 19
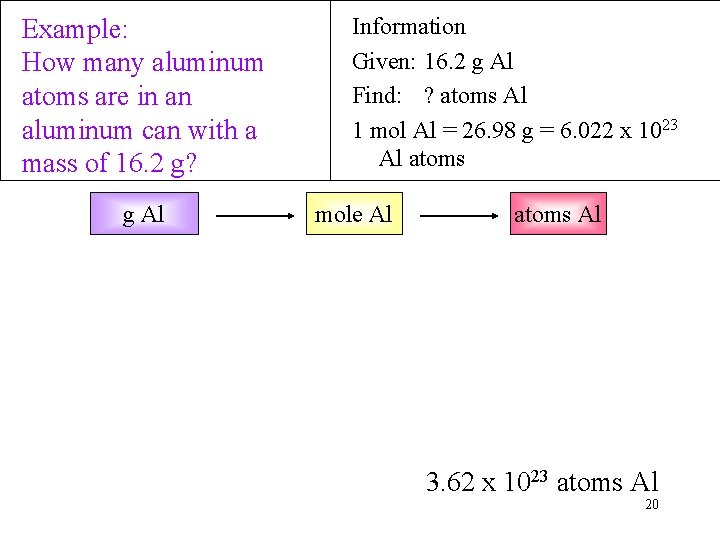
Example: How many aluminum atoms are in an aluminum can with a mass of 16. 2 g? g Al Information Given: 16. 2 g Al Find: ? atoms Al 1 mol Al = 26. 98 g = 6. 022 x 1023 Al atoms mole Al atoms Al 3. 62 x 1023 atoms Al 20

Example: What is the mass of 1. 34 x 1020 silver atoms? Given: 1. 34 x 1020 Ag Find: ? grams Ag 1 mol Ag = 107. 87 g Ag = 6. 022 x 1023 Ag atoms SM: atoms mol g 0. 0240 g Ag 21
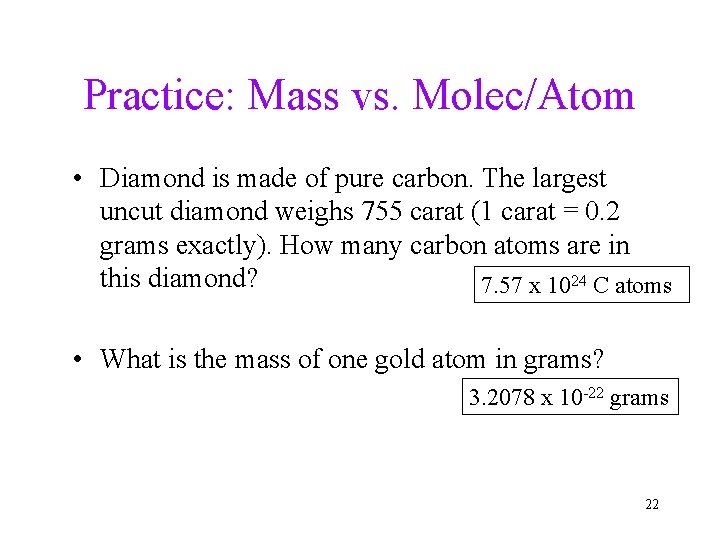
Practice: Mass vs. Molec/Atom • Diamond is made of pure carbon. The largest uncut diamond weighs 755 carat (1 carat = 0. 2 grams exactly). How many carbon atoms are in this diamond? 7. 57 x 1024 C atoms • What is the mass of one gold atom in grams? 3. 2078 x 10 -22 grams 22
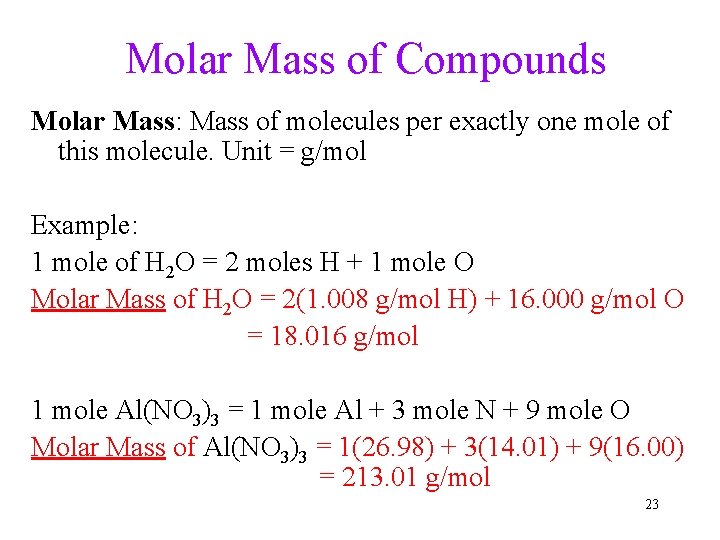
Molar Mass of Compounds Molar Mass: Mass of molecules per exactly one mole of this molecule. Unit = g/mol Example: 1 mole of H 2 O = 2 moles H + 1 mole O Molar Mass of H 2 O = 2(1. 008 g/mol H) + 16. 000 g/mol O = 18. 016 g/mol 1 mole Al(NO 3)3 = 1 mole Al + 3 mole N + 9 mole O Molar Mass of Al(NO 3)3 = 1(26. 98) + 3(14. 01) + 9(16. 00) = 213. 01 g/mol 23

Practice: Find the Molar Mass • • • Sulfuric acid Aluminum sulfate Ammonium chlorite Hydrobromic acid Nickel(II) phosphate 24

Mass of Compound ↔ Moles of Compound
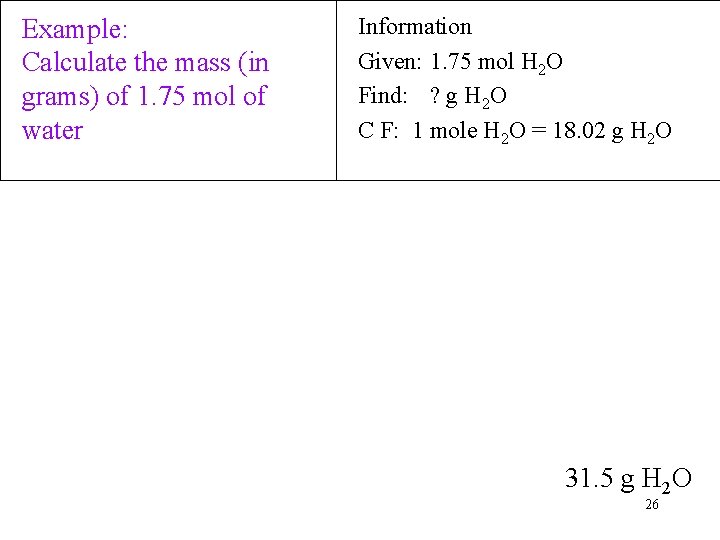
Example: Calculate the mass (in grams) of 1. 75 mol of water Information Given: 1. 75 mol H 2 O Find: ? g H 2 O C F: 1 mole H 2 O = 18. 02 g H 2 O 31. 5 g H 2 O 26
Example: Calculate the mole of CO 2 in 454 g dry ice (solid carbon dioxide). Information Given: 454 g CO 2 Find: ? mol CO 2 C F: 1 mole CO 2 = 44. 01 g CO 2 10. 3 mol CO 2 27
Mass of Compound ↔ Number of molecules
Example: Find the mass of 4. 8 x 1024 NO 2 molecules Information Given: 4. 8 x 1024 molec NO 2 Find: ? g NO 2 1 mole NO 246. 01 g NO 2 3. 7 x 102 g 29
Example: How many water molecules in 1. 00 m. L DI water? Information Given: 1. 00 m. L H 2 O Find: #H 2 O molecules 1 mole H 2 O = 18. 02 g H 2 O Density of water = ______ g/m. L 3. 34 x 1022 H 2 O molecules 30

Practice • Online: http: //www. sciencegeek. net/Chemistry/taters/Unit 4 Gram. Mole. Volume. htm • How many moles of water in 10. 00 m. L pure water if the density of water is 1. 00 g/m. L? • Determine the mass of 2. 0 × 103 mole Na. Cl. 31
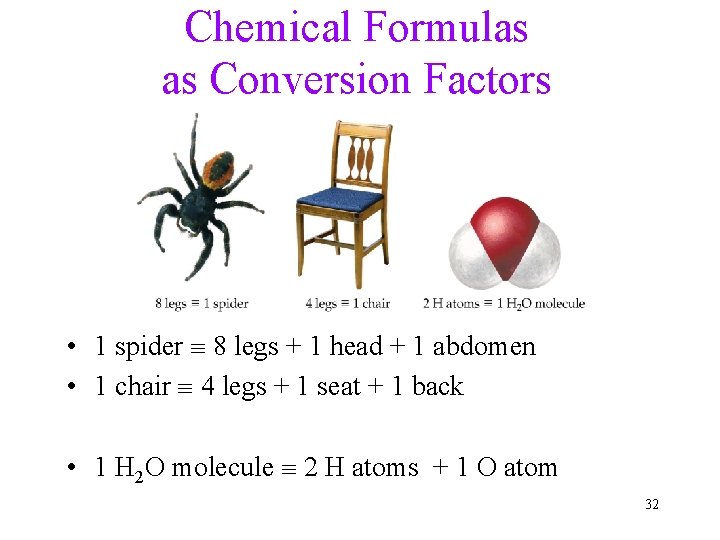
Chemical Formulas as Conversion Factors • 1 spider 8 legs + 1 head + 1 abdomen • 1 chair 4 legs + 1 seat + 1 back • 1 H 2 O molecule 2 H atoms + 1 O atom 32
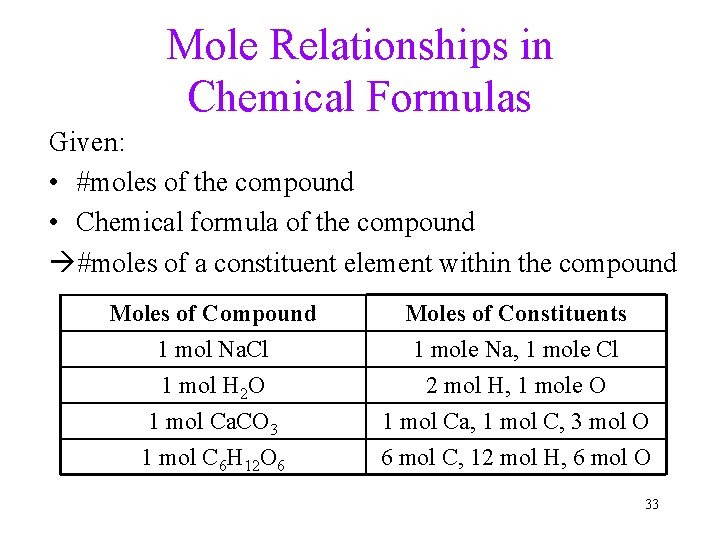
Mole Relationships in Chemical Formulas Given: • #moles of the compound • Chemical formula of the compound #moles of a constituent element within the compound Moles of Compound 1 mol Na. Cl 1 mol H 2 O 1 mol Ca. CO 3 Moles of Constituents 1 mole Na, 1 mole Cl 2 mol H, 1 mole O 1 mol Ca, 1 mol C, 3 mol O 1 mol C 6 H 12 O 6 6 mol C, 12 mol H, 6 mol O 33
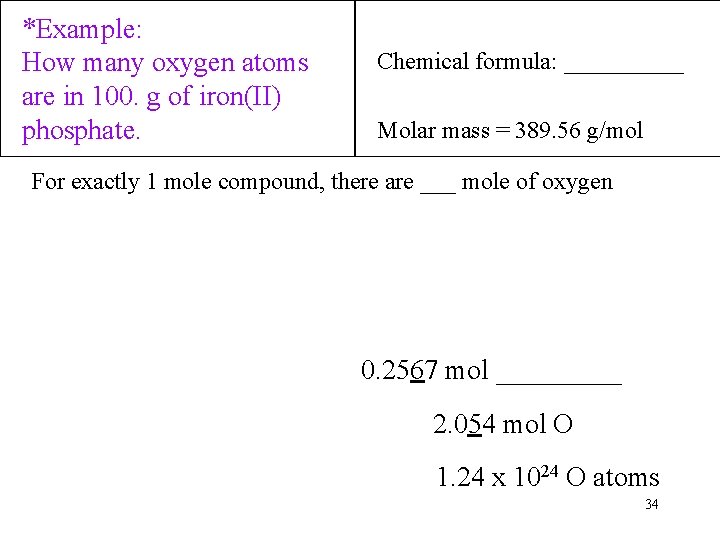
*Example: How many oxygen atoms are in 100. g of iron(II) phosphate. Chemical formula: _____ Molar mass = 389. 56 g/mol For exactly 1 mole compound, there are ___ mole of oxygen 0. 2567 mol _____ 2. 054 mol O 1. 24 x 1024 O atoms 34
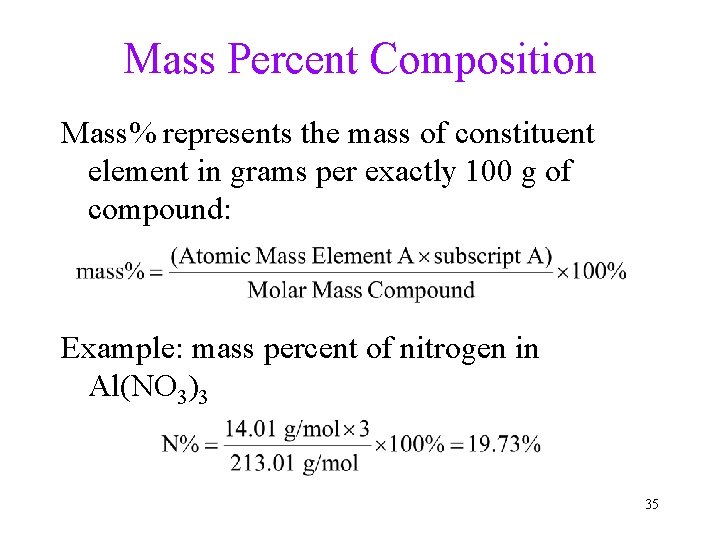
Mass Percent Composition Mass% represents the mass of constituent element in grams per exactly 100 g of compound: Example: mass percent of nitrogen in Al(NO 3)3 35
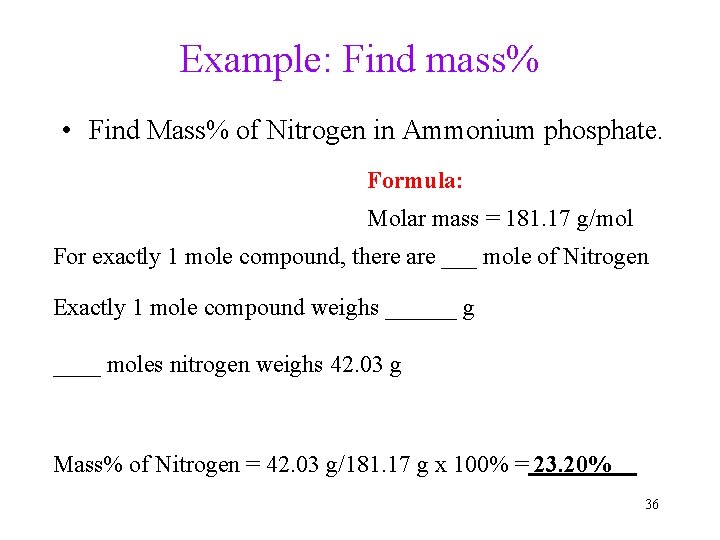
Example: Find mass% • Find Mass% of Nitrogen in Ammonium phosphate. Formula: Molar mass = 181. 17 g/mol For exactly 1 mole compound, there are ___ mole of Nitrogen Exactly 1 mole compound weighs ______ g ____ moles nitrogen weighs 42. 03 g Mass% of Nitrogen = 42. 03 g/181. 17 g x 100% = 23. 20% 36
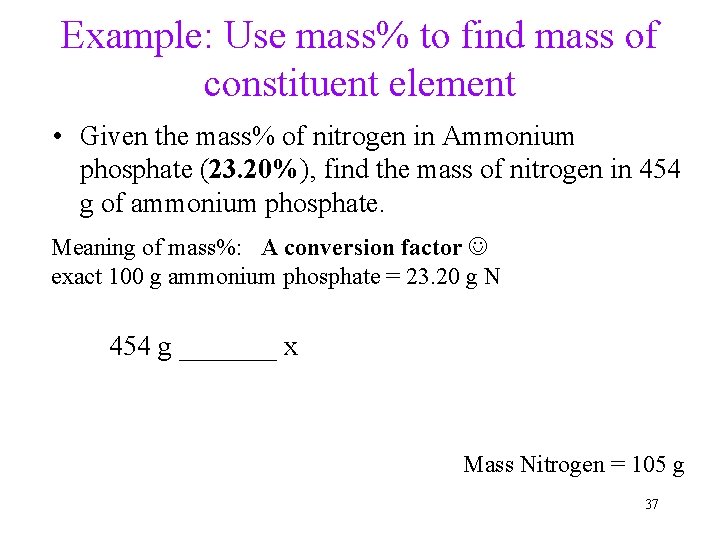
Example: Use mass% to find mass of constituent element • Given the mass% of nitrogen in Ammonium phosphate (23. 20%), find the mass of nitrogen in 454 g of ammonium phosphate. Meaning of mass%: A conversion factor exact 100 g ammonium phosphate = 23. 20 g N 454 g _______ x Mass Nitrogen = 105 g 37
Empirical Formula Chemical Formula Mass% of each element Chemical Formula? Empirical Formula: The simplest, whole-number ratio of atoms in a molecule • It shows the mole ratio in the compound • It can be determined from percent composition or combining masses 38
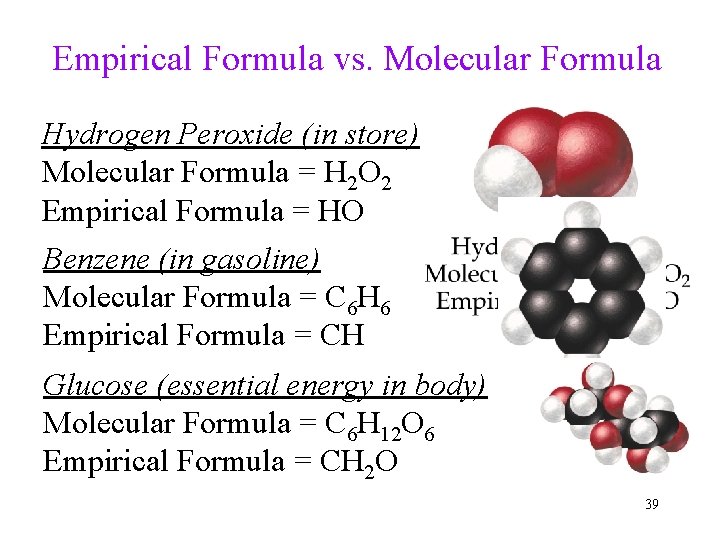
Empirical Formula vs. Molecular Formula Hydrogen Peroxide (in store) Molecular Formula = H 2 O 2 Empirical Formula = HO Benzene (in gasoline) Molecular Formula = C 6 H 6 Empirical Formula = CH Glucose (essential energy in body) Molecular Formula = C 6 H 12 O 6 Empirical Formula = CH 2 O 39
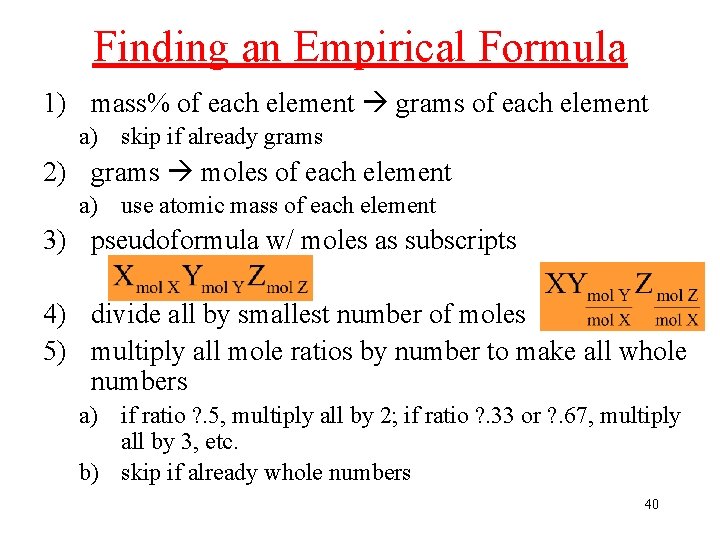
Finding an Empirical Formula 1) mass% of each element grams of each element a) skip if already grams 2) grams moles of each element a) use atomic mass of each element 3) pseudoformula w/ moles as subscripts 4) divide all by smallest number of moles 5) multiply all mole ratios by number to make all whole numbers a) if ratio ? . 5, multiply all by 2; if ratio ? . 33 or ? . 67, multiply all by 3, etc. b) skip if already whole numbers 40
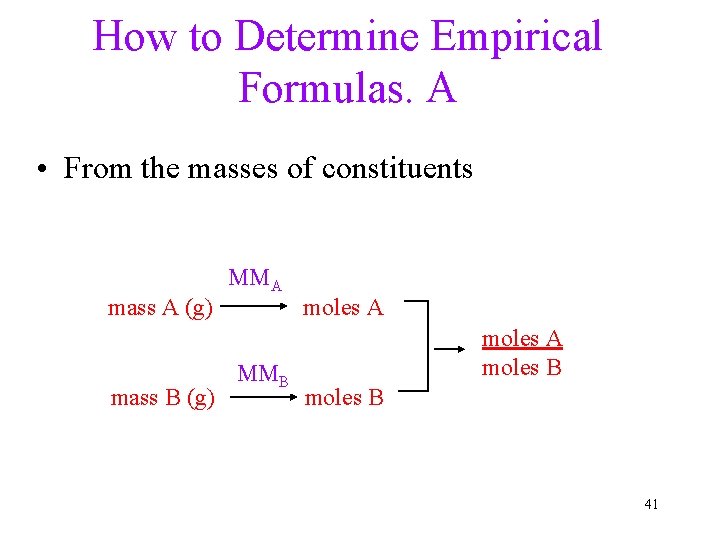
How to Determine Empirical Formulas. A • From the masses of constituents MMA mass A (g) moles A MMB mass B (g) moles B moles A moles B 41
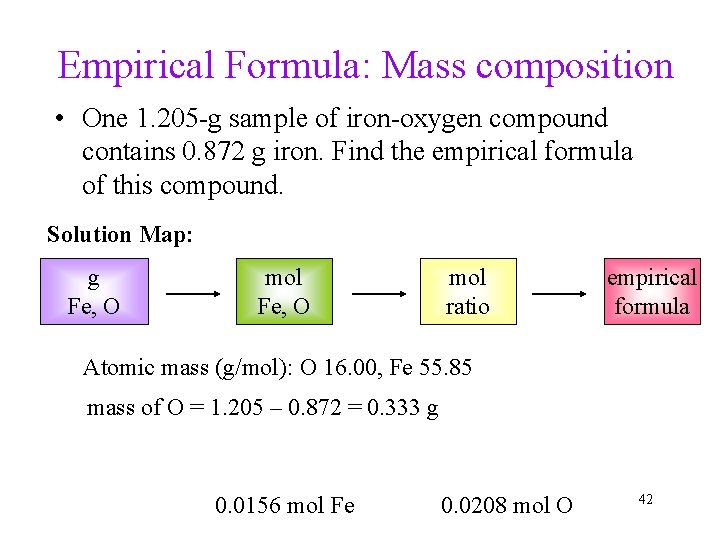
Empirical Formula: Mass composition • One 1. 205 -g sample of iron-oxygen compound contains 0. 872 g iron. Find the empirical formula of this compound. Solution Map: g Fe, O mol ratio empirical formula Atomic mass (g/mol): O 16. 00, Fe 55. 85 mass of O = 1. 205 – 0. 872 = 0. 333 g 0. 0156 mol Fe 0. 0208 mol O 42
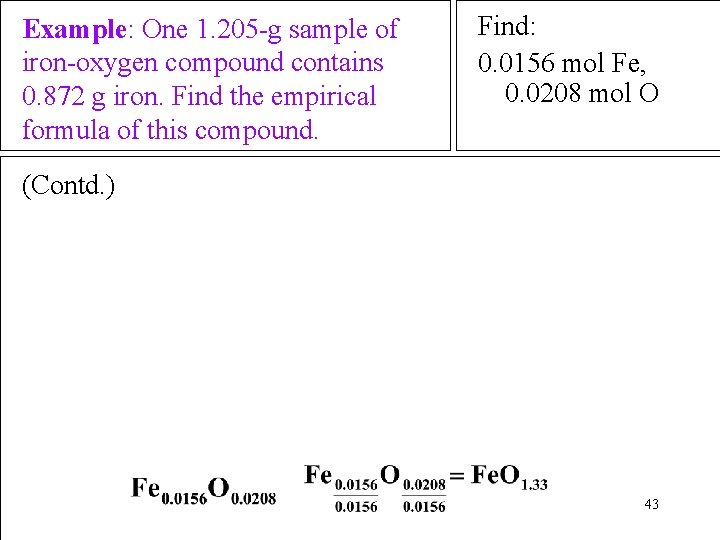
Example: One 1. 205 -g sample of iron-oxygen compound contains 0. 872 g iron. Find the empirical formula of this compound. Find: 0. 0156 mol Fe, 0. 0208 mol O (Contd. ) 43
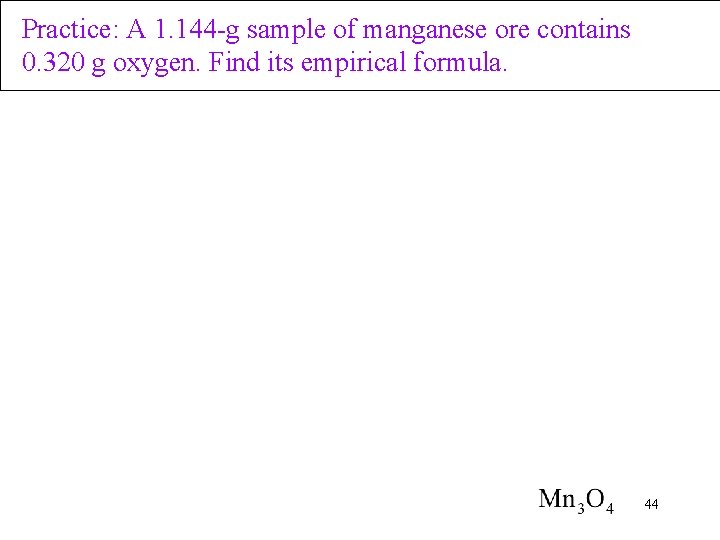
Practice: A 1. 144 -g sample of manganese ore contains 0. 320 g oxygen. Find its empirical formula. 44
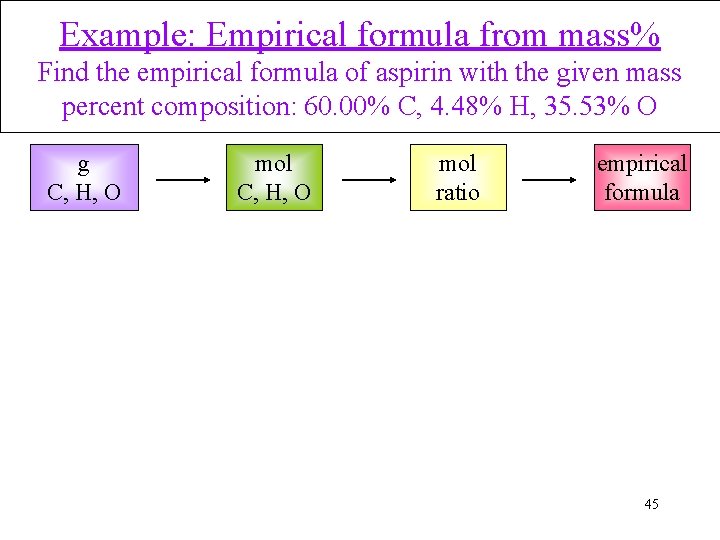
Example: Empirical formula from mass% Find the empirical formula of aspirin with the given mass percent composition: 60. 00% C, 4. 48% H, 35. 53% O g C, H, O mol ratio empirical formula 45
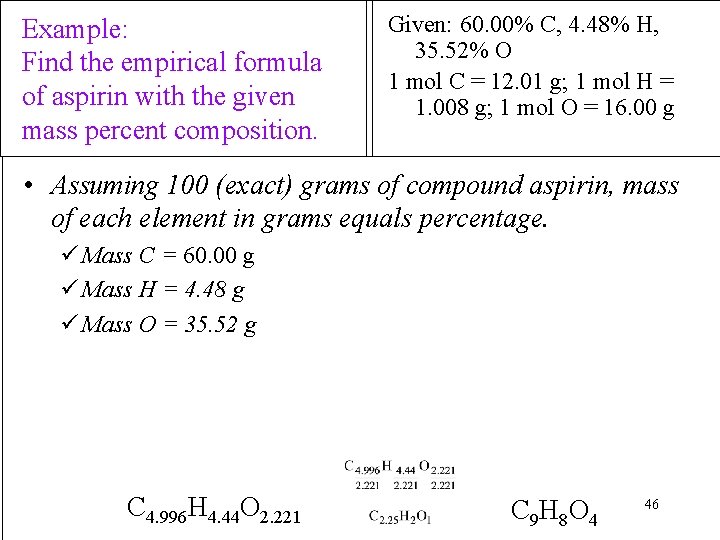
Example: Find the empirical formula of aspirin with the given mass percent composition. Given: 60. 00% C, 4. 48% H, 35. 52% O 1 mol C = 12. 01 g; 1 mol H = 1. 008 g; 1 mol O = 16. 00 g • Assuming 100 (exact) grams of compound aspirin, mass of each element in grams equals percentage. ü Mass C = 60. 00 g ü Mass H = 4. 48 g ü Mass O = 35. 52 g C 4. 996 H 4. 44 O 2. 221 C 9 H 8 O 4 46
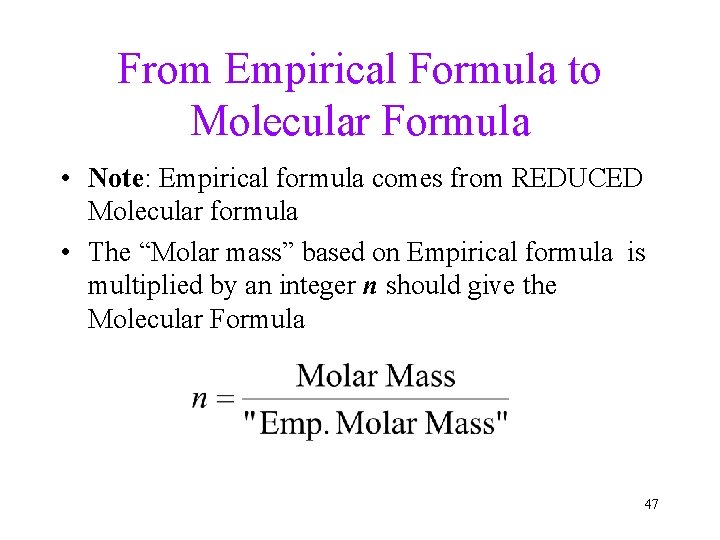
From Empirical Formula to Molecular Formula • Note: Empirical formula comes from REDUCED Molecular formula • The “Molar mass” based on Empirical formula is multiplied by an integer n should give the Molecular Formula 47
Example: Find Empirical Formula and Molecular Formula • Vitamic C has the following mass percent composition: C 40. 91%, H 4. 58%, O 54. 41%. Find the empirical formula. ü Ans: C 3 H 4 O 3 • The molar mass of vitamin C is 176. 1 g/mol. Find the molecular formula of vitamin C. ü Ans: solve n = 2, so molecular formula C 6 H 8 O 6 48
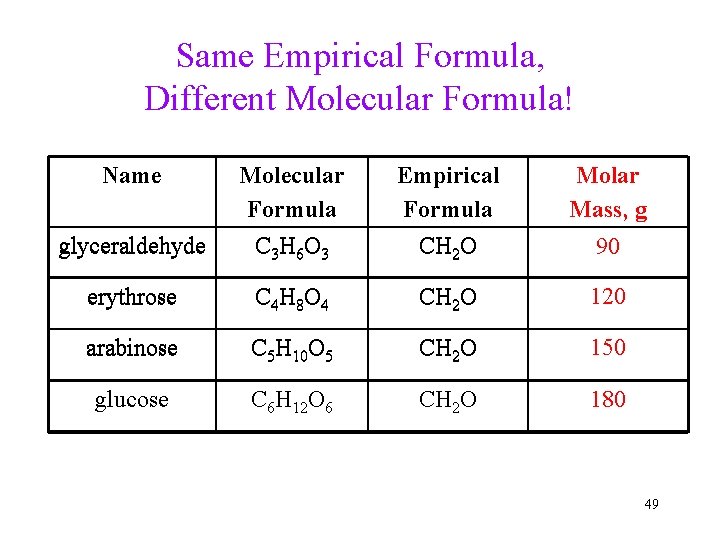
Same Empirical Formula, Different Molecular Formula! Name Empirical Formula CH 2 O Molar Mass, g glyceraldehyde Molecular Formula C 3 H 6 O 3 erythrose C 4 H 8 O 4 CH 2 O 120 arabinose C 5 H 10 O 5 CH 2 O 150 glucose C 6 H 12 O 6 CH 2 O 180 90 49

Answer key: Molar Mass (all in g/mol) • • • Sulfuric acid: 98. 09 g/mol Aluminum sulfate: 342. 17 g/mol Ammonium chlorite: 181. 70 g/mol Hydrobromic acid: 80. 91 g/mol Nickel(II) phosphate: 366. 01 g/mol 50
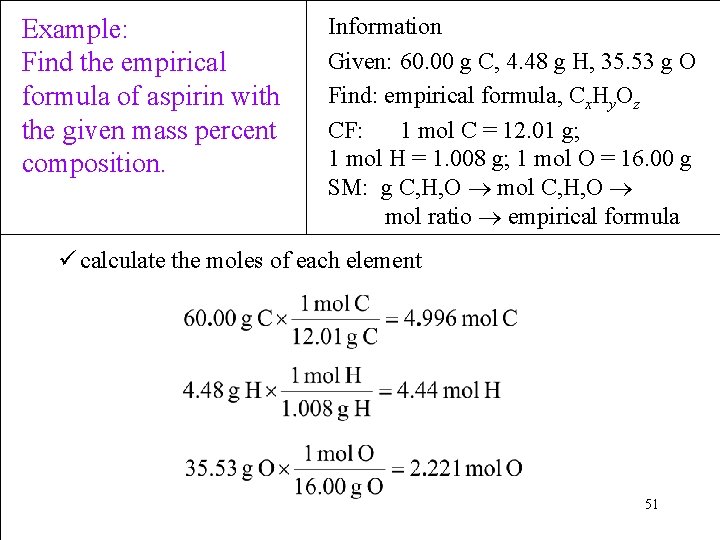
Example: Find the empirical formula of aspirin with the given mass percent composition. Information Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz CF: 1 mol C = 12. 01 g; 1 mol H = 1. 008 g; 1 mol O = 16. 00 g SM: g C, H, O mol ratio empirical formula ü calculate the moles of each element 51
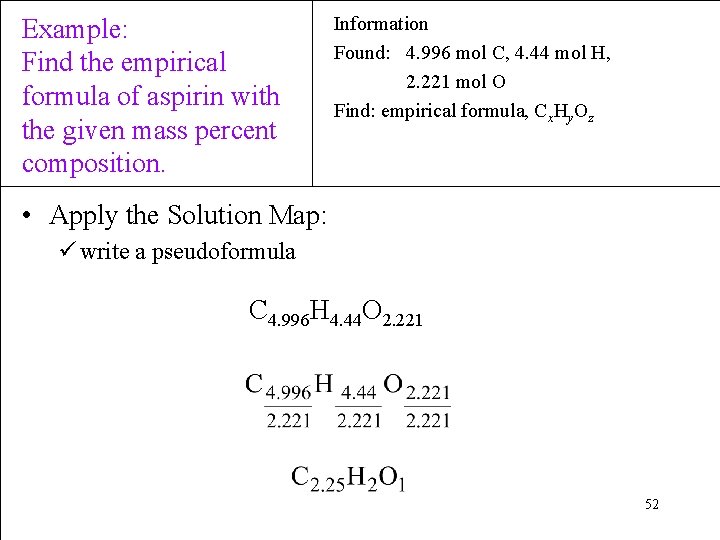
Example: Find the empirical formula of aspirin with the given mass percent composition. Information Found: 4. 996 mol C, 4. 44 mol H, 2. 221 mol O Find: empirical formula, Cx. Hy. Oz • Apply the Solution Map: ü write a pseudoformula C 4. 996 H 4. 44 O 2. 221 52
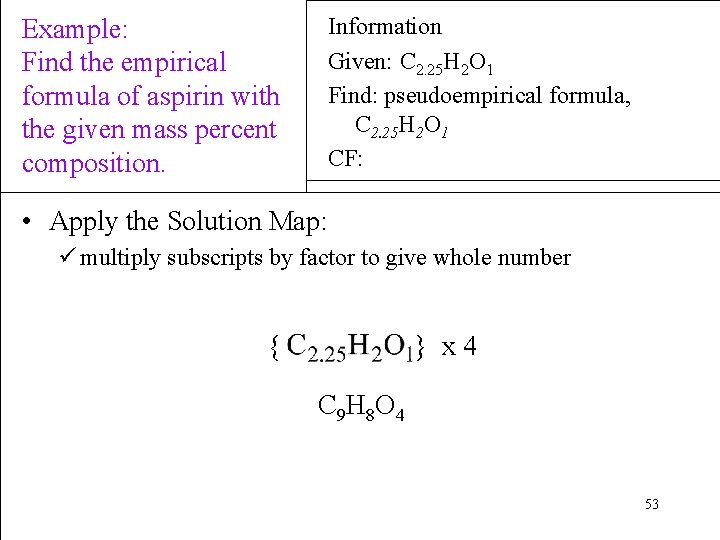
Example: Find the empirical formula of aspirin with the given mass percent composition. Information Given: C 2. 25 H 2 O 1 Find: pseudoempirical formula, C 2. 25 H 2 O 1 CF: • Apply the Solution Map: ü multiply subscripts by factor to give whole number { } x 4 C 9 H 8 O 4 53
- Slides: 53