Mass Moles and Molar Mass Read pp 80
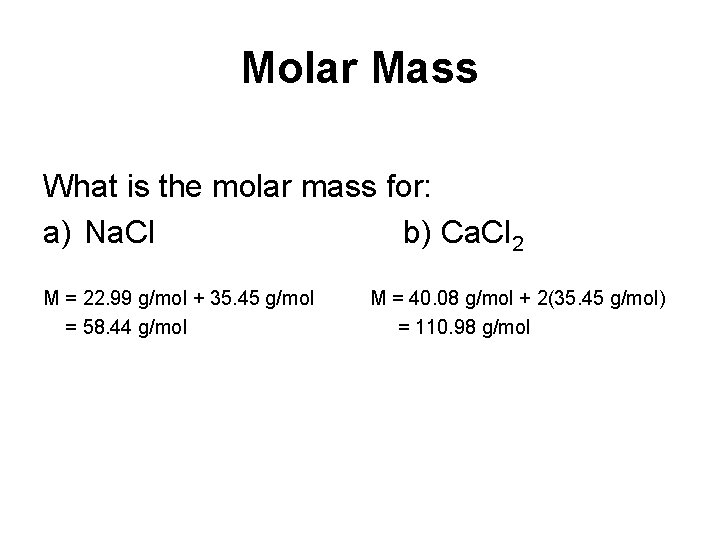
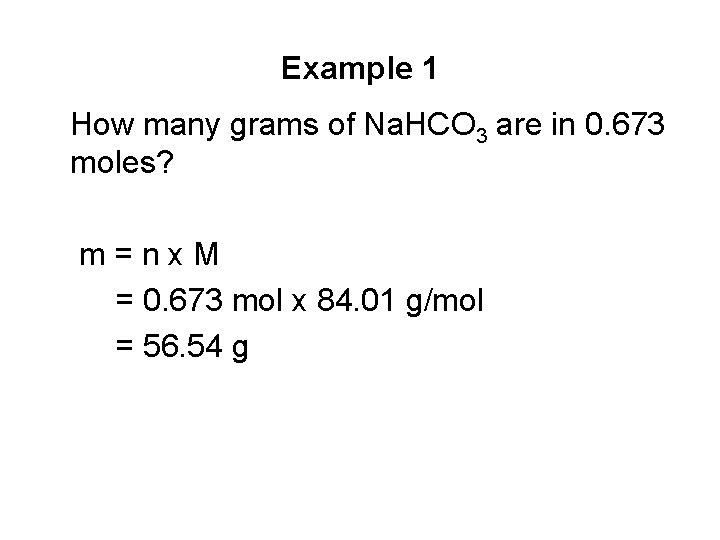




- Slides: 15

Mass, Moles, and Molar Mass Read pp. 80 -105 (2. 1 and 22) Extension Questions p. 92 #1, 4, 8 -10 p. 106 #1 -10

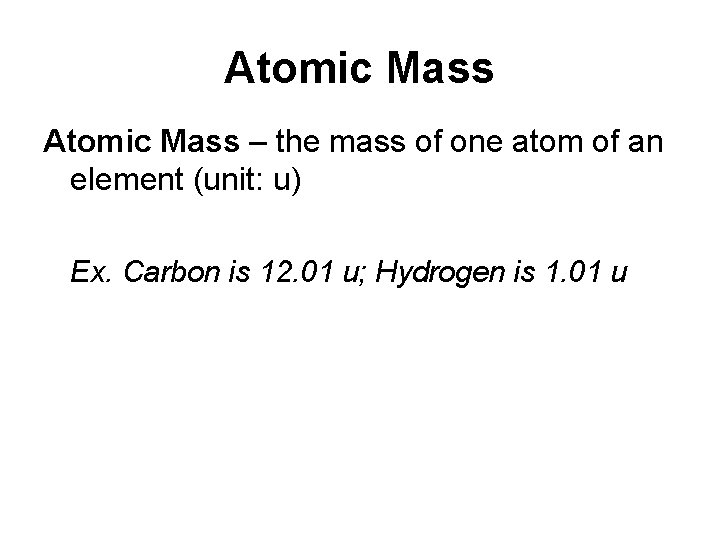
Atomic Mass – the mass of one atom of an element (unit: u) Ex. Carbon is 12. 01 u; Hydrogen is 1. 01 u

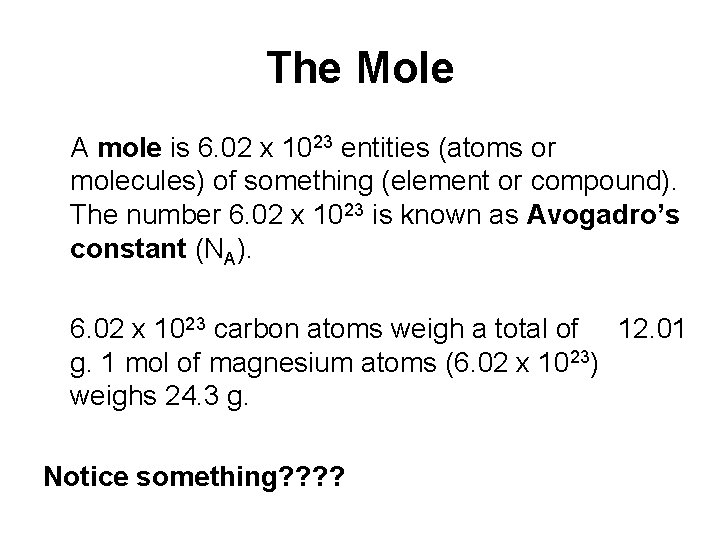
The Mole A mole is 6. 02 x 1023 entities (atoms or molecules) of something (element or compound). The number 6. 02 x 1023 is known as Avogadro’s constant (NA). 6. 02 x 1023 carbon atoms weigh a total of 12. 01 g. 1 mol of magnesium atoms (6. 02 x 1023) weighs 24. 3 g. Notice something? ?

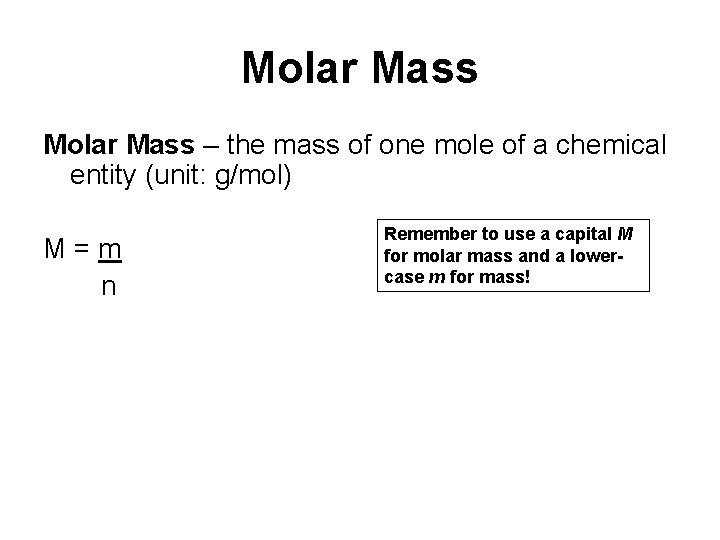
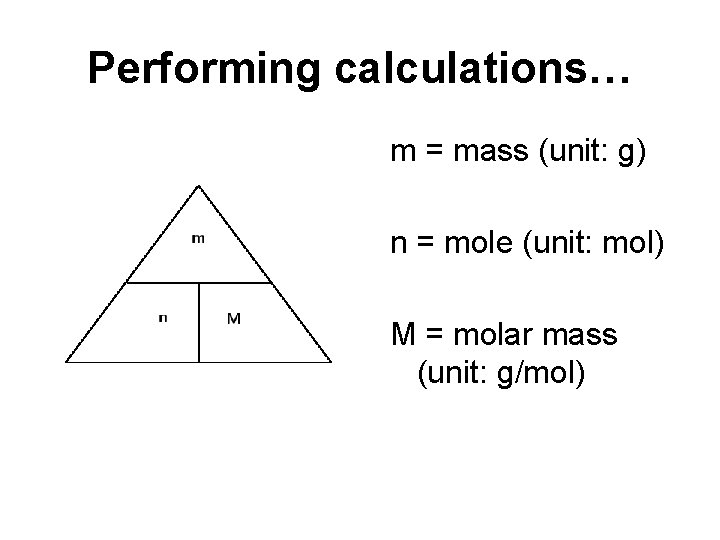
Molar Mass – the mass of one mole of a chemical entity (unit: g/mol) M=m n Remember to use a capital M for molar mass and a lowercase m for mass!
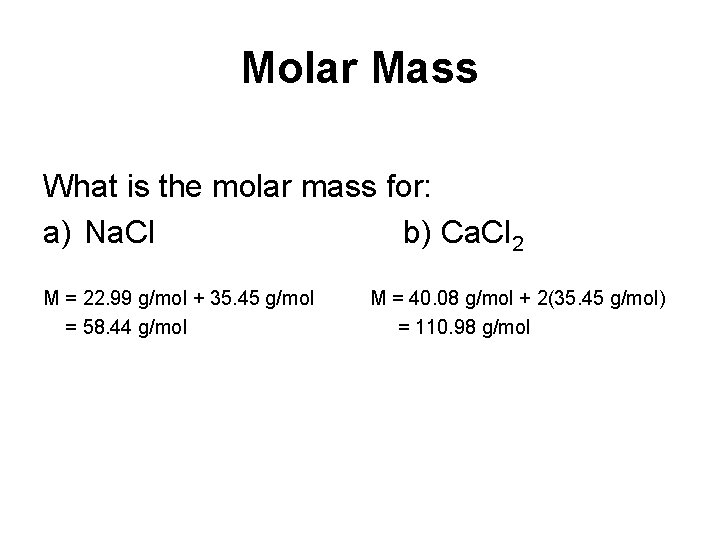
Molar Mass What is the molar mass for: a) Na. Cl b) Ca. Cl 2 M = 22. 99 g/mol + 35. 45 g/mol = 58. 44 g/mol M = 40. 08 g/mol + 2(35. 45 g/mol) = 110. 98 g/mol

Performing calculations… m = mass (unit: g) n = mole (unit: mol) M = molar mass (unit: g/mol)
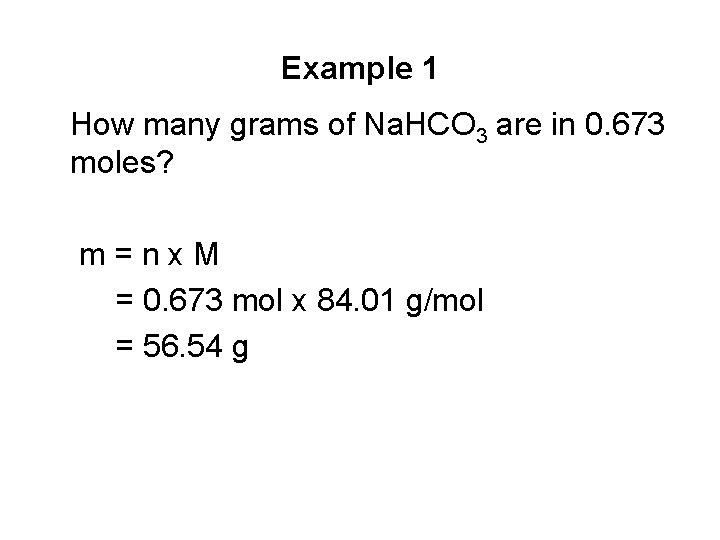
Example 1 How many grams of Na. HCO 3 are in 0. 673 moles? m=nx. M = 0. 673 mol x 84. 01 g/mol = 56. 54 g

Example 2 How many moles of KMn. O 4 are there if you have 250 g of it? n=m/M = 250 g / 158. 04 g/mol = 1. 58 mol

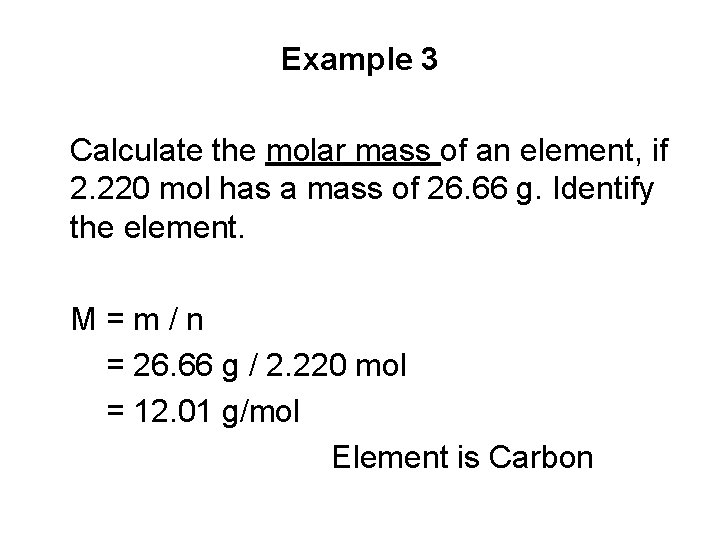
Example 3 Calculate the molar mass of an element, if 2. 220 mol has a mass of 26. 66 g. Identify the element. M=m/n = 26. 66 g / 2. 220 mol = 12. 01 g/mol Element is Carbon

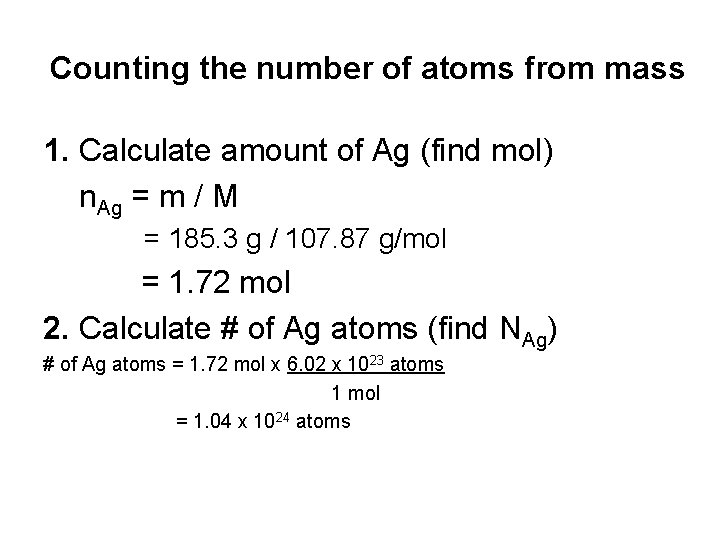
Counting the number of atoms from mass How many atoms of silver are in a 185. 3 g lump of pure silver? Two steps: 1. Calculate amount of X (find mol) 2. Calculate # of X atoms (find NX)

Counting the number of atoms from mass 1. Calculate amount of Ag (find mol) n. Ag = m / M = 185. 3 g / 107. 87 g/mol = 1. 72 mol 2. Calculate # of Ag atoms (find NAg) # of Ag atoms = 1. 72 mol x 6. 02 x 1023 atoms 1 mol = 1. 04 x 1024 atoms

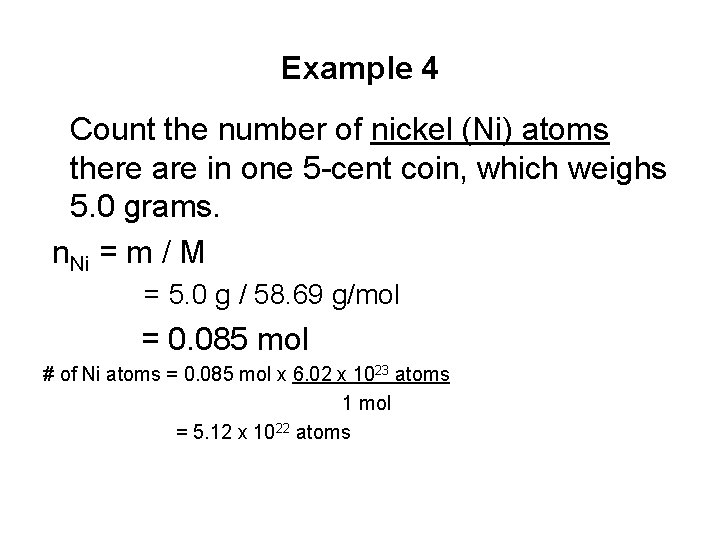
Example 4 Count the number of nickel (Ni) atoms there are in one 5 -cent coin, which weighs 5. 0 grams. n. Ni = m / M = 5. 0 g / 58. 69 g/mol = 0. 085 mol # of Ni atoms = 0. 085 mol x 6. 02 x 1023 atoms 1 mol = 5. 12 x 1022 atoms

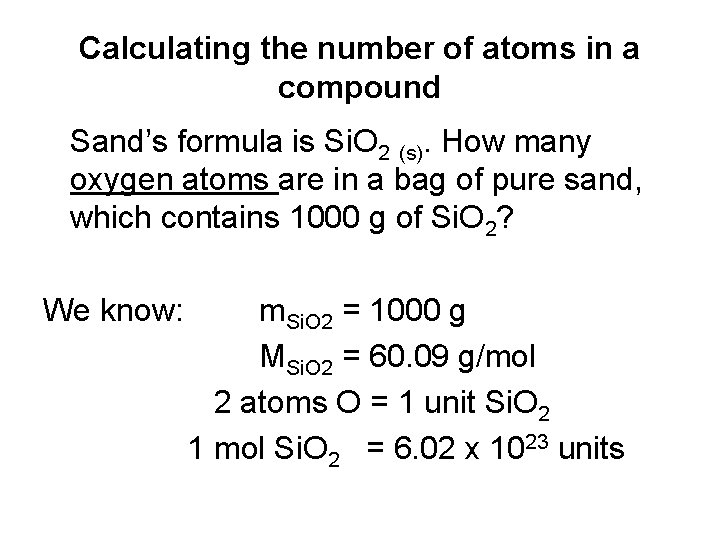
Calculating the number of atoms in a compound Sand’s formula is Si. O 2 (s). How many oxygen atoms are in a bag of pure sand, which contains 1000 g of Si. O 2? We know: m. Si. O 2 = 1000 g MSi. O 2 = 60. 09 g/mol 2 atoms O = 1 unit Si. O 2 1 mol Si. O 2 = 6. 02 x 1023 units

We want NO (# of oxygen atoms) NO = n. Si. O 2 x 6. 02 x 1023 units Si. O 2 x 2 atoms O 1 mol Si. O 2 1 unit Si. O 2 NO = 1000 g x 6. 02 x 1023 units Si. O 2 x 2 atoms O 60. 09 g/mol 1 mol Si. O 2 1 unit Si. O 2 NO = 2. 00 x 1025 atoms Note: Same principle as finding # of atoms for an element, but in this, you are adding in the # of atoms of the element in 1 unit of the compound (eg. always 2 O atoms in 1 unit of Si. O 2).

Example 5 How many atoms of nitrogen are there in 1. 26 kg of nitrogen gas? n. N 2 = m / M = 1260 g / 28. 02 g/mol = 44. 97 mol # of N atoms = 44. 97 mol x 6. 02 x 1023 molecules N 2 x 2 atoms N 1 molecule N 2 = 5. 41 x 1025 atoms