Ideal Gas Law 1 Avogadros Principle n How



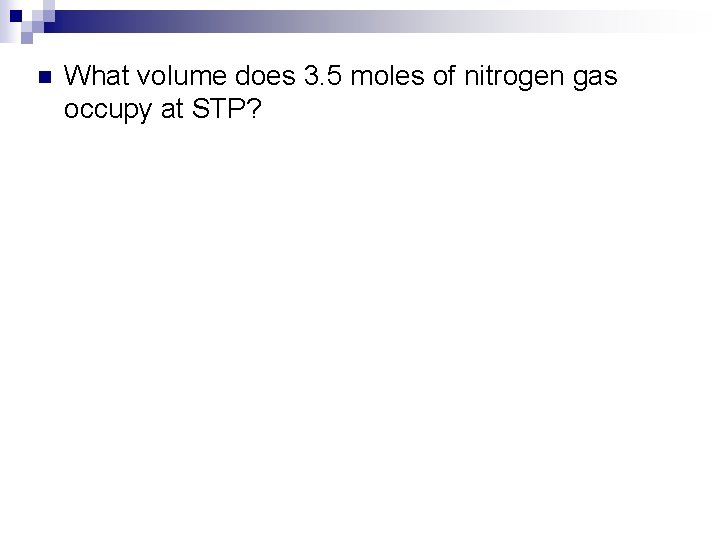
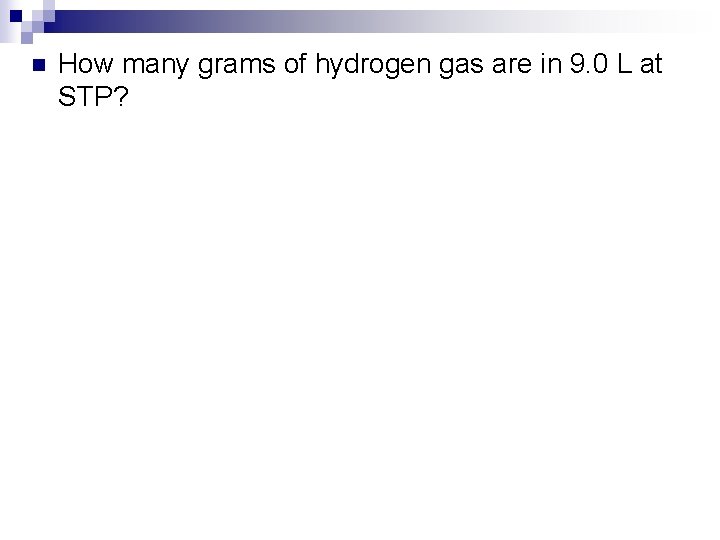





















- Slides: 46

Ideal Gas Law

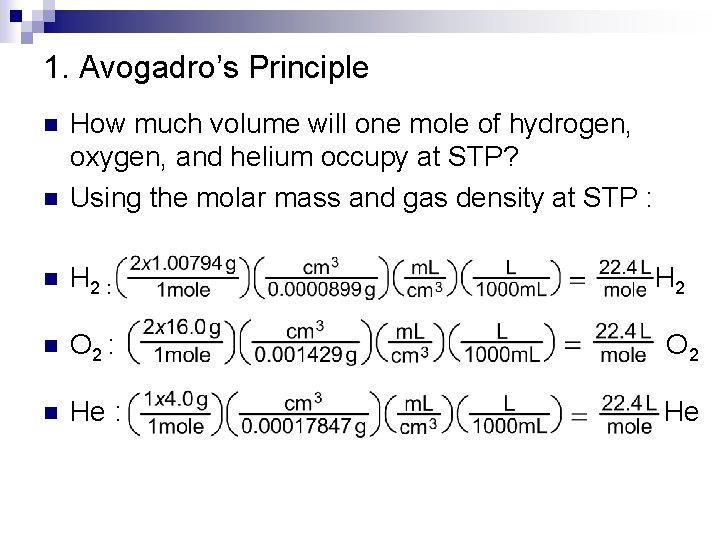
1. Avogadro’s Principle n How much volume will one mole of hydrogen, oxygen, and helium occupy at STP? Using the molar mass and gas density at STP : n H 2 : H 2 n O 2 : O 2 n He : He n

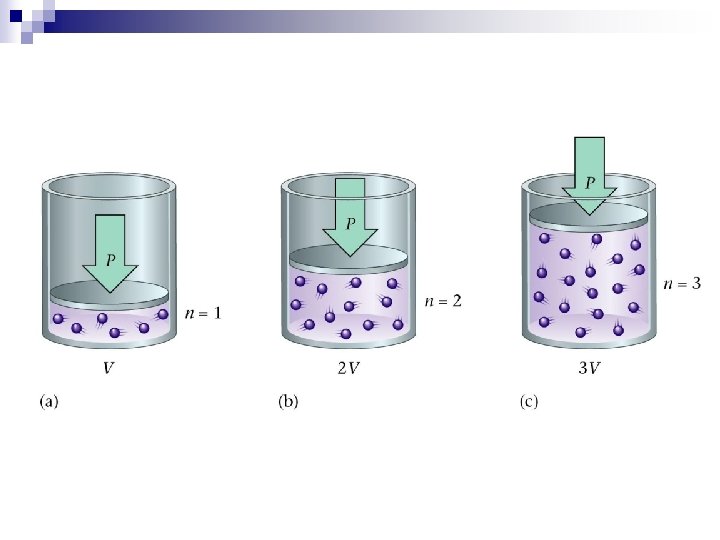
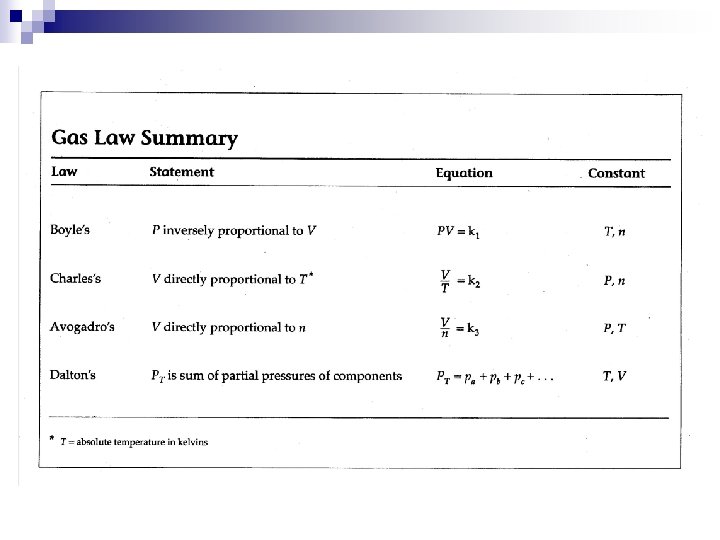
n n n It appears that : ¨ 1 mole of a gas, at STP occupies 22. 4 L ¨ STP – standard temperature and pressure ¨ STP - 0ºC, 101. 3 k. Pa (1 atm, 760 mm. Hg) ¨ 6. 02 x 1023 gas particles, at STP, 22. 4 L Avogadro’s Principle – At equal temperature and pressure, equal volumes of gases contain equal number of molecules. = constant V = volume, n = moles


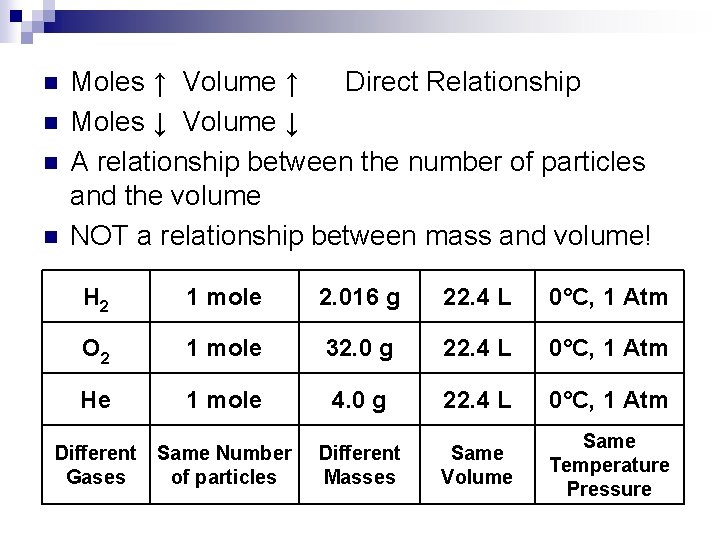
n n Moles ↑ Volume ↑ Direct Relationship Moles ↓ Volume ↓ A relationship between the number of particles and the volume NOT a relationship between mass and volume! H 2 1 mole 2. 016 g 22. 4 L 0°C, 1 Atm O 2 1 mole 32. 0 g 22. 4 L 0°C, 1 Atm He 1 mole 4. 0 g 22. 4 L 0°C, 1 Atm Different Gases Same Number of particles Different Masses Same Volume Same Temperature Pressure

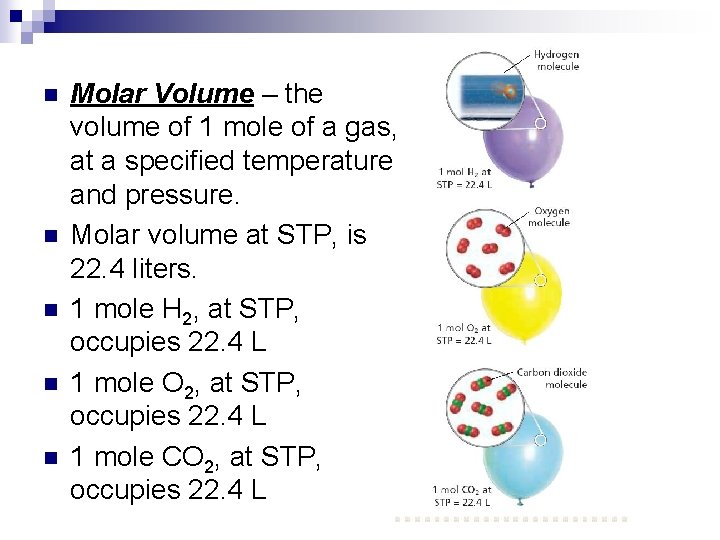
n n n Molar Volume – the volume of 1 mole of a gas, at a specified temperature and pressure. Molar volume at STP, is 22. 4 liters. 1 mole H 2, at STP, occupies 22. 4 L 1 mole O 2, at STP, occupies 22. 4 L 1 mole CO 2, at STP, occupies 22. 4 L

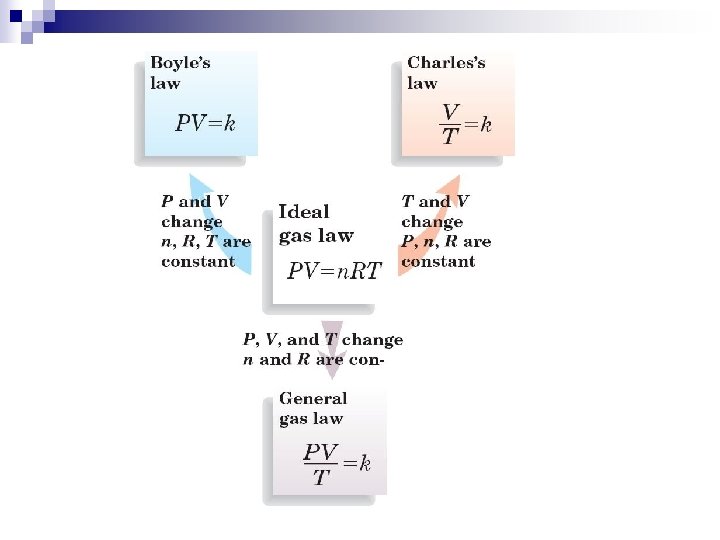
2. Ideal Gas Law n Avogadro’s Principle : = constant ¨ V = volume n = moles n Combined Gas Law : n Ideal Gas Law : or PV = n. RT Where R = Ideal Gas Constant



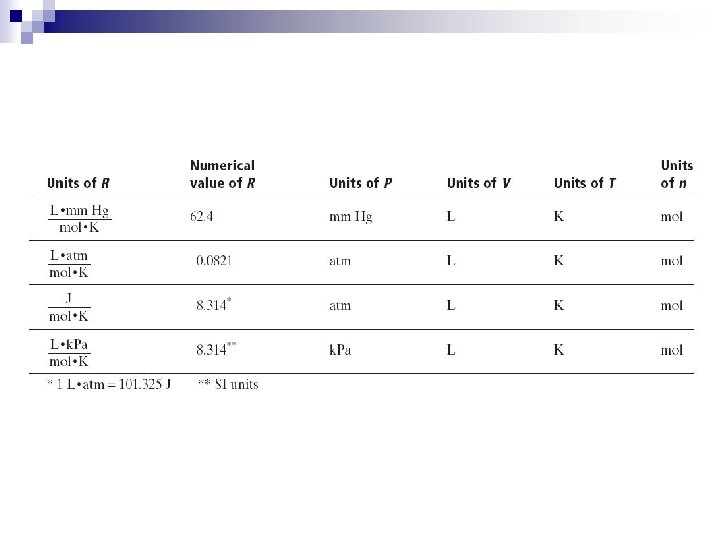
n PV = n. RT ¨ P = pressure, kilopascals, k. Pa ¨ V = volume, Liters, L ¨ n = number of moles n (convert grams to moles using Molar Mass) ¨ T = temperature, kelvin, K ¨ R = Ideal Gas Constant, n Convert all units to the above, and you will only have to memorize one ideal gas constant!


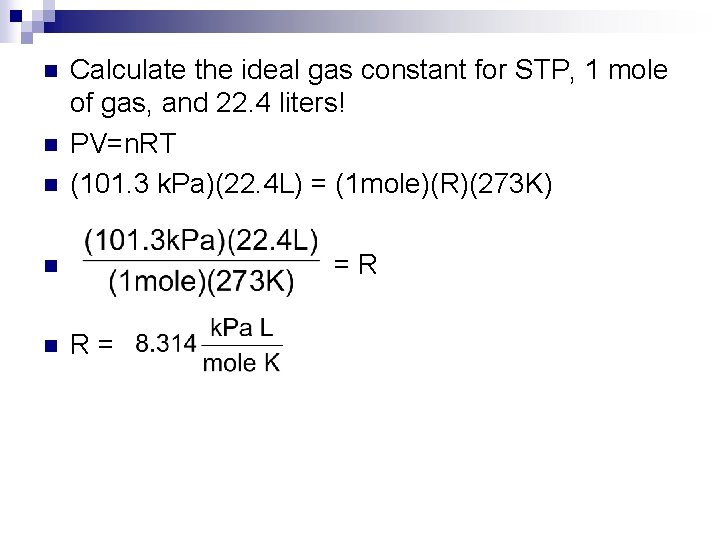
n Calculate the ideal gas constant for STP, 1 mole of gas, and 22. 4 liters! PV=n. RT (101. 3 k. Pa)(22. 4 L) = (1 mole)(R)(273 K) n = R n R = n n

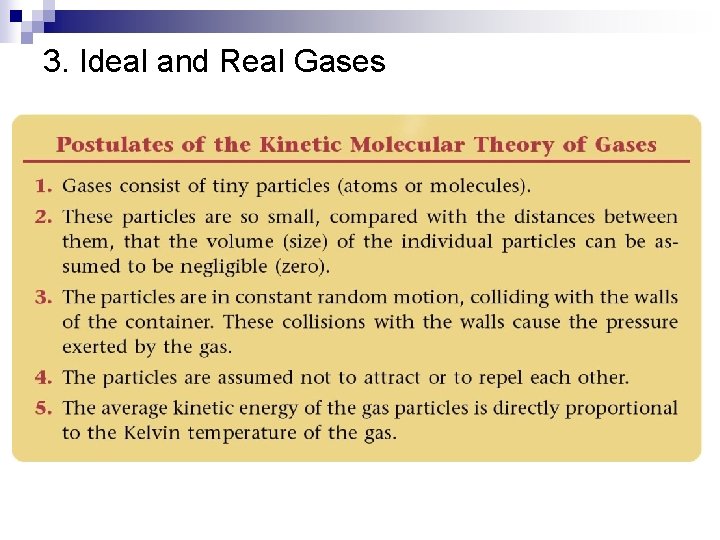
3. Ideal and Real Gases
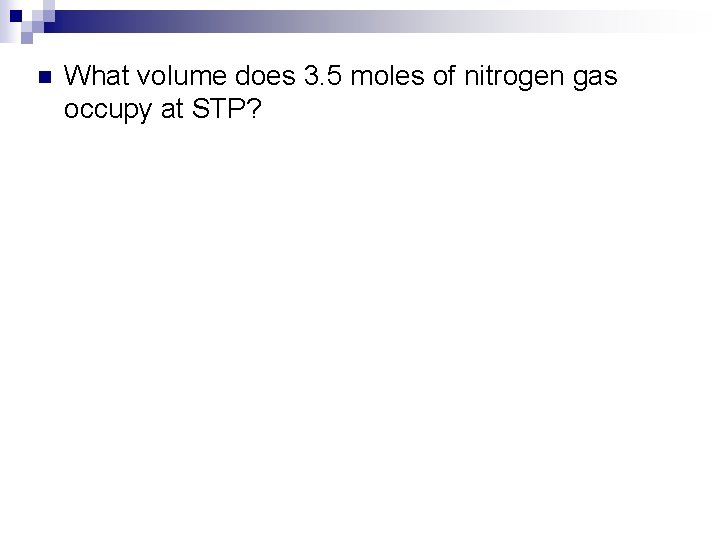
n What volume does 3. 5 moles of nitrogen gas occupy at STP?
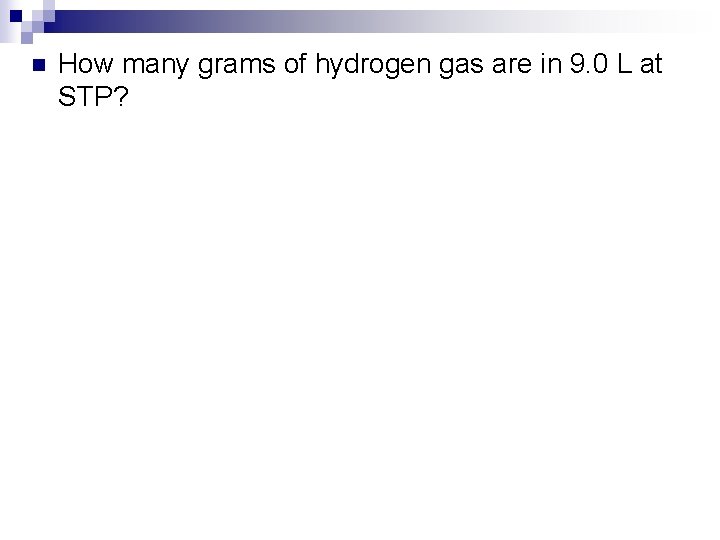
n How many grams of hydrogen gas are in 9. 0 L at STP?
n What volume will 125 g of carbon dioxide occupy at STP?

n What is the volume (in liters) of 2. 00 g CS 2 vapor at 276 mm Hg and 70°C?

n How many grams are in a sample of ammonia gas at 786 mm Hg, 2. 5 L, and 28°C?

n What pressure, in k. Pa, is exerted by 1. 75 g of hydrogen gas in a 4. 08 liter container at 35°C?

n What is the volume of a gas that is 0. 023 mole of nitrogen gas at STP?
n How many moles of air are in a 6. 0 L tire at STP?

n How many moles of oxygen are in a 5. 5 L canister at STP?
n What mass of helium is in a 2. 00 L balloon at STP?

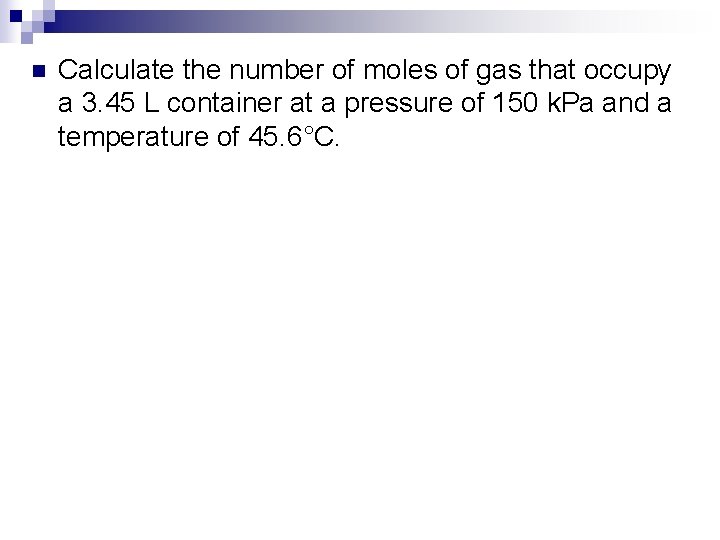
n Calculate the number of moles of gas that occupy a 3. 45 L container at a pressure of 150 k. Pa and a temperature of 45. 6°C.

n What is the pressure in mm. Hg that a 0. 44 g sample of carbon dioxide gas will exert at a temperature of 46. 2°C when it occupies a volume of 5. 00 L?

n Calculate the mass of oxygen gas present in a 2. 50 L sample kept at 1. 66 atm pressure and a temperature of 10. 0°C.

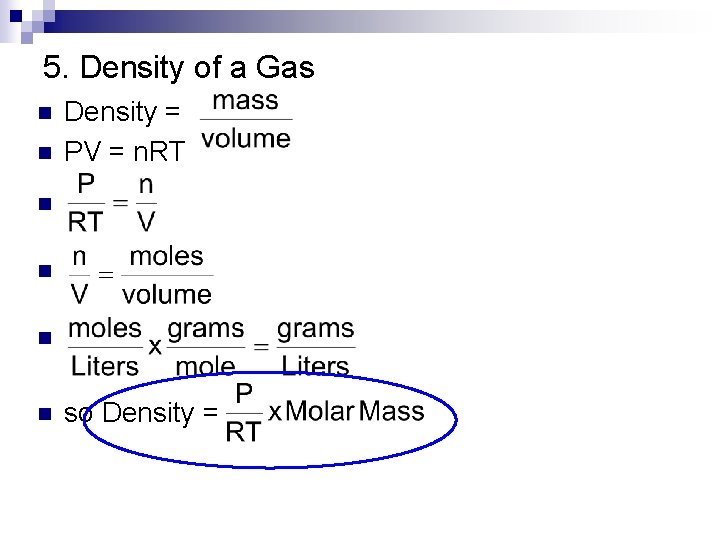
5. Density of a Gas n Density = PV = n. RT n n so Density = n

n What is the density of ammonia gas if the pressure is 700. 0 mm. Hg and the temperature is 63. 0°C?

n What is the density of sulfur dioxide at STP?

n What is the density of carbon dioxide at 26. 0°C and 1. 15 atm?

6. Molar Mass of Gases n Molar mass = n Used to identify a gas. PV=n. RT Solve for moles (n) from given pressure, volume, and temperature. Divide given grams by calculated moles (n). n n n

n What is the molar mass of a 1. 25 g sample of gas with a volume of 1. 00 L, at 730. 0 mm. Hg, and 27. 0°C?

n What is the molar mass of 0. 427 g of a gas that occupies 125 m. L at STP?

n What is the molar mass of a sample of gas that has a density of 0. 285 g/L at 101 k. Pa and 29°C?

n What is the molar mass of a gas if 142 g of the gas occupies a volume of 45. 1 L at 28. 4°C and 94. 6 k. Pa?

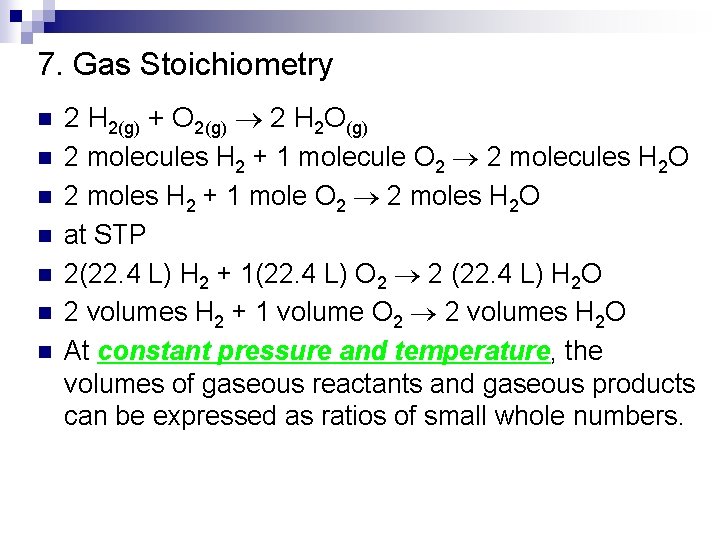
7. Gas Stoichiometry n n n n 2 H 2(g) + O 2(g) 2 H 2 O(g) 2 molecules H 2 + 1 molecule O 2 2 molecules H 2 O 2 moles H 2 + 1 mole O 2 2 moles H 2 O at STP 2(22. 4 L) H 2 + 1(22. 4 L) O 2 2 (22. 4 L) H 2 O 2 volumes H 2 + 1 volume O 2 2 volumes H 2 O At constant pressure and temperature, the volumes of gaseous reactants and gaseous products can be expressed as ratios of small whole numbers.

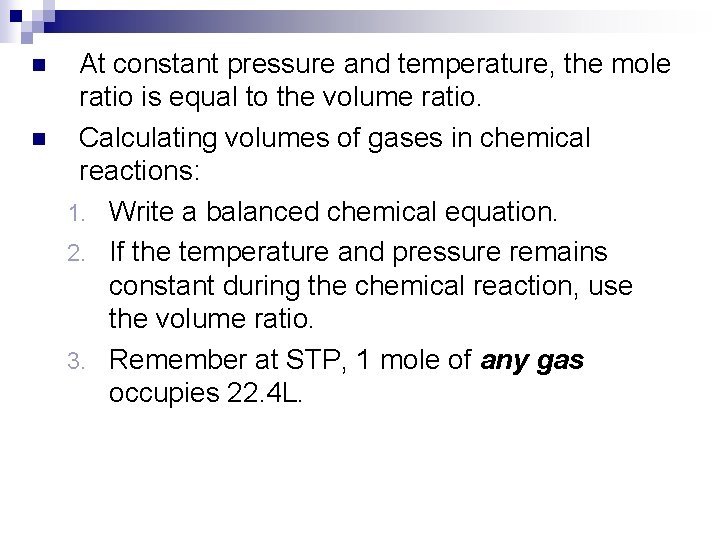
n n At constant pressure and temperature, the mole ratio is equal to the volume ratio. Calculating volumes of gases in chemical reactions: 1. Write a balanced chemical equation. 2. If the temperature and pressure remains constant during the chemical reaction, use the volume ratio. 3. Remember at STP, 1 mole of any gas occupies 22. 4 L.

n n If you are dealing with chemical reactions where: ¨ Mixture of solids, liquids, and gases ¨ Change in temperature and/or pressure for gases You will have to use the Ideal Gas Law. 1. 2. 3. 4. Write a balanced chemical equation. Calculate moles of gas from PV=n. RT Use mole ratio. Convert moles of product to volume using PV=n. RT.

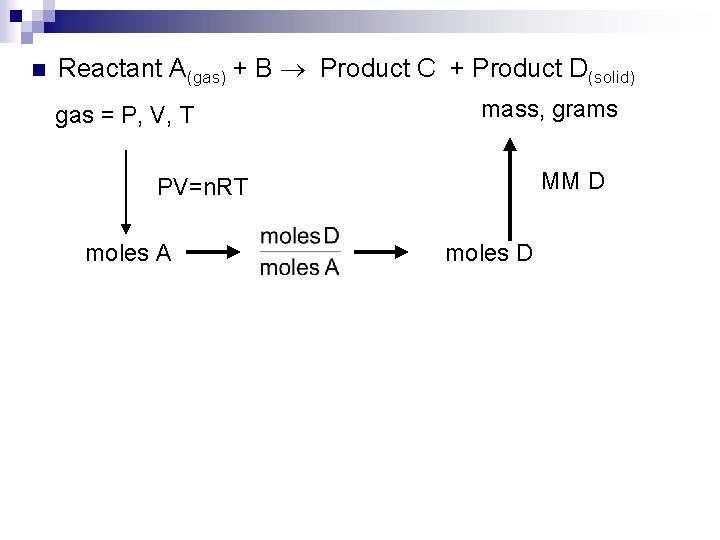
n Reactant A(gas) + B Product C + Product D(solid) gas = P, V, T mass, grams MM D PV=n. RT moles A moles D

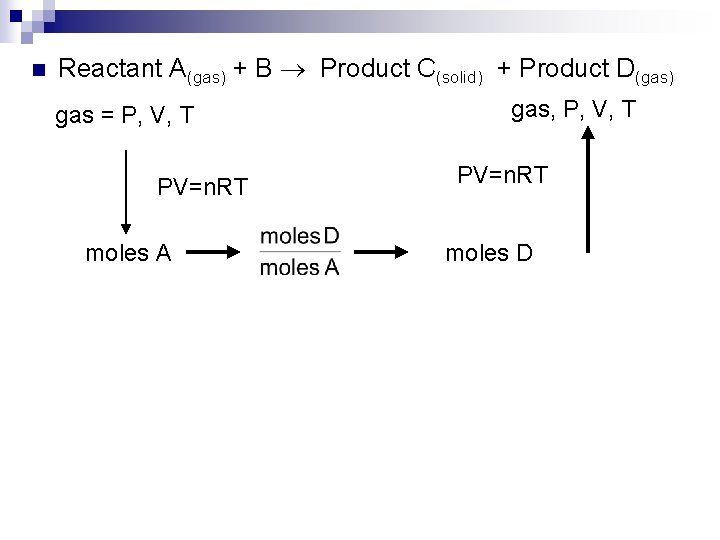
n Reactant A(gas) + B Product C(solid) + Product D(gas) gas = P, V, T PV=n. RT moles A gas, P, V, T PV=n. RT moles D

n If I have 27. 0 L of hydrogen gas that reacts with an excess amount of nitrogen gas, how many liters of ammonia will be produced at the same temperature and pressure?

n How many liters of carbon monoxide, at 27. 0°C and 25. 0 k. Pa can be produced from burning 65. 5 g of carbon?

n What volume of oxygen can be collected at 100. 0 k. Pa and 25°C when 30. 6 g KCl. O 3 decomposes?

n What volume of bromine gas (at 0. 00°C, 98. 0 k. Pa) is produced when 95. 0 L of chlorine (at 50. 0°C, 50. 5 k. Pa) react with excess HBr?

n Calculate the mass of hydrogen peroxide needed to obtain 0. 460 L of oxygen gas at STP.

n Magnesium metal will “burn” in carbon dioxide to produce elemental carbon and magnesium oxide. What mass of magnesium will “burn” in a 255 m. L container of CO 2 at 77. 0°C and 65. 0 k. Pa?