Experiment 6 Fractional Distillation Reading Assignment Experiment 6













- Slides: 36

Experiment 6: Fractional Distillation • Reading Assignment – Experiment 6 (pp. 58 -64) – Operation 29

Key Point! • When conducting a distillation, the vapor should be richer in the lower boiling component than what you started with.

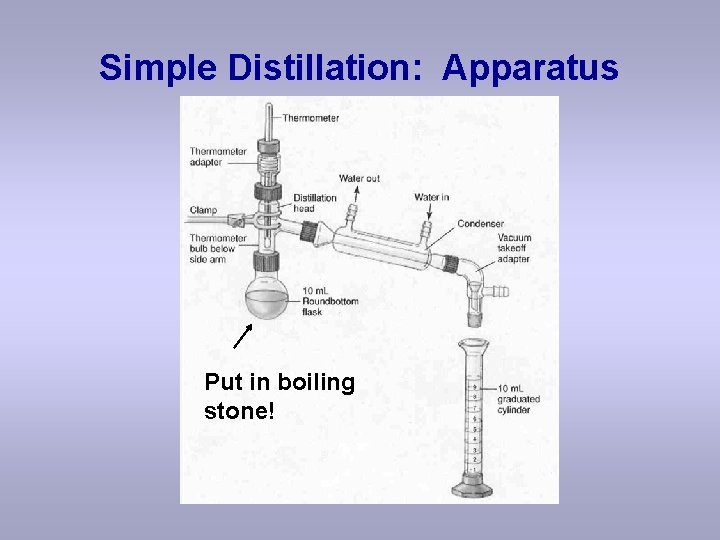
Simple Distillation: Apparatus Put in boiling stone!

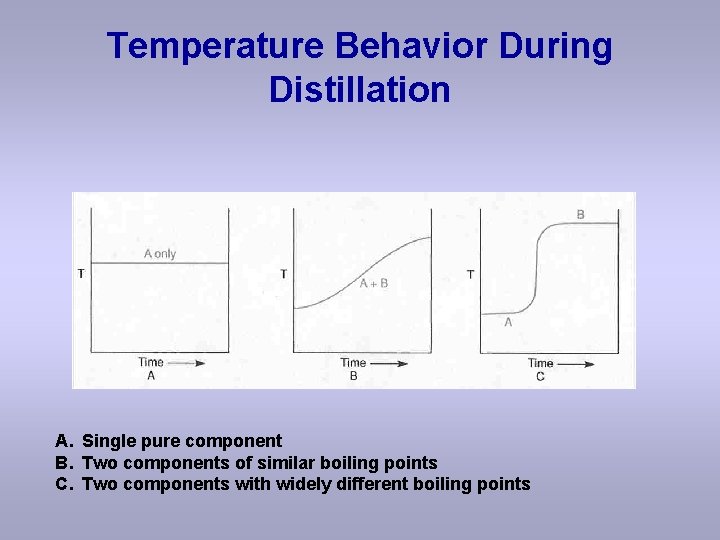
Temperature Behavior During Distillation A. Single pure component B. Two components of similar boiling points C. Two components with widely different boiling points

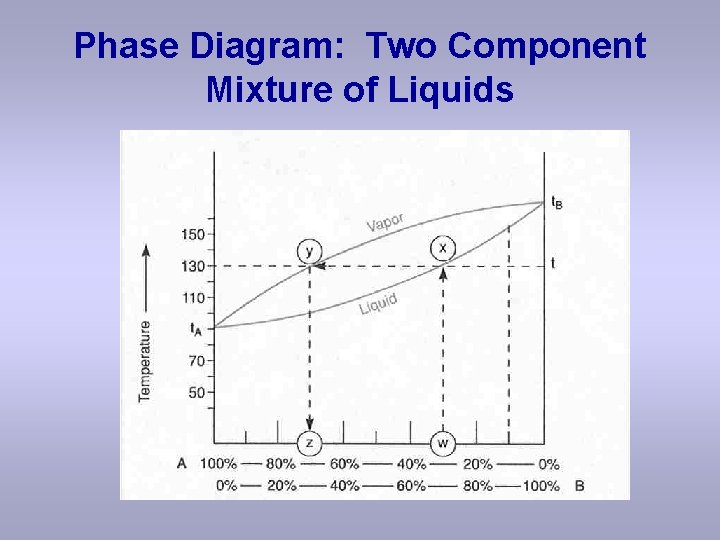
Phase Diagram: Two Component Mixture of Liquids

Questions based upon the previous slide: a) What is the bp of pure A? b) What is the bp of pure B? c) What is the bp of a solution with the composition of 30 % B, assuming a simple distilllation apparatus? d) What is the composition of the vapor assuming a simple distillation apparatus? e) What is the composition of the distillate collected assuming a simple distillation apparatus? f) What does the “tie-line, ” x-y represent? Hint: the upper curve is the vapor curve and the lower curve is the liquid curve. “Composition of the vapor and liquid that are in equilibriuim with each other at 130 o. C. ”

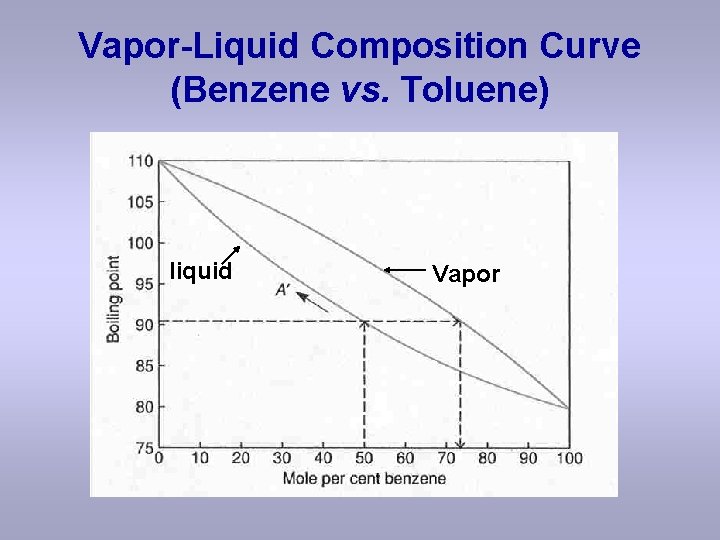
Vapor-Liquid Composition Curve (Benzene vs. Toluene) liquid Vapor

Questions based upon the previous slide: a) What is the bp of pure toluene? b) What is the bp of pure benzene? c) What is the bp of a solution with the composition of 50 % benzene, assuming a simple distilllation apparatus? d) What is the composition of the distillate assuming a simple distillation apparatus? e) How many theoretical plates would be necessary for a fractional distillation starting with a 50 % benzene solution?

When will simple distillation do a reasonable job of separating a mixture? 1) When the difference in boiling points is over 100 o 2) When there is a fairly small amount of impurity, say less than 10 %. 3) When one of the components will not distil because of a lack of volatility (i. e. sugar dissolved in water).

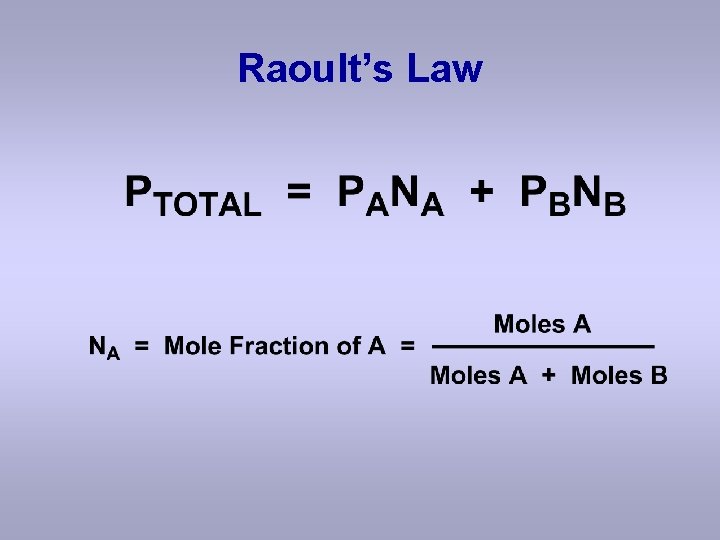
Raoult’s Law

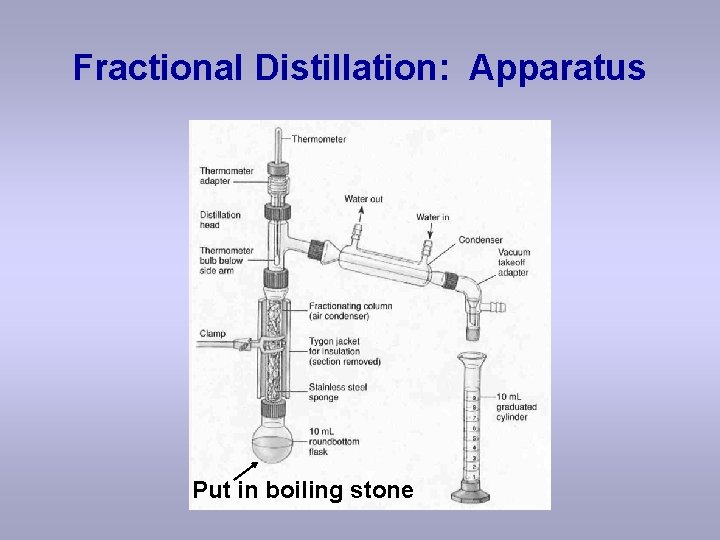
Fractional Distillation: Apparatus Put in boiling stone

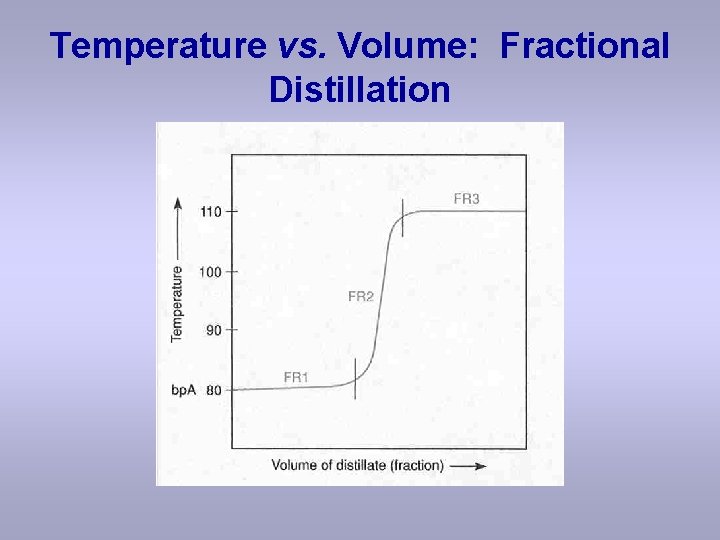
Temperature vs. Volume: Fractional Distillation

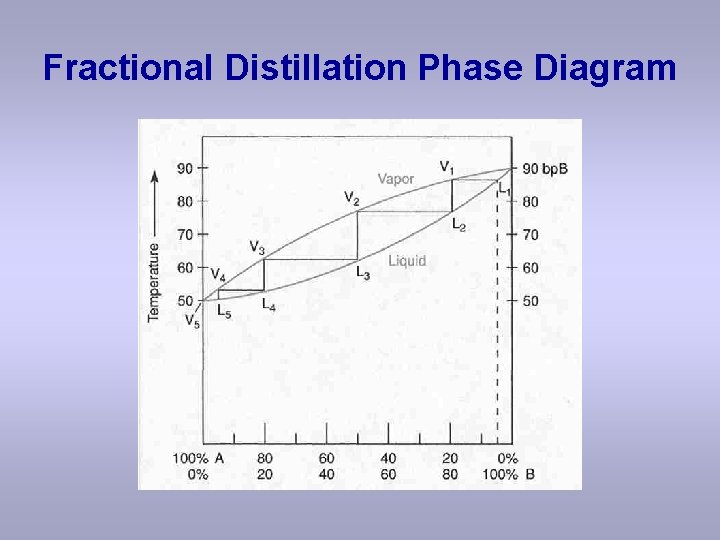
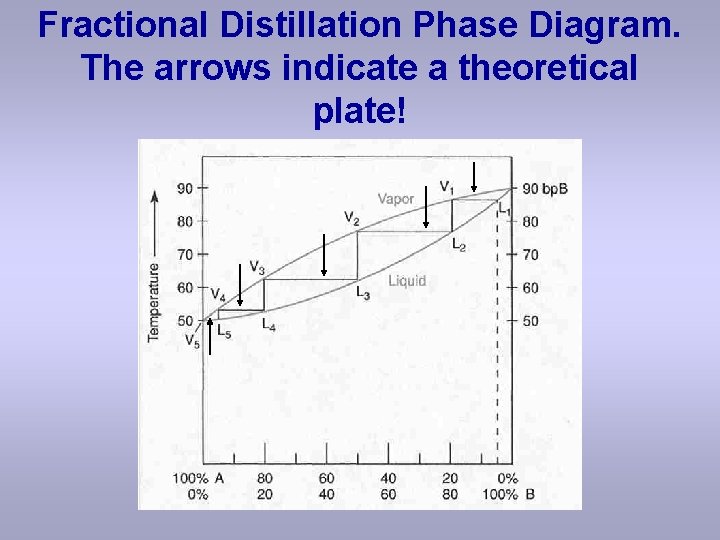
Fractional Distillation Phase Diagram

How many theoretical plates are need to separate a mixture starting at L? • Looks like about 5 plates are needed to separate the mixture on the previous slide! • Count the “tie-lines” (horizontal lines) to come up with the 5 plates (labelled with arrows on the next slide)!

Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate!

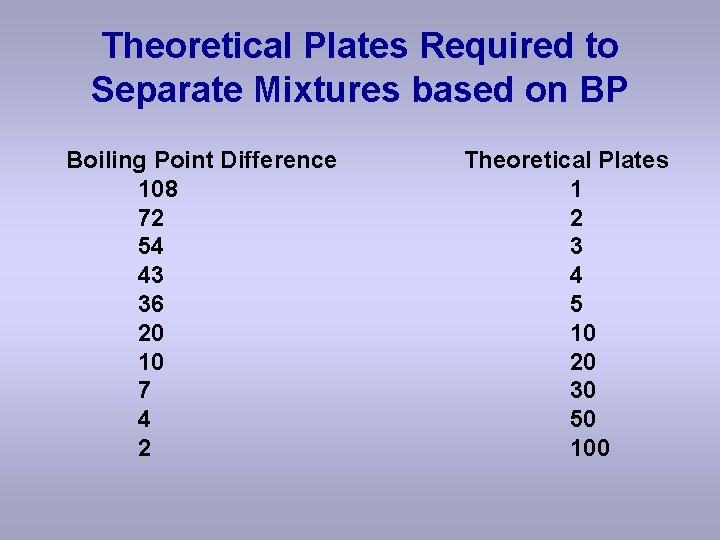
Theoretical Plates Required to Separate Mixtures based on BP Boiling Point Difference 108 72 54 43 36 20 10 7 4 2 Theoretical Plates 1 2 3 4 5 10 20 30 50 100

Azeotrope • Some mixtures of liquids, because of attractions or repulsions between the molecules, do not behave ideally • These mixtures do not obey Raoult’s Law • An azeotrope is a mixture with a fixed composition that cannot be altered by either simple or fractional distillation • An azeotrope behaves as if it were a pure compound, and it distills from beginning to end at a constant temperature.

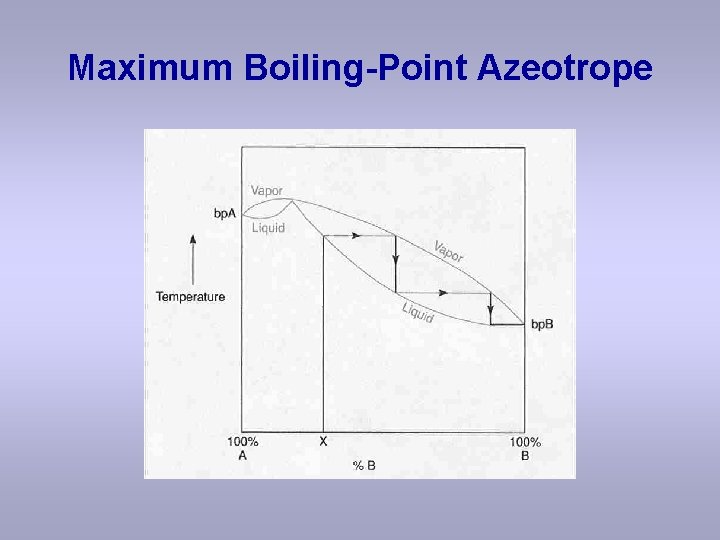
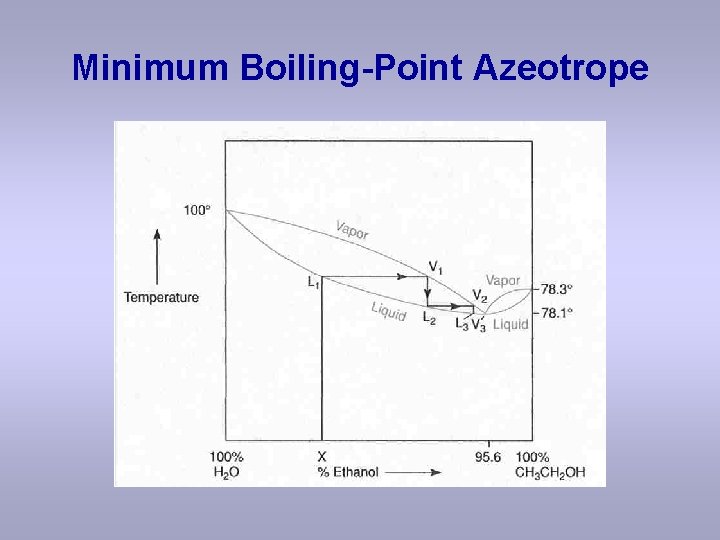
Types of Azeotropes • There are two types of non-ideal behavior: – Minimum-boiling-point • Boiling point of the mixture is lower than the boiling point of either pure component – Maximum-boiling-point • Boiling point of the mixture is higher than the boiling point of either pure component

Maximum Boiling-Point Azeotrope

Observations with maximum boiling azeotrope On the right side of the diagram: Compound B will distill (lowest bp). Once B has been removed, the azeotrope will distill (highest bp). On the left side of the diagram: Compound A will distill (lowest bp) Once A has been removed, the azeotrope will distill. (highest bp) The azeotrope acts like a pure “compound”

Minimum Boiling-Point Azeotrope

Observations with minimum boiling azeotrope On the right side of the diagram: The azeotrope is the lower boiling “compound, ” and it will be removed first. Pure ethanol will distill once the azeotrope has distilled. On the left side of the diagram: the azeotrope is the lower boiling “compound, ” and it will distill first. Once the azeotrope has been removed, then pure water will distill. The azeotrope acts like a pure “compound”

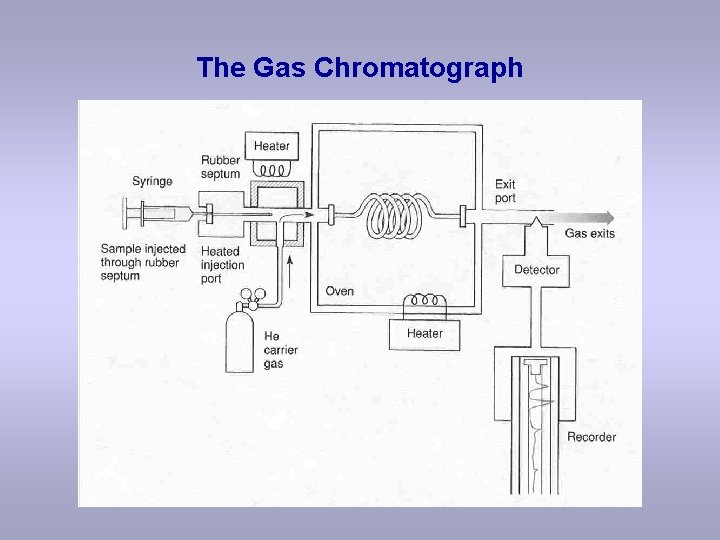
The Gas Chromatograph

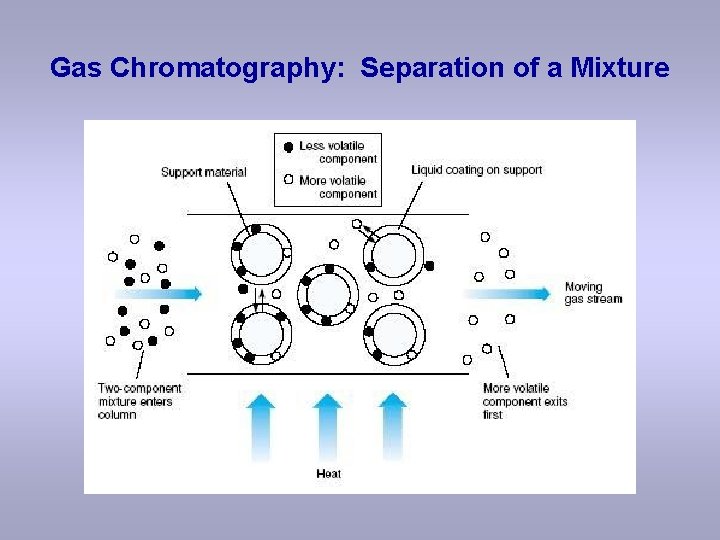
Gas Chromatography: Separation of a Mixture

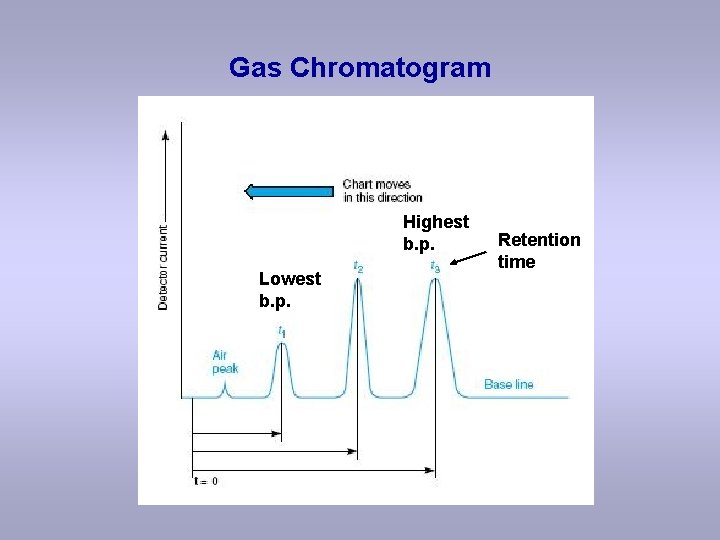
Gas Chromatogram Highest b. p. Lowest b. p. Retention time

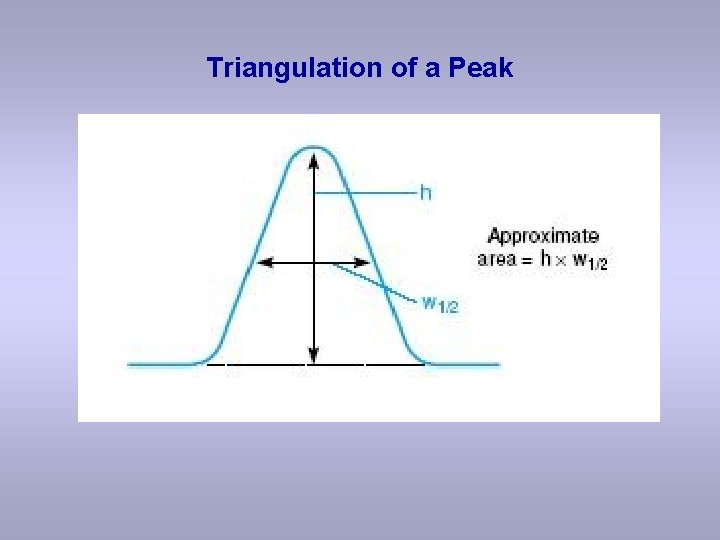
Triangulation of a Peak

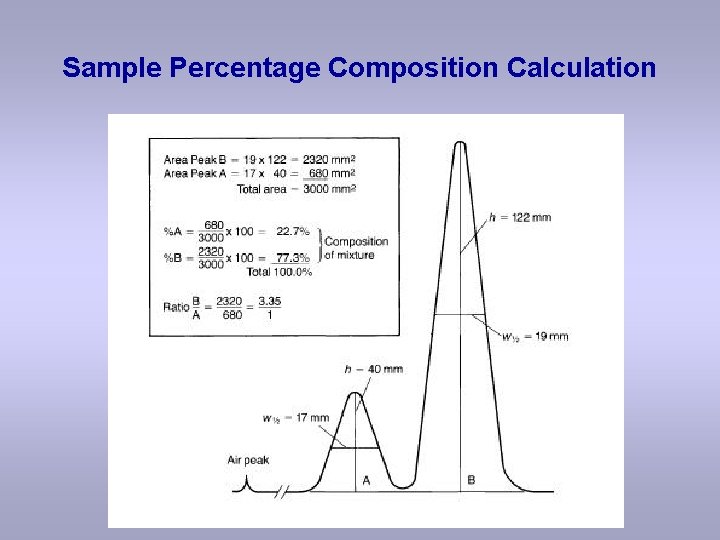
Sample Percentage Composition Calculation

Gas Chromatography: Results In a modern gas chromatography instrument, the results are displayed analyzed using a computerized data station. It is no longer necessary to calculate peak areas by triangulation; this determination is made electronically. Our analysis will be conducted on a modern data station.

Compounds in mixture: boiling points. Cyclohexane 80 o. C Toluene 110 o. C Mixture separates by distillation according to the boiling point. Compounds with the lower bp come off first! The same is true on the gas chromatographic column; the lower boiling compound comes off first!

How to identify the components in your unknown mixture Use the retention time information from your gas chromatograms to provide a positive identification of each of the components in the mixture. Don’t rely on the distillation plot to determine the composition of your mixture!

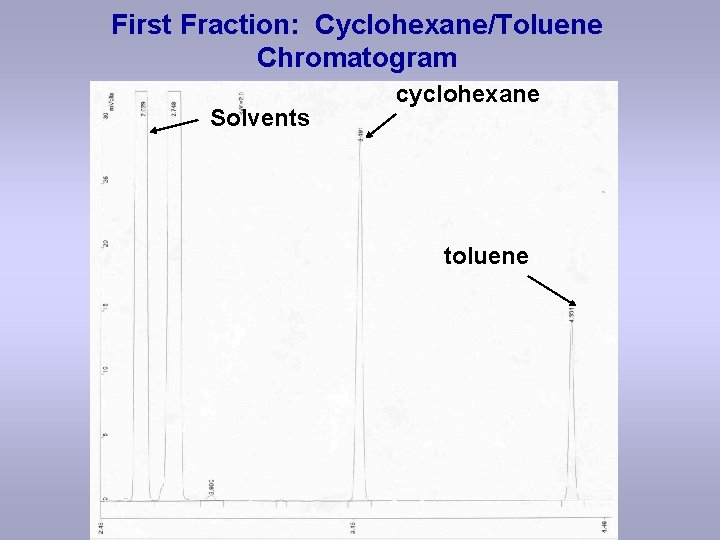
First Fraction: Cyclohexane/Toluene Chromatogram Solvents cyclohexane toluene

Data: Cyclohexane/Toluene First Fraction solvents cyclohexane toluene ?

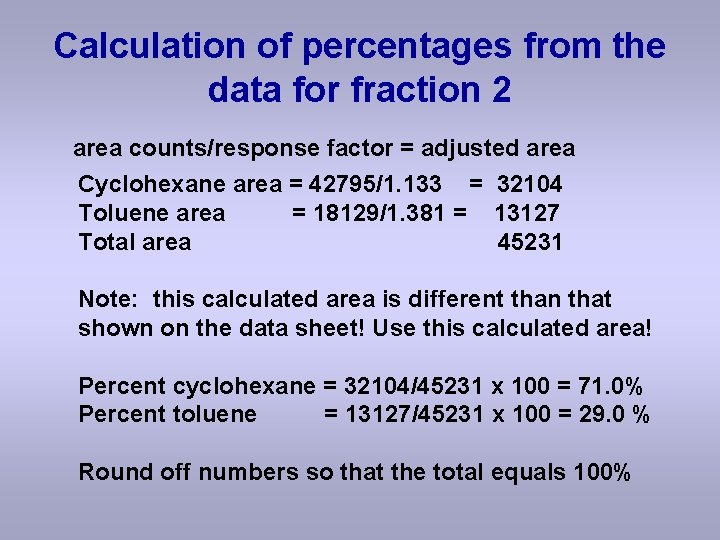
Calculation of percentages from the data for fraction 2 area counts/response factor = adjusted area Cyclohexane area = 42795/1. 133 = 32104 Toluene area = 18129/1. 381 = 13127 Total area 45231 Note: this calculated area is different than that shown on the data sheet! Use this calculated area! Percent cyclohexane = 32104/45231 x 100 = 71. 0% Percent toluene = 13127/45231 x 100 = 29. 0 % Round off numbers so that the total equals 100%

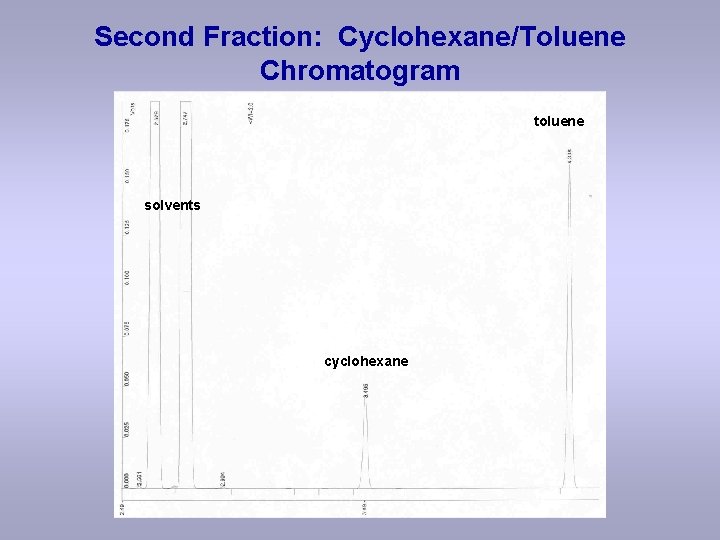
Second Fraction: Cyclohexane/Toluene Chromatogram toluene solvents cyclohexane

Data: Cyclohexane/Toluene Second Fraction solvents cyclohexane toluene ?

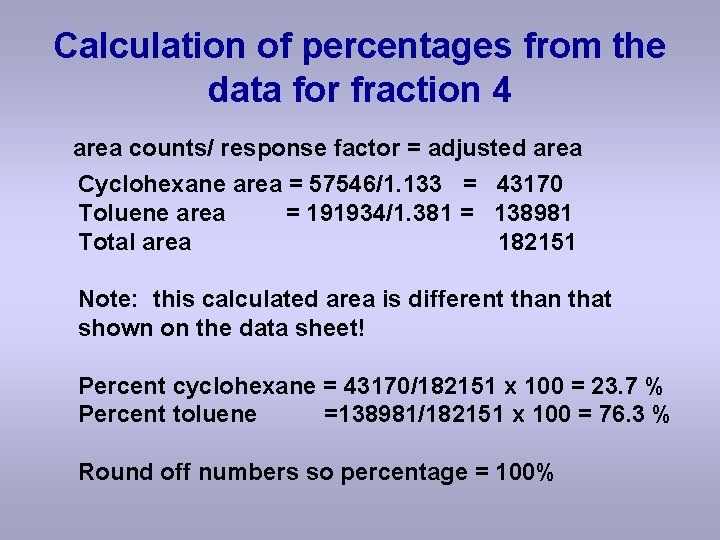
Calculation of percentages from the data for fraction 4 area counts/ response factor = adjusted area Cyclohexane area = 57546/1. 133 = 43170 Toluene area = 191934/1. 381 = 138981 Total area 182151 Note: this calculated area is different than that shown on the data sheet! Percent cyclohexane = 43170/182151 x 100 = 23. 7 % Percent toluene =138981/182151 x 100 = 76. 3 % Round off numbers so percentage = 100%