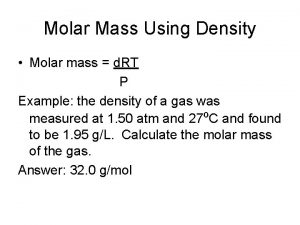
Molar Mass Molar mass means to calculate the


























- Slides: 32

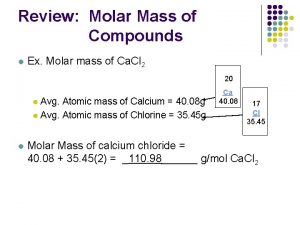
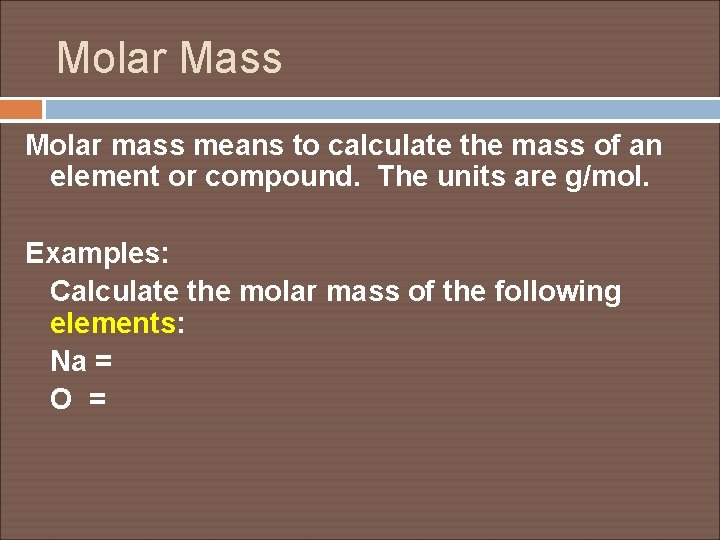
Molar Mass Molar mass means to calculate the mass of an element or compound. The units are g/mol. Examples: Calculate the molar mass of the following elements: Na = O =

Calculate molar mass for the following compounds: Na. Cl = Ca(OH)2 = Copper(II) nitride = Aluminum sulfate =

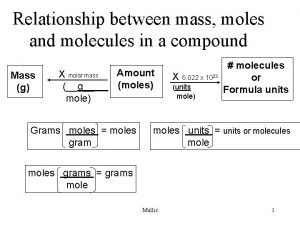
Converting to Moles What is a mole? A unit of measurement. How do I calculate the amount of moles? Use the molar mass.

How do I convert to moles? Use the molar mass; this is the purpose of calculating it in the first place!

Create a Flow Chart Grams moles

Converting to Moles 1. Determine the number of moles of Na. Cl in 50 g of Na. Cl.

Converting to Moles 2. You are given 362. 8 g of the compound C 5 H 12. Determine the number of moles.

Converting to Moles 3. How many moles are in 25 g of water?

Try on Your Own 4. 5. 6. Determine the moles in 5 g of sodium oxide. Determine the moles in 10 g of iron (III) chloride. Determine the moles of C 3 H 8 in 2. 3 g of C 3 H 8.

Converting to Grams This is just the reverse process Grams moles 7. Determine the mass of 1. 112 mol of sodium fluoride.

Moles to Grams 8. Find the mass of 0. 89 mol of Ca. Cl 2.

Moles to Grams 9. How many grams are in 4. 50 moles of lithium oxide?

Practice: Show all your work! 1. 64 g Na. Cl = _____mol 2. 10. 2 mol Sr = ______g 3. 0. 49 g iron (III) chloride = ____mol 4. Calculate the moles in your copper sample. Show all work. Number of sample_____ Moles =____

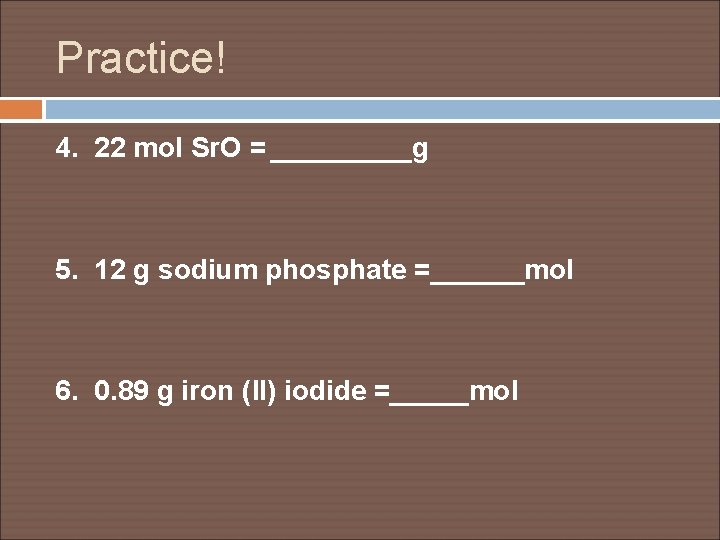
Practice! 4. 22 mol Sr. O = _____g 5. 12 g sodium phosphate =______mol 6. 0. 89 g iron (II) iodide =_____mol

Recognize the pattern! There is only one of two ways to solve these problems.

Practice! 34. . 05 mole lead (IV) sulfate = __g 35. 16 g silver cyanide = __mol 36. 25. 3 mol vanadium (V) nitride =_g 37. 100 g strontium acetate = _mol

On Your Own 38. 5 g molybdenum sulfate = _mol 39. 325 mol platinum (II) sulfide = _g 40. 26 g ammonium sulfate = _mol

Exit Questions 1. 2. 3. Calculate the grams in 33 moles of Ba. Cl 2. Name this compound. Is this an ionic compound or a covalent compound? Explain how to tell the two types of bonding apart?

Measurement Activity Measurement of Grams and Moles Using the copper sample provided (copper #_____), determine the number of moles in the sample. Weigh out. 05 moles of sugar (C 6 H 12 O 6). Weigh out. 03 moles of aluminum.

Avogadro’s Number Avogadro’s number relates moles to atoms 6. 02 x 1023 atoms in 1 mole OR 6. 02 x 1023 atoms = 1 mole

Here is what that looks like! 6020000000. atoms in 1 mole

Using Avogadro’s Number 1. How many moles are in 23 3. 45 x 10 atoms of H 2 SO 4?

Example 2 2. Calculate the amount of moles contained in a salt dispenser that has 23. 5 x 1023 atoms?

Example 3 3. How many atoms are in 67 moles of Ca. Cl 2?

On Your Own! 1. 2. How many atoms are in 32 moles of ammonia (NH 3)? How many atoms are in 23 moles of propane (C 3 H 8)?

Going from Grams->moles->atoms 1. How many atoms are in 22 g of salt?

Going from Grams->moles->atoms 2. Calculate the number of molecules in 72. 3 g of potassium sulfide.

Equations and Stoichiometry 1. A solution of sodium hydroxide is reacted with hydrochloric acid. Given that the reaction is started with 2 g of sodium hydroxide, what mass of water can be expected?

Equations and Stoichiometry 2. Nitrogen gas and hydrogen gas combine to make ammonia. Given 5 grams of hydrogen, what mass of ammonia is produced?

Equations and Stoichiometry 3. Magnesium and hydrofluoric acid react in a beaker. If the reaction is started with 55 g of hydrofluoric acid, what mass of magnesium fluoride can be produced?

Exit Question Glucose is burned in the presence of air. If the reaction is started with 0. 0103 moles of oxygen, how much glucose is required for the reaction?

Metric and Moles 4. 6. 8 g Mg. CO 3 = _______mol 5. 84. 6 dg Hg. O = _______mol 6. 74 mol KOH = _____Dg 7. 0. 52 mg Mg. S = ____mol
 What is the molar mass equation
What is the molar mass equation Calculate moles from grams
Calculate moles from grams How to find molar mass
How to find molar mass How to calculate molar mass from freezing point
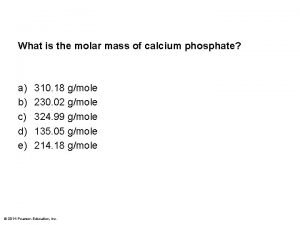
How to calculate molar mass from freezing point Calculate the molar mass of calcium phosphate
Calculate the molar mass of calcium phosphate Volume to mols
Volume to mols Mass to moles conversion factor
Mass to moles conversion factor Molar mass units
Molar mass units Converting mass to moles
Converting mass to moles Unit of molar mass
Unit of molar mass Formula mass vs molar mass
Formula mass vs molar mass Atomic mass vs molar mass
Atomic mass vs molar mass What is molar enthalpy of fusion
What is molar enthalpy of fusion Triangle quadrilateral pentagon hexagon
Triangle quadrilateral pentagon hexagon Morphe means in metamorphism
Morphe means in metamorphism Meta and morph means
Meta and morph means Biodiversity conservation definition
Biodiversity conservation definition Bio means life logy means
Bio means life logy means الكتلة المولية
الكتلة المولية N=pv/rt
N=pv/rt Is atomic mass a whole number
Is atomic mass a whole number Cl- molar mass
Cl- molar mass What are the units for molar mass
What are the units for molar mass Barium phosphite formula
Barium phosphite formula How to get mass percent
How to get mass percent Molar mass of potassium permanganate
Molar mass of potassium permanganate Moles of atoms
Moles of atoms How to calcuate moles
How to calcuate moles Molar mass of fructose
Molar mass of fructose Molar mass of butane
Molar mass of butane Cl- molar mass
Cl- molar mass Carbon dioxide symbol
Carbon dioxide symbol Calculate it
Calculate it