STOICHIOMETRY Molar mass Percent composition Moles Conversions Empirical


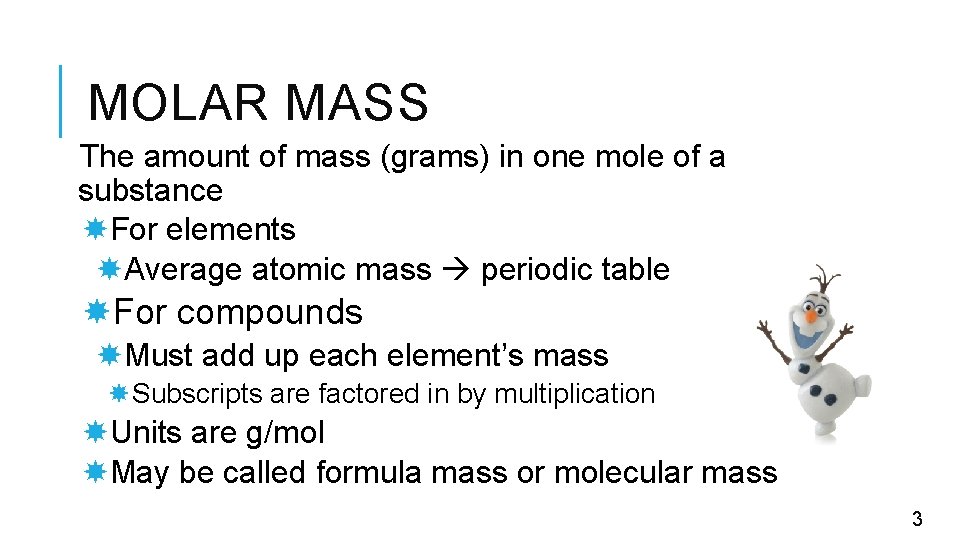




- Slides: 20

STOICHIOMETRY Molar mass, Percent composition, Moles, Conversions, Empirical formulas, Molecular formulas 1

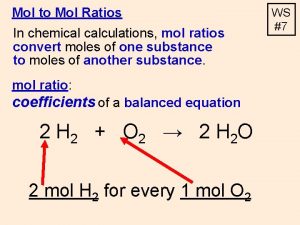
THE MOLE SI unit for amount A counting unit for measuring large quantities of small items Atoms molecules Particles 1 mole = 6. 022 x 10 23 atoms = molar mass 2
MOLAR MASS The amount of mass (grams) in one mole of a substance For elements Average atomic mass periodic table For compounds Must add up each element’s mass Subscripts are factored in by multiplication Units are g/mol May be called formula mass or molecular mass 3

STEPS TO SOLVE 1. Find elements on periodic table 2. Write down the mass 3. Multiply the mass by the subscript 4. Add together 4

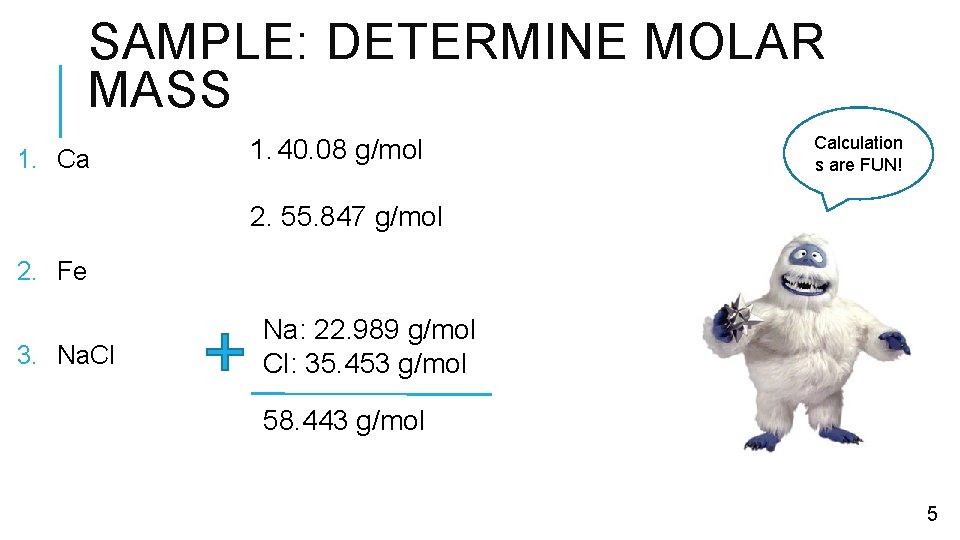
SAMPLE: DETERMINE MOLAR MASS 1. Ca 1. 40. 08 g/mol Calculation s are FUN! 2. 55. 847 g/mol 2. Fe 3. Na. Cl Na: 22. 989 g/mol Cl: 35. 453 g/mol 58. 443 g/mol 5

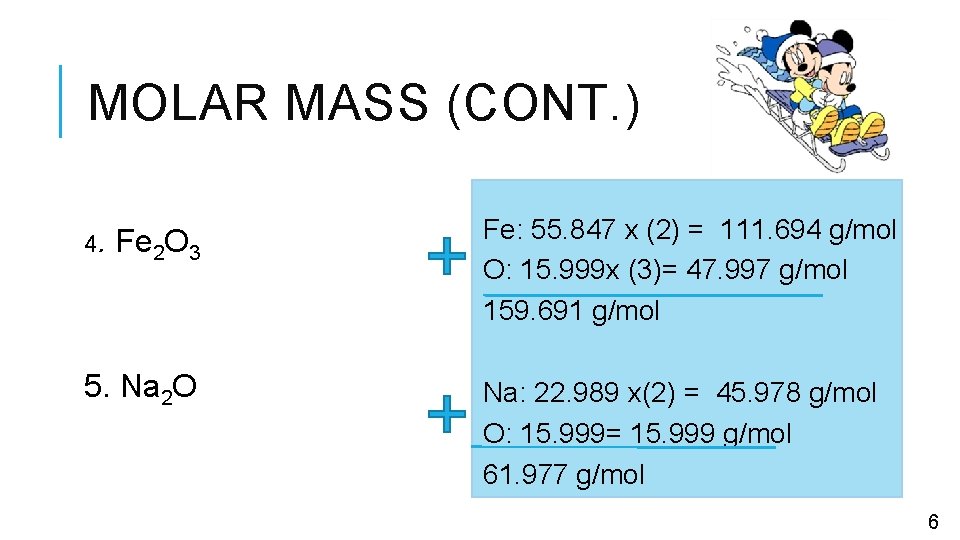
MOLAR MASS (CONT. ) 4. Fe 2 O 3 5. Na 2 O Fe: 55. 847 x (2) = 111. 694 g/mol O: 15. 999 x (3)= 47. 997 g/mol 159. 691 g/mol Na: 22. 989 x(2) = 45. 978 g/mol O: 15. 999= 15. 999 g/mol 61. 977 g/mol 6

PERCENT COMPOSITION Steps to solve 1. Find the total mass of the COMPOUND 2. Take the mass of each element and divide it by the total mass 3. Multiply by 100 7

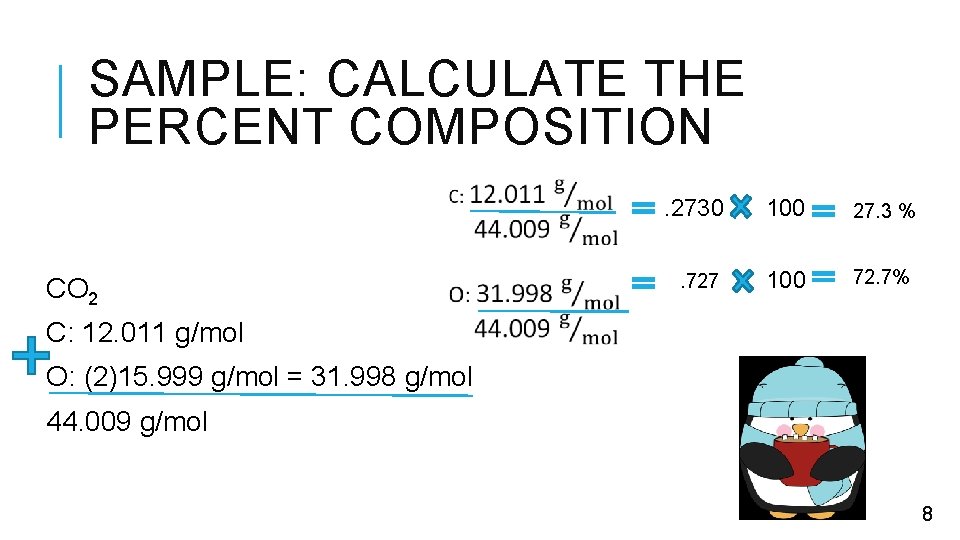
SAMPLE: CALCULATE THE PERCENT COMPOSITION CO 2 . 2730 100 27. 3 % . 727 100 72. 7% C: 12. 011 g/mol O: (2)15. 999 g/mol = 31. 998 g/mol 44. 009 g/mol 8

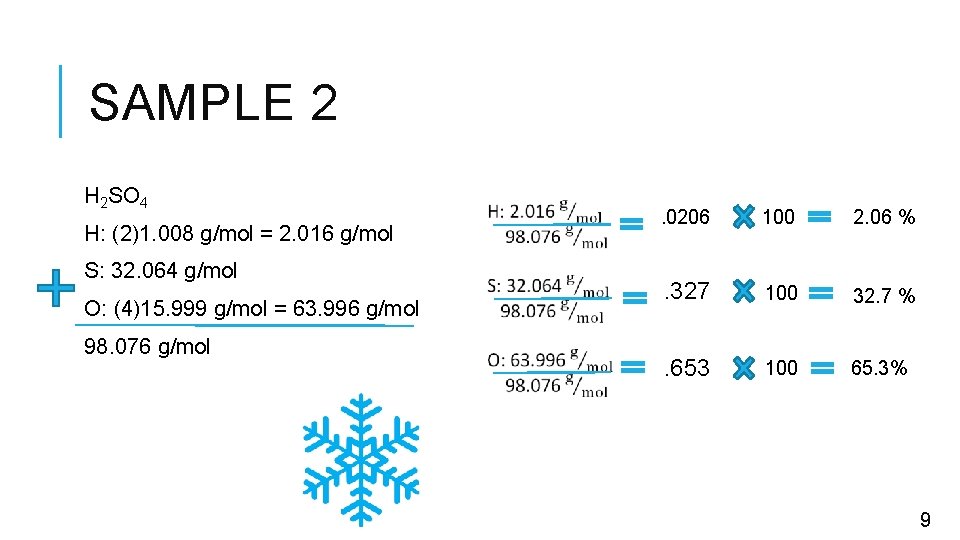
SAMPLE 2 H 2 SO 4 H: (2)1. 008 g/mol = 2. 016 g/mol S: 32. 064 g/mol O: (4)15. 999 g/mol = 63. 996 g/mol 98. 076 g/mol . 0206 100 2. 06 % . 327 100 32. 7 % . 653 100 65. 3% 9

COMPOSITION OF HYDRATES Hydrate: crystal contains water within • Water can fit into the salt crystal • Happens in fixed ratios • Each salt that forms a hydrate has a DIFFERENT water ratio • Each salt crystal is unique Anhydrous: water has been removed Steps to solve 1. Calculate the total mass of the compound INCLUDING THE water 2. Divide the water mass by total mass 3. Multiply by 100 • Achieved through drying 10

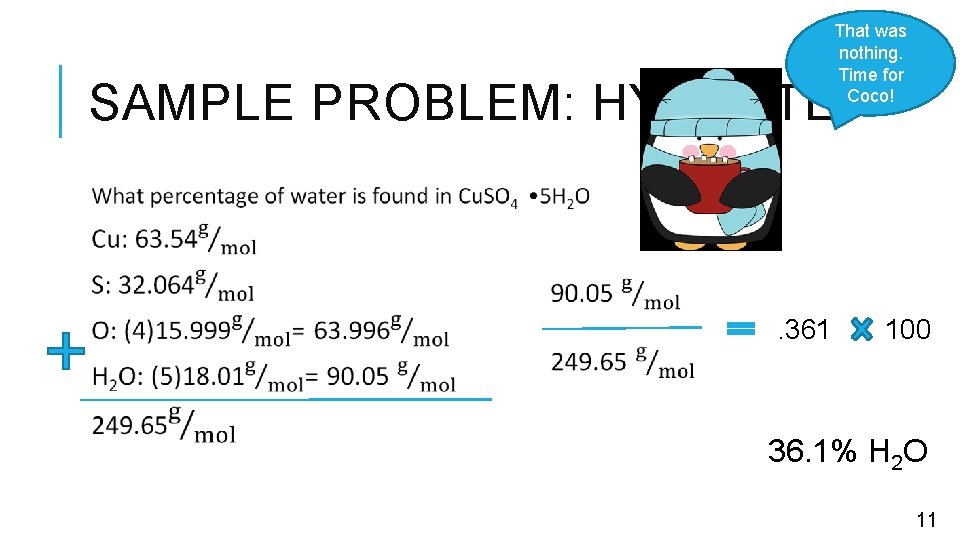
That was nothing. Time for Coco! SAMPLE PROBLEM: HYDRATE . 361 100 36. 1% H 2 O 11

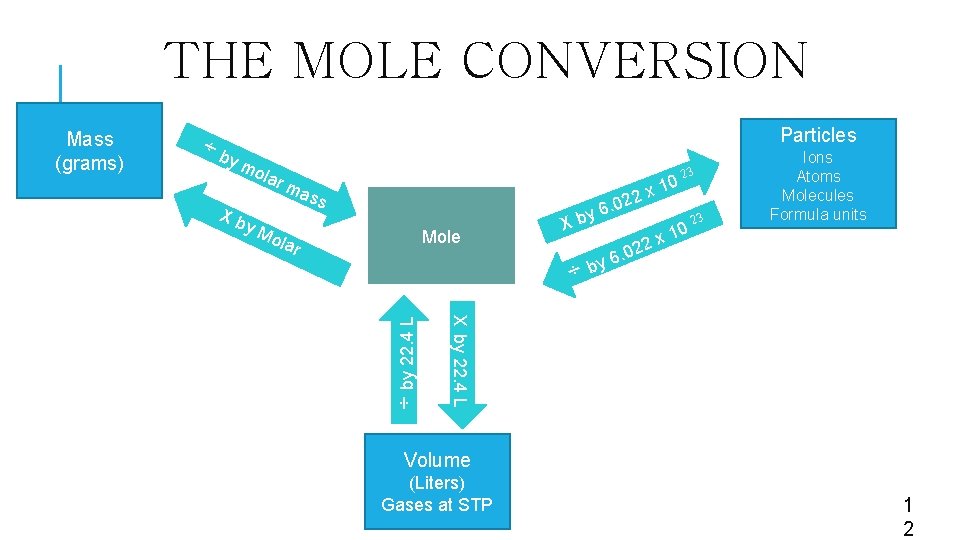
THE MOLE CONVERSION by Particles mo lar m 23 X b ma y Mol ar ss ass 10 x 2 2 Mole . 0 6 y X b 3 2 2 0 6. ÷ by 2 10 Ions Atoms Molecules Formula units x x by 22. 4 L ÷ Mass (grams) Volume (Liters) Gases at STP 1 2

STOICHIOMETRIC CONVERSIONS: MASS TO MOLES • You can relate any unit to another by this method • Relate one unit to another • conversion factors Steps to convert 1. Identify what you start with 2. Determine what units you end in A. Might have to do side calculations 3. Set up conversion factor 4. Evaluate A. Do the Math! 1 3

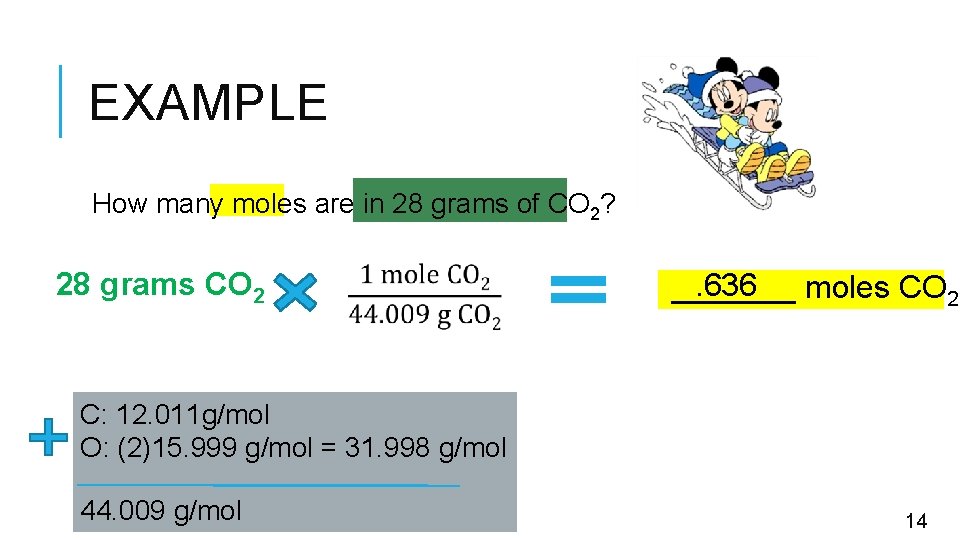
EXAMPLE How many moles are in 28 grams of CO 2? 28 grams CO 2 . 636 _______ moles CO 2 C: 12. 011 g/mol O: (2)15. 999 g/mol = 31. 998 g/mol 44. 009 g/mol 14

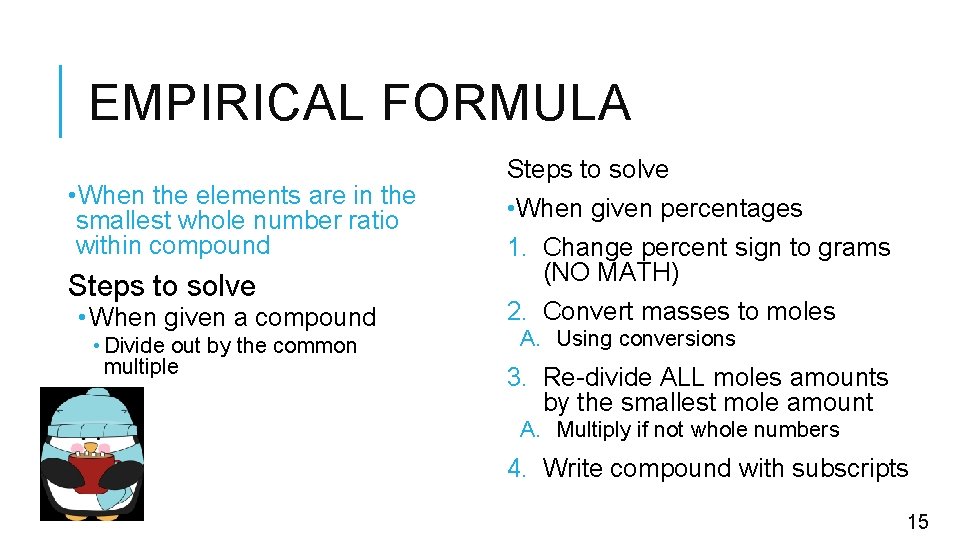
EMPIRICAL FORMULA • When the elements are in the smallest whole number ratio within compound Steps to solve • When given a compound • Divide out by the common multiple Steps to solve • When given percentages 1. Change percent sign to grams (NO MATH) 2. Convert masses to moles A. Using conversions 3. Re-divide ALL moles amounts by the smallest mole amount A. Multiply if not whole numbers 4. Write compound with subscripts 15

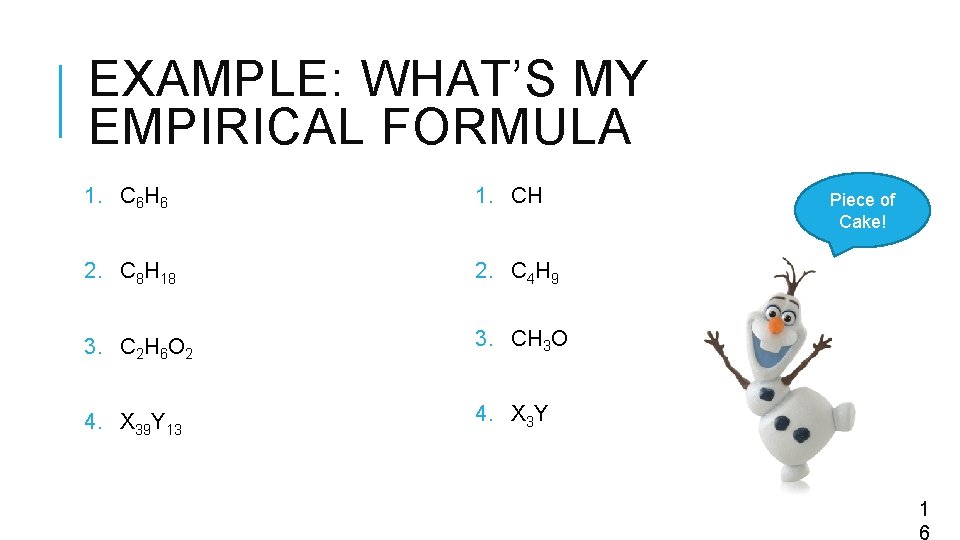
EXAMPLE: WHAT’S MY EMPIRICAL FORMULA 1. C 6 H 6 1. CH 2. C 8 H 18 Piece of Cake! 2. C 4 H 9 3. C 2 H 6 O 2 3. CH 3 O 4. X 39 Y 13 4. X 3 Y 1 6

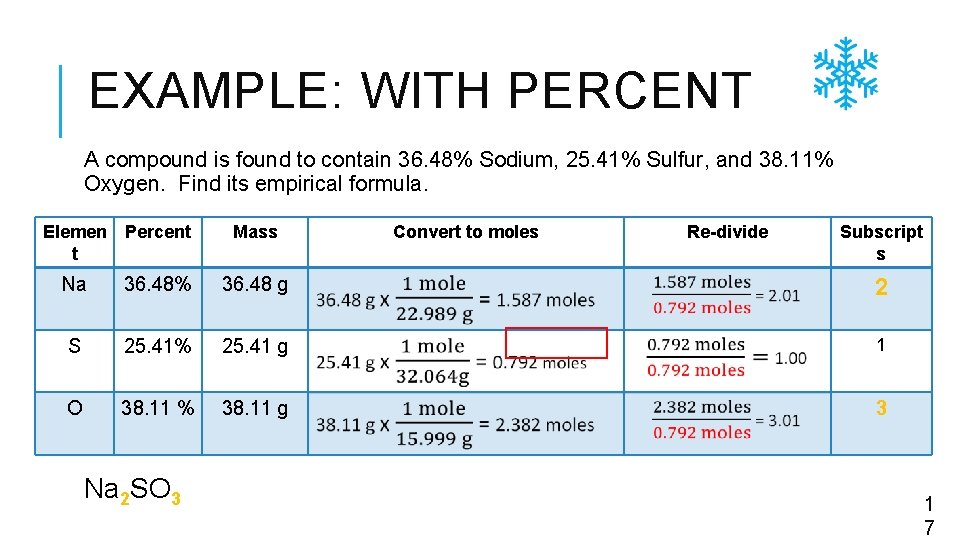
EXAMPLE: WITH PERCENT A compound is found to contain 36. 48% Sodium, 25. 41% Sulfur, and 38. 11% Oxygen. Find its empirical formula. Elemen t Percent Mass Na 36. 48% 36. 48 g 2 S 25. 41% 25. 41 g 1 O 38. 11 % 38. 11 g 3 Na 2 SO 3 Convert to moles Re-divide Subscript s 1 7

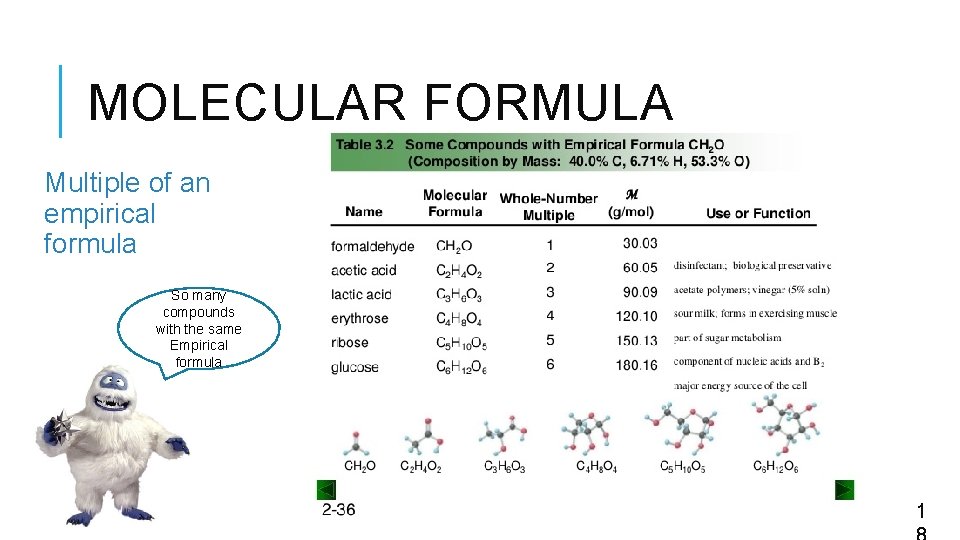
MOLECULAR FORMULA Multiple of an empirical formula So many compounds with the same Empirical formula 1

STEPS TO SOLVE 1. Complete an empirical formula process if needed 2. Find the mass of the EMPIRICAL FORMULA 3. Divide the Empirical formula mass by the molecular mass A. Molecular mass is normally given in the problem B. Answer is the common multiple 4. Multiple the SUBSCRIPTS by the common multiple 1 9

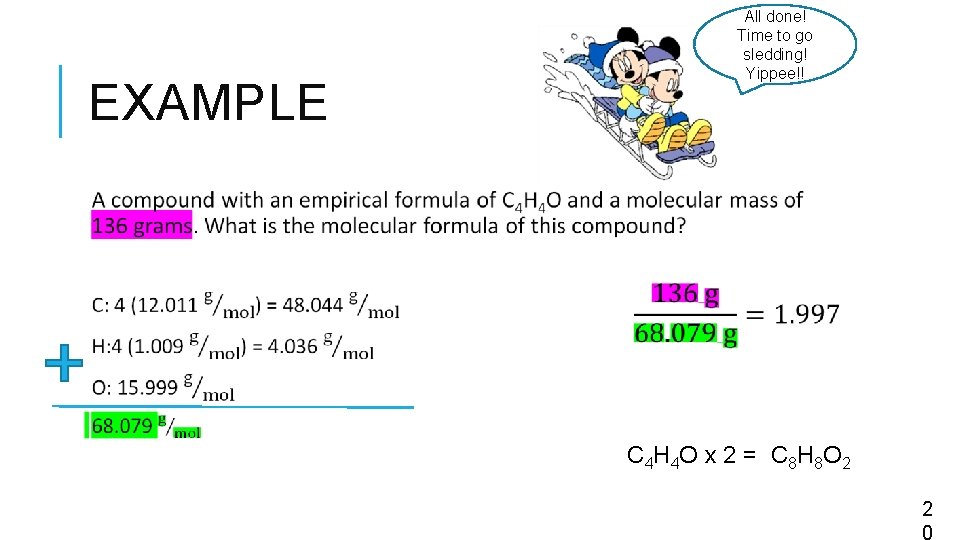
All done! Time to go sledding! Yippee!! EXAMPLE C 4 H 4 O x 2 = C 8 H 8 O 2 2 0
 How to find percent mass
How to find percent mass Convert from mass to moles
Convert from mass to moles Empirical formula of lactic acid
Empirical formula of lactic acid How to find empirical formula from percent
How to find empirical formula from percent Empirical formula from percent composition
Empirical formula from percent composition How to calculate empirical formula using percentages
How to calculate empirical formula using percentages How to find empirical formula of a hydrate
How to find empirical formula of a hydrate How to find the empirical formula from percent composition
How to find the empirical formula from percent composition How to find molecular formula
How to find molecular formula Empirical formula to percent composition
Empirical formula to percent composition Molar mass of chocolate chips
Molar mass of chocolate chips Calculate moles from grams
Calculate moles from grams Mmol formula
Mmol formula Mass by mass percentage formula
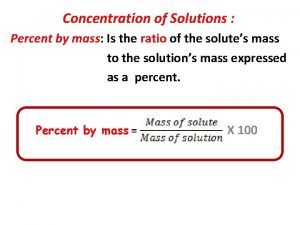
Mass by mass percentage formula Percent by mass
Percent by mass Percent composition of nitrogen
Percent composition of nitrogen How to find mass percent
How to find mass percent A part of a whole is
A part of a whole is Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Stoichiometry examples
Stoichiometry examples Percent concentration formula
Percent concentration formula