AVOGADROS LAW WHAT IS AVOGADROS LAW v Avogadros









- Slides: 10

AVOGADRO’S LAW

WHAT IS AVOGADRO’S LAW v Avogadro’s Principle – equal volumes of gases at the same temperature and pressure contain equal numbers of particles

AVOGADRO’S FORMULA * n represents the amount of gas V 1= V 2 n 1 n 2 This is a direct relationship! v So if the amount of gas increases, then the volume will increase ______. If the amount of gas decreases, then the decreas e volume will _____.

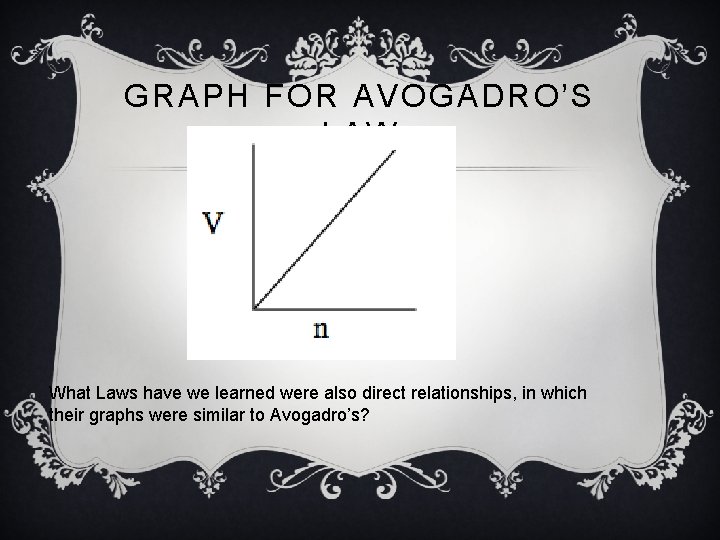
GRAPH FOR AVOGADRO’S LAW What Laws have we learned were also direct relationships, in which their graphs were similar to Avogadro’s?


AVOGADRO’S LAW v. Molar Volume – for a gas is the volume that one mole of that gas occupies at STP Avogadro showed experimentally that 1 mole of any gas will occupy a volume of 22. 4 L at STP **Conversion Factor: 1 mol (any gas) = 22. 4 L at STP **

AVOGADRO’S LAW: EXAMPLE 1 Calculate the volume that 0. 881 moles of oxygen gas at STP will occupy.

AVOGADRO’S LAW: EXAMPLE 1 ANSWER v Formula: V 1 = V 2 n 1 V 1 = ? L n 1 = 0. 881 mol O 2 V 2 = 22. 4 L n 2 = 1 mol O 2 n 2 Remember, at STP: 22. 4 L/mol V 1. 881 mol O 2 = 22. 4 L 1 mol O 2 After cross multiplying you end up with ---------V 1(1 mol O 2) = (22. 4 L)(. 881 -----mol O 2) -----1 mol O 2 19. 7 L 1 mol O 2 V 1 = ______

AVOGADRO’S LAW: EXAMPLE 2 How many grams of N 2 will be contained in a 2. 0 L flask at STP? (Remember- one mole WEIGHS the formula mass in grams)

AVOGADRO’S LAW: EXAMPLE 2 ANSWERRemember, at STP: 22. 4 L/mol v Formula: V 1 = V 2 n 1 First , solve for the number of moles of N 2: 2 L N 2 | 1 mol N 2 | 22. 4 L N 2 =. 089 n 2 V 1 = 2. 0 L n 1 = ? g N 2 V 2 = 22. 4 L n 2 = 1 mol N 2 Then, use dimensional analysis to convert from moles of N 2 to grams of N 2: . 089 mol N 2 | 28. 014 g N 2 = | 1 mol N 2 2. 5 g N 2
 Avogadro’s law
Avogadro’s law Lesson 64 stp the mole and avogadro law answer key
Lesson 64 stp the mole and avogadro law answer key Avogadro's law example
Avogadro's law example What is the symbol of the avogadro’s number?
What is the symbol of the avogadro’s number? Percent mass formula
Percent mass formula Avogadros tal formel
Avogadros tal formel What is avogadro's number used for
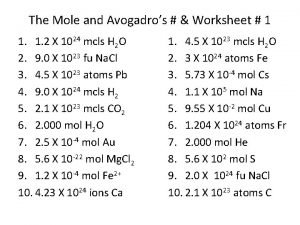
What is avogadro's number used for The mole and avogadro's law worksheet answers
The mole and avogadro's law worksheet answers What is avogadros hypothesis
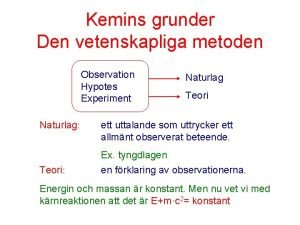
What is avogadros hypothesis Syra bas reaktion
Syra bas reaktion Avogadros principle
Avogadros principle