The Mole 1 amu 1 66 x 10














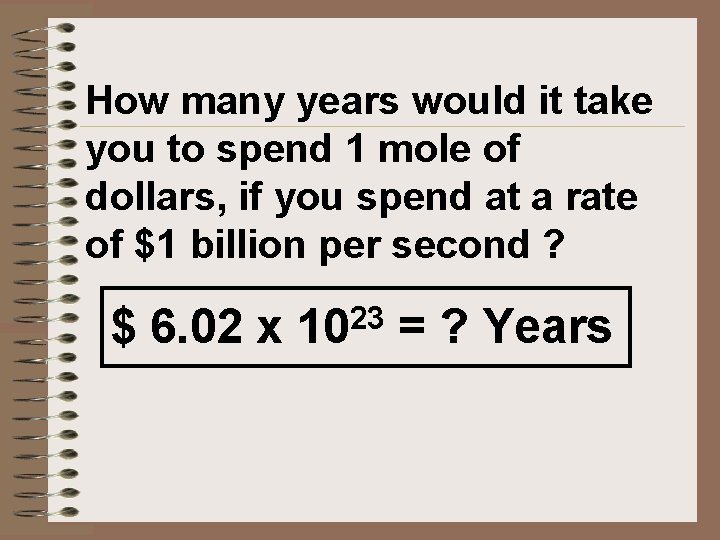



















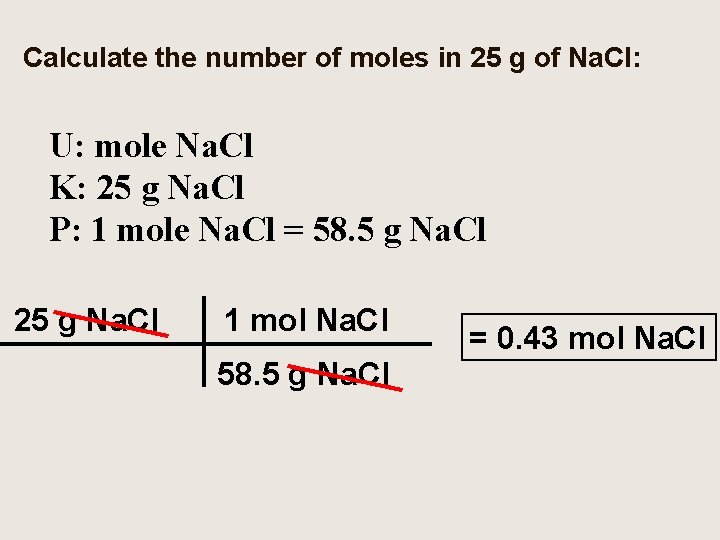


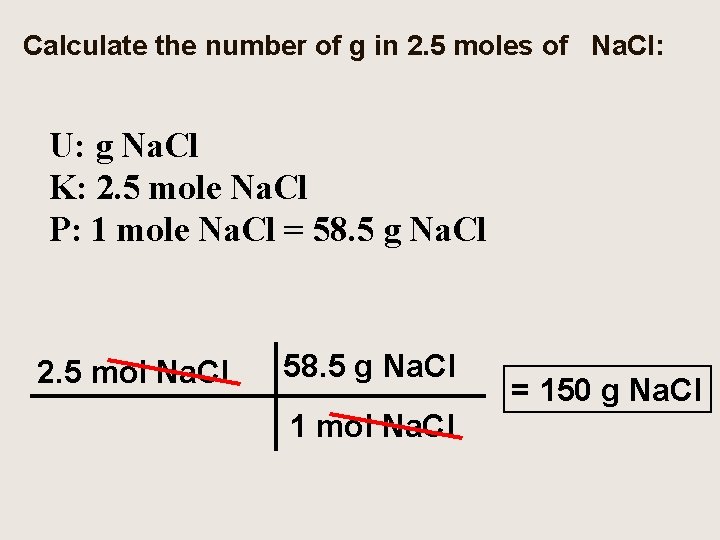










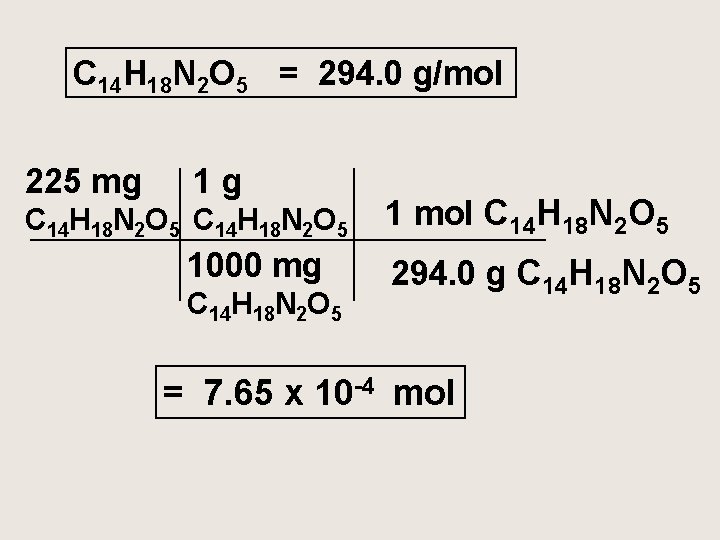




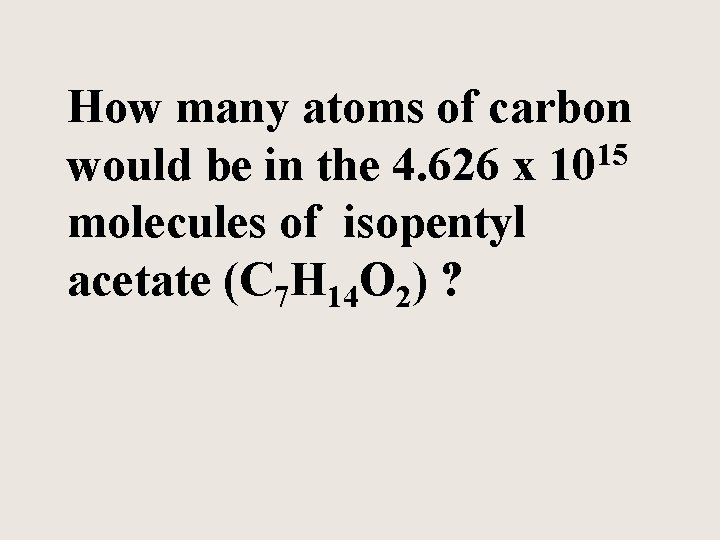















- Slides: 130

The Mole

• • 1 amu = 1. 66 x 10 -24 grams 1 C-12 atom = 12 amu (12)(1. 66 x 10 -24 g) = 1. 992 x 10 -23 grams is the mass of one carbon-12 atom • But … we do not work with single atoms or with gram values this small • We need reasonable numbers to work with in the laboratory

The Mole Concept

STOICHIOMETRY - the study of the quantitative aspects of chemical reactions.

What is the mole? We’re not talking about this kind of mole!

The Mole • The mole is a number…. . • A HUGE number…… • But still just a number…. . • A mole is a collection of 6. 02 x 1023 particles 602, 000, 000, 000 These particles may be atoms, molecules, ions, or electrons

1 dozen = 12 1 gross = 144 1 mole = 6. 02 x 1023 Just how large is this number? 602, 000, 000, 000 It is really hard to relate to a number this large ………. but let’s try and see if we can make sense the enormity of the number.

If all 7 billion people on Earth were to do nothing but count the atoms in 1 mole of an element, 24 hours a day, at the rate of 1 atom per second…….

It would take 4 million years !!

1 mole of seconds represents a span of time…. .

4 million times as long as the Earth has already existed !!

1 mole of marbles is enough marbles to cover the entire Earth…

to a depth of 50 miles

1 mole of rice grains has a mass equal to….

each of the 7 billion people on earth having 1 million cars each

A mole of dollars ? ?
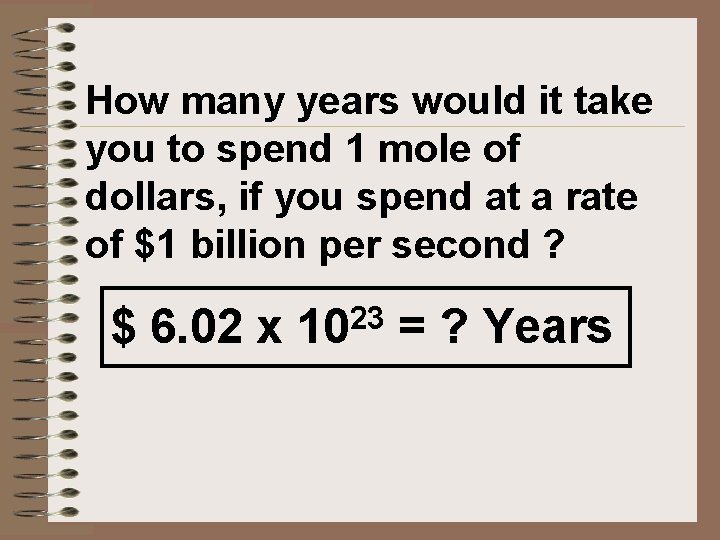
How many years would it take you to spend 1 mole of dollars, if you spend at a rate of $1 billion per second ? $ 6. 02 x 1023 = ? Years

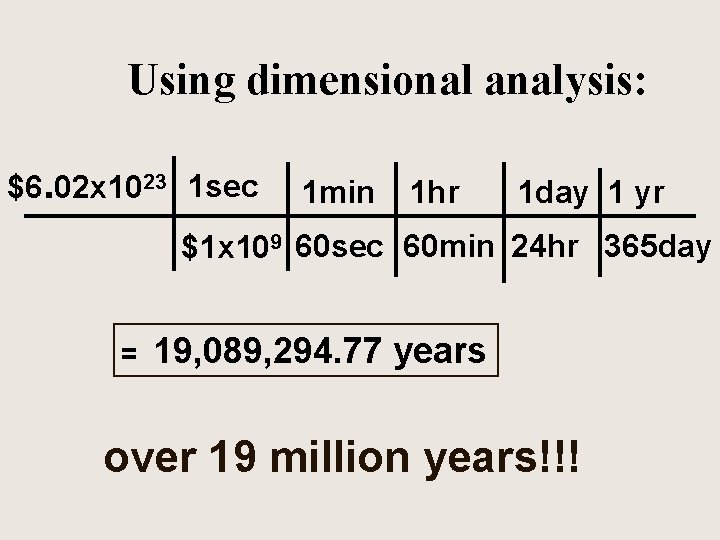
Using dimensional analysis: $6. 02 x 1023 1 sec 1 min 1 hr 1 day 1 yr $1 x 109 60 sec 60 min 24 hr 365 day = 19, 089, 294. 77 years over 19 million years!!!

And yet there is 1 mole of water 23 molecules – 6. 022 x 10 molecules of water in only 18 m. L of water !!



Say you had a mole of paper and stacked it toward the sky, Paper's really thin, but that pile wou get so high, It'd reach up into outer space, in fact I think you'd find, It'd go up to the moon and back, eighty billion times.

Say you had a mole of atoms, would the pile be immense? Should I say the answer now, or leave you in suspense? Well, atoms are so very, very small, you understand, You could hold a mole of atoms in the palm of your hand.

So shake a little sugar in the middle of your palm Now you don't want to spill it, so try and stay calm. You hardly can imagine and barely realize, There are more atoms in that sugar than stars up in the sky.

• Why is this number so special? • The mole is defined as the number of atoms of 12 C in exactly 12. 000 grams of pure 12 C • There are exactly 1 mole of atoms in the atomic mass of an element when that mass is expressed in grams

Avogadro’s Number • 1 mole = 6. 022 x particles • 1 mole = Avogadro’s number • 1 mole = the atomic mass of an element expressed in grams 23 10

What is the mass of 1 mole of carbon ? 12. 011 grams How many atoms? 6. 022 x 1023 atoms What is the mass of ½ mole of carbon ? 6. 0055 grams

12. 011 grams of carbon contains the same # of atoms as 1 mole of carbon

What is the mass of 1 mole of gold ? 196. 967 grams How many atoms? 6. 022 x 1023 atoms What is the mass of ½ mole of gold ? 98. 4835 grams

12. 011 grams of carbon contains the same # of atoms as 196. 967 grams of gold.

What is the mass of 1 mole of Mg ? 24. 305 grams What is the mass of 2 moles of Mg ? 48. 610 grams How many atoms? 1. 204 x 1024 atoms

12. 011 grams of carbon contains the same # of atoms as 196. 967 grams of gold and the same # of atoms as 24. 305 grams of magnesium.


Molar Mass: The mass of 1 mole of a substance in grams The substance can be a compound or an element

Molar Mass § Molar mass is the generic term for the mass of one mole of any substance (expressed in grams/mol) § The same as: § 1) Gram Molecular Mass (for molecules) § 2) Gram Formula Mass (ionic compounds) § 3) Gram Atomic Mass (for elements) molar mass is just a much broader term than the others

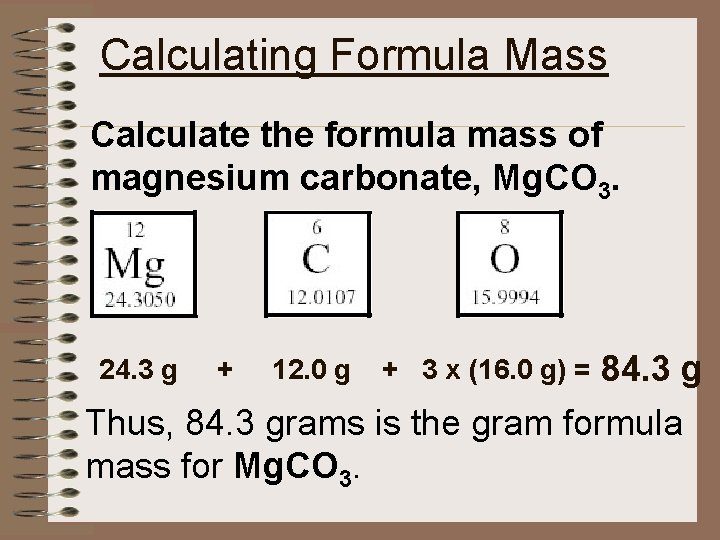
Calculating Formula Mass Calculate the formula mass of magnesium carbonate, Mg. CO 3. 84. 3 g Thus, 84. 3 grams is the gram formula mass for Mg. CO 3. 24. 3 g + 12. 0 g + 3 x (16. 0 g) =

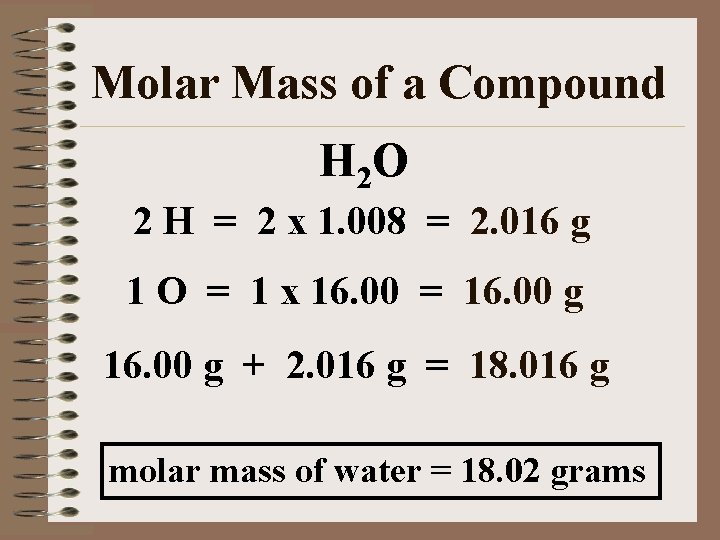
Molar Mass of a Compound H 2 O 2 H = 2 x 1. 008 = 2. 016 g 1 O = 1 x 16. 00 = 16. 00 g + 2. 016 g = 18. 016 g molar mass of water = 18. 02 grams

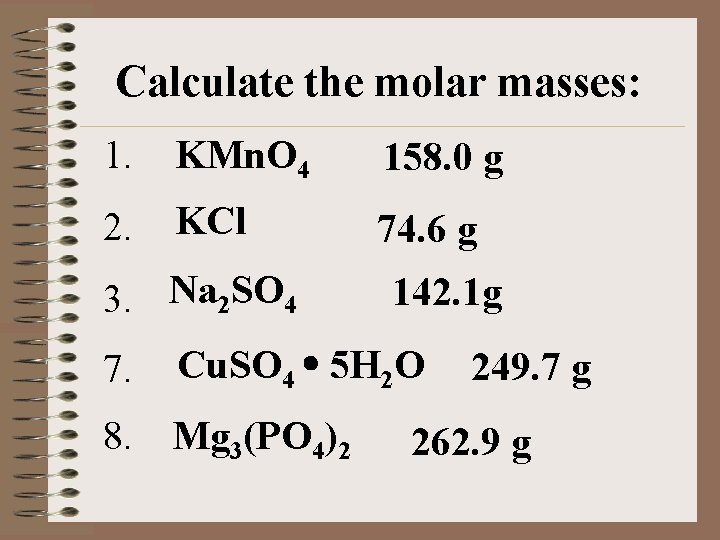
Calculate the molar masses: 1. KMn. O 4 158. 0 g 2. KCl 74. 6 g 3. Na 2 SO 4 7. 142. 1 g Cu. SO 4 5 H 2 O 8. Mg 3(PO 4)2 249. 7 g 262. 9 g


Mole Conversions

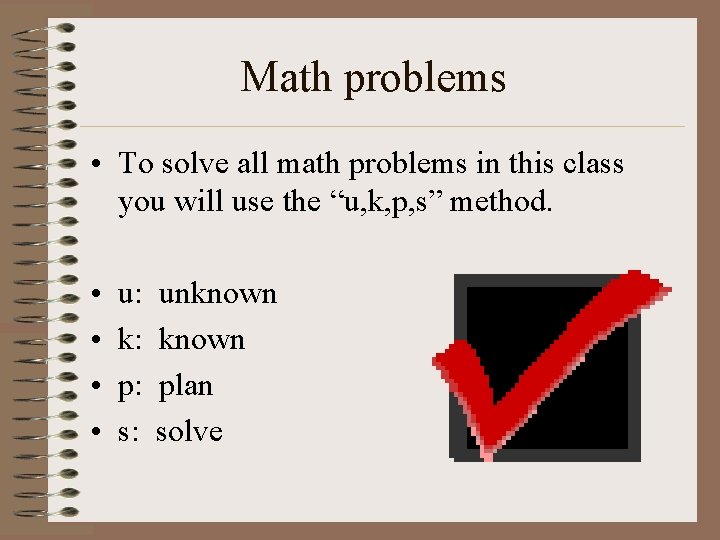
Math problems • To solve all math problems in this class you will use the “u, k, p, s” method. • • u: k: p: s: unknown plan solve

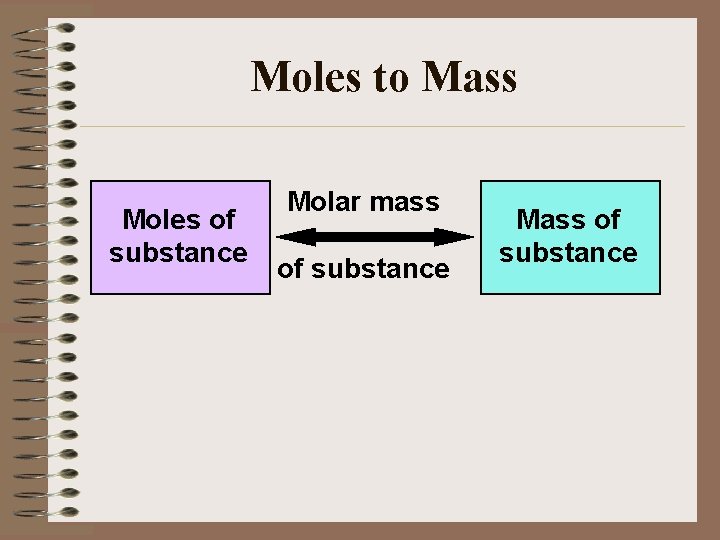
Moles to Mass Moles of substance Molar mass of substance Mass of substance

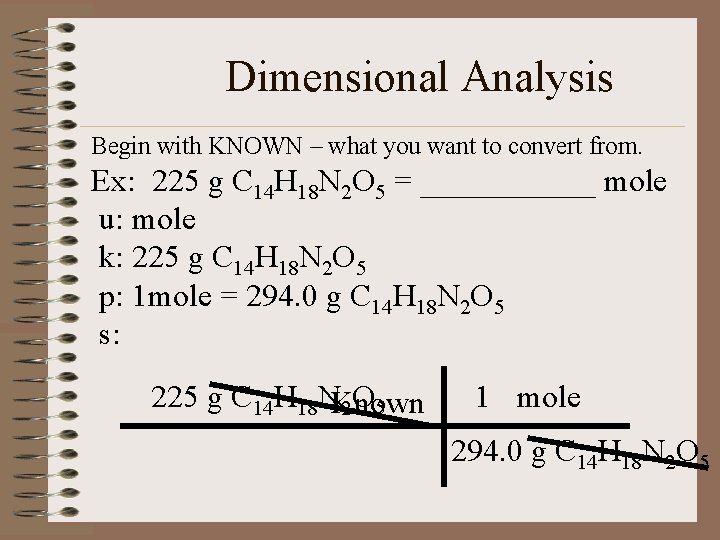
Learning Check! The artificial sweetener aspartame (Nutra-Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame?

Dimensional Analysis Begin with KNOWN – what you want to convert from. Ex: 225 g C 14 H 18 N 2 O 5 = ______ mole u: mole k: 225 g C 14 H 18 N 2 O 5 p: 1 mole = 294. 0 g C 14 H 18 N 2 O 5 s: 225 g C 14 H 18 NKnown 2 O 5 1 mole 294. 0 g C 14 H 18 N 2 O 5

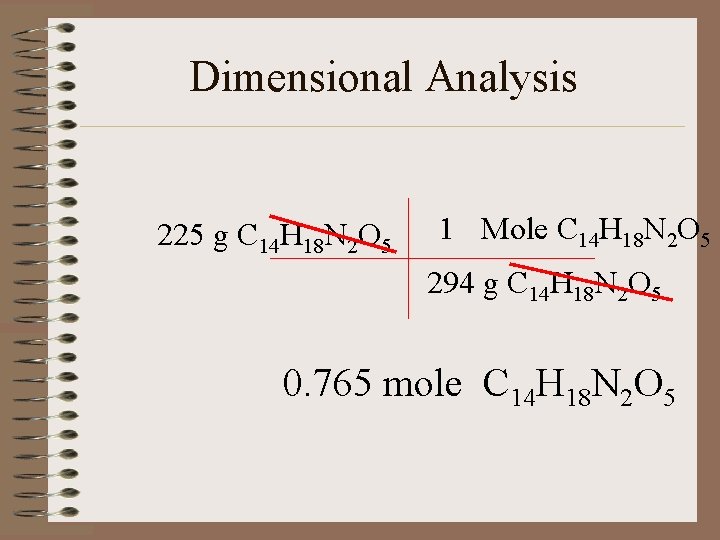
Dimensional Analysis 225 g C 14 H 18 N 2 O 5 1 Mole C 14 H 18 N 2 O 5 294 g C 14 H 18 N 2 O 5 0. 765 mole C 14 H 18 N 2 O 5
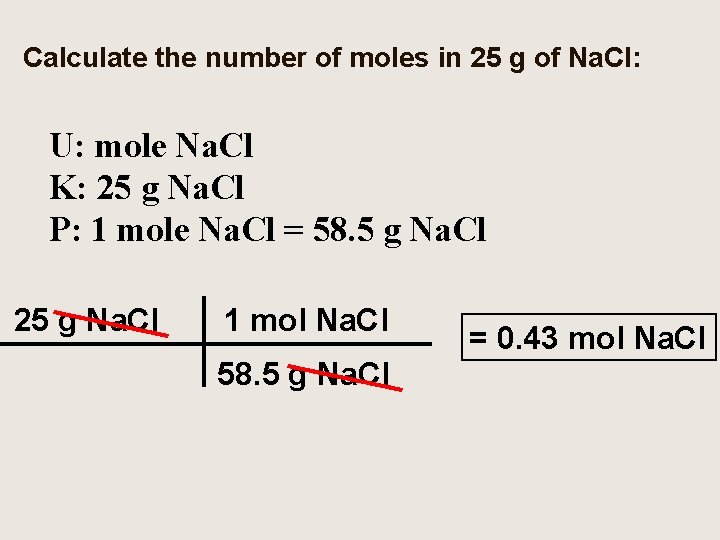
Calculate the number of moles in 25 g of Na. Cl: U: mole Na. Cl K: 25 g Na. Cl P: 1 mole Na. Cl = 58. 5 g Na. Cl 25 g Na. Cl 1 mol Na. Cl 58. 5 g Na. Cl = 0. 43 mol Na. Cl

Continue working problems from worksheet 2: 2. Determine the number of moles of present in 125. 0 g of H 2 SO 4

Continue working problems from worksheet 2: 2. Determine the number of moles of present in 125. 0 g of H 2 SO 4 1. 3 mole
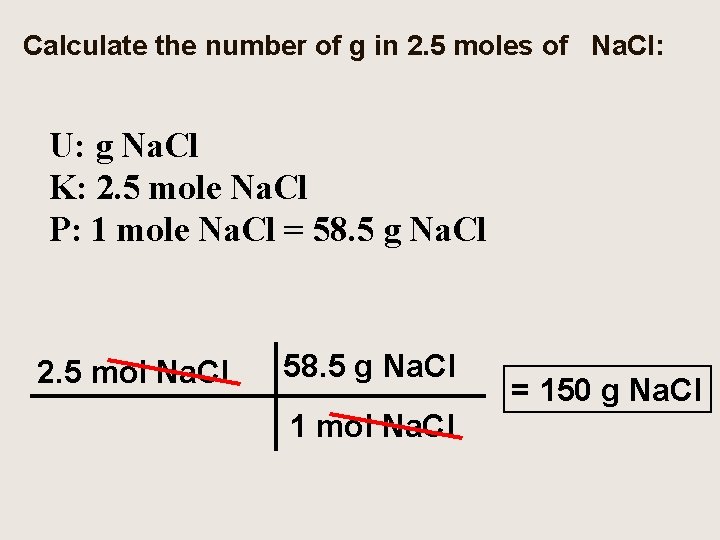
Calculate the number of g in 2. 5 moles of Na. Cl: U: g Na. Cl K: 2. 5 mole Na. Cl P: 1 mole Na. Cl = 58. 5 g Na. Cl 2. 5 mol Na. Cl 58. 5 g Na. Cl 1 mol Na. Cl = 150 g Na. Cl

Continue working problems from worksheet 2: 7. Determine the number of grams 0. 50 moles of H 2 SO 4

Continue working problems from worksheet 2: 7. Determine the number of grams 0. 50 moles of H 2 SO 4 49 g H 2 SO 4

Molar Volume The volumes of gases can be compared at STP, (Standard Temperature and Pressure) Standard temperature (T) • 0°C or 273 K Standard pressure (P) • 1 atm (760 mm Hg)

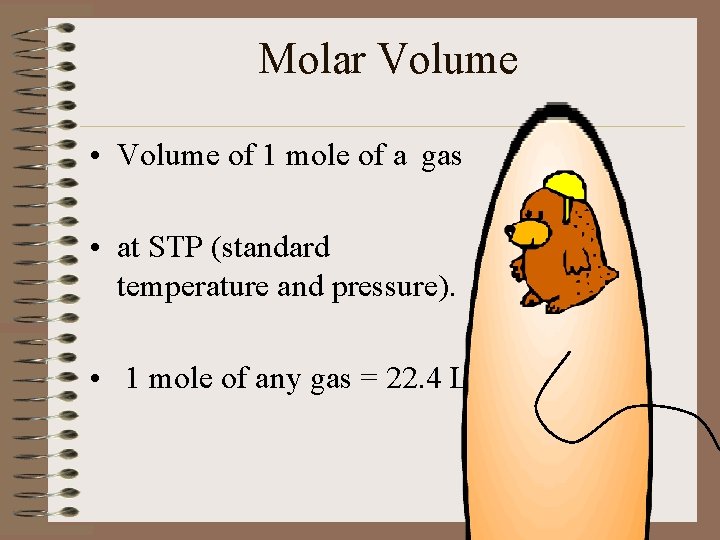
Molar Volume • Volume of 1 mole of a gas • at STP (standard temperature and pressure). • 1 mole of any gas = 22. 4 L

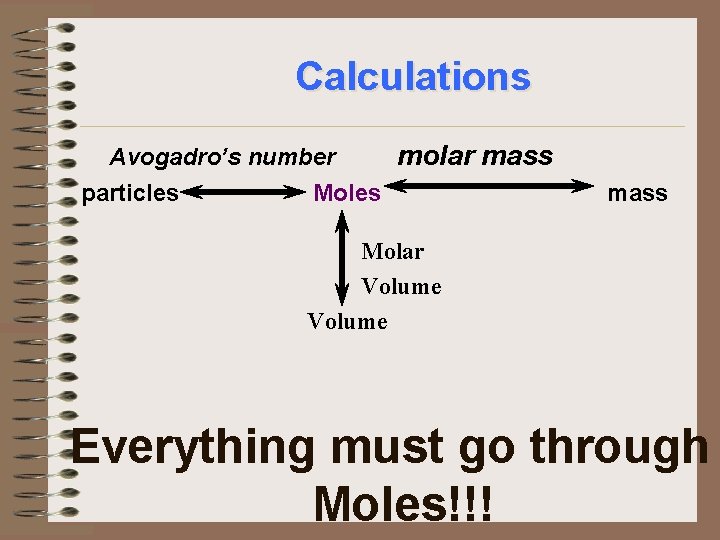
Calculations Moles of substance Molar Volume of substance Everything must go through Moles!!!

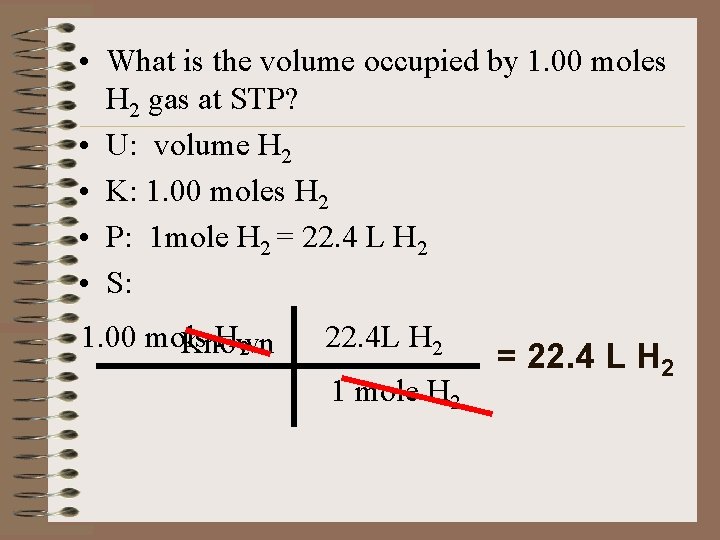
• What is the volume occupied by 1. 00 moles H 2 gas at STP? • U: volume H 2 • K: 1. 00 moles H 2 • P: 1 mole H 2 = 22. 4 L H 2 • S: 1. 00 mols H 2 Known 22. 4 L H 2 1 mole H 2 = 22. 4 L H 2

Continue working problems from worksheet 3: 2. What volume is occupied by 3. 20 mole of O 2

Continue working problems from worksheet 3: 2. What volume is occupied by 3. 20 mole of O 2 71. 7 L O 2

Moles to Number of Entities Number of atoms or molecules Avogadro’s number Moles of substance

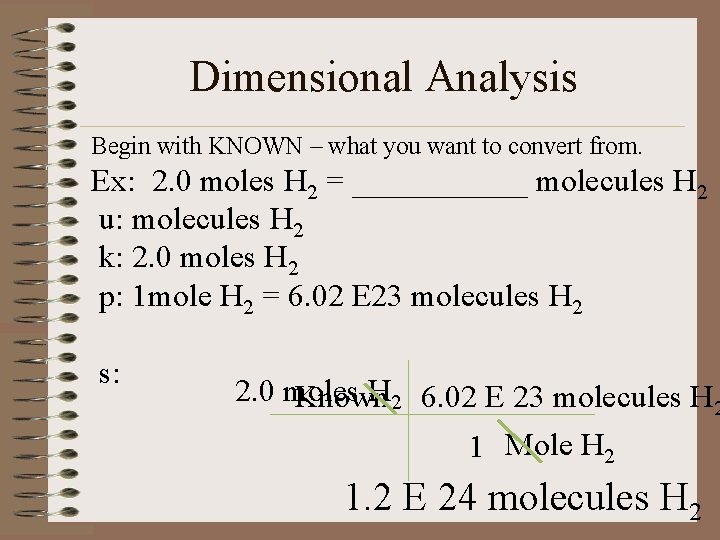
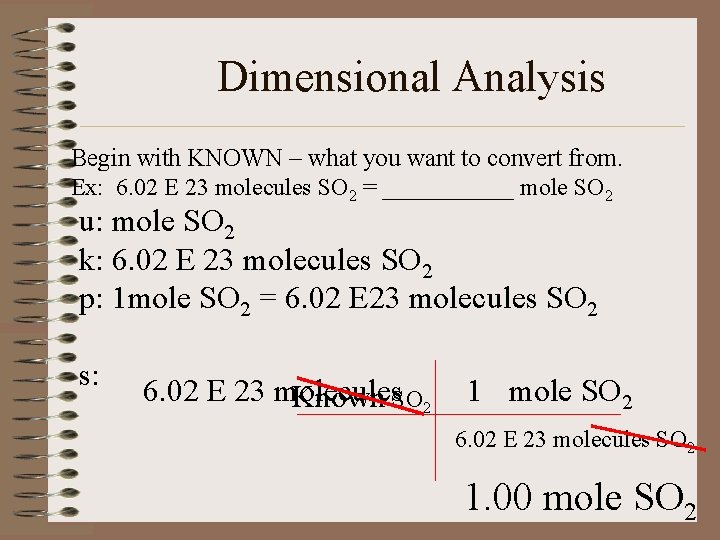
Dimensional Analysis Begin with KNOWN – what you want to convert from. Ex: 2. 0 moles H 2 = ______ molecules H 2 u: molecules H 2 k: 2. 0 moles H 2 p: 1 mole H 2 = 6. 02 E 23 molecules H 2 s: 2. 0 moles H 2 6. 02 E 23 molecules H 2 Known 1 Mole H 2 1. 2 E 24 molecules H 2

Dimensional Analysis Begin with KNOWN – what you want to convert from. Ex: 6. 02 E 23 molecules SO 2 = ______ mole SO 2 u: mole SO 2 k: 6. 02 E 23 molecules SO 2 p: 1 mole SO 2 = 6. 02 E 23 molecules SO 2 s: 6. 02 E 23 molecules Known SO 2 1 mole SO 2 6. 02 E 23 molecules SO 2 1. 00 mole SO 2

Atoms/Molecules and Grams • Since 6. 02 X 1023 particles = 1 mole AND 1 mole = molar mass (grams) • You can convert atoms/molecules to moles and then moles to grams! (Two step process) • You can’t go directly from atoms to grams!!!! You MUST go thru MOLES. • That’s like asking what is the weight in ounces of 2 dozen cookies, if 1 cookie weighs 4 oz? You have to convert to dozen first!

Calculations Avogadro’s number molar mass particles Moles mass Molar Volume Everything must go through Moles!!!


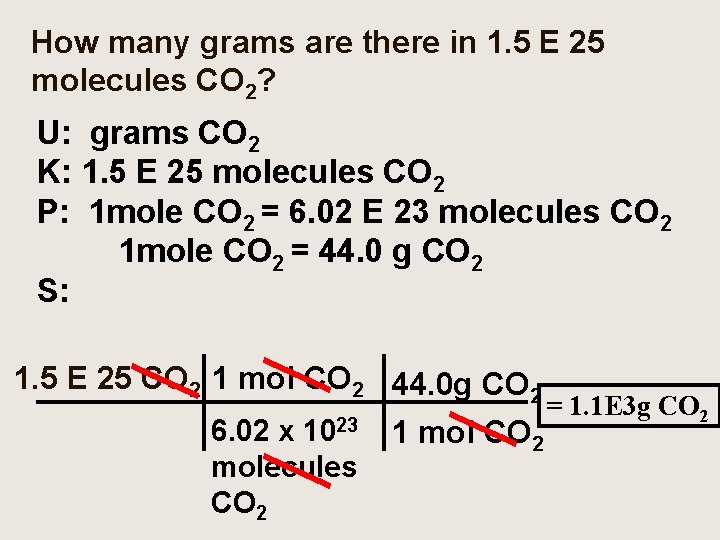
How many grams are there in 1. 5 E 25 molecules CO 2? U: grams CO 2 K: 1. 5 E 25 molecules CO 2 P: 1 mole CO 2 = 6. 02 E 23 molecules CO 2 1 mole CO 2 = 44. 0 g CO 2 S: 1. 5 E 25 CO 2 1 mol CO 2 44. 0 g CO 2 = 1. 1 E 3 g CO 2 6. 02 x 1023 1 mol CO 2 molecules CO 2

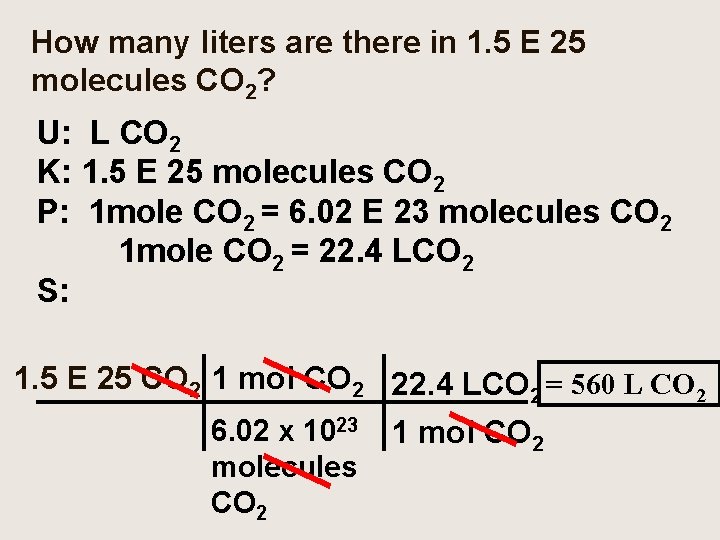
How many liters are there in 1. 5 E 25 molecules CO 2? U: L CO 2 K: 1. 5 E 25 molecules CO 2 P: 1 mole CO 2 = 6. 02 E 23 molecules CO 2 1 mole CO 2 = 22. 4 LCO 2 S: 1. 5 E 25 CO 2 1 mol CO 2 22. 4 LCO 2 = 560 L CO 2 6. 02 x 1023 1 mol CO 2 molecules CO 2


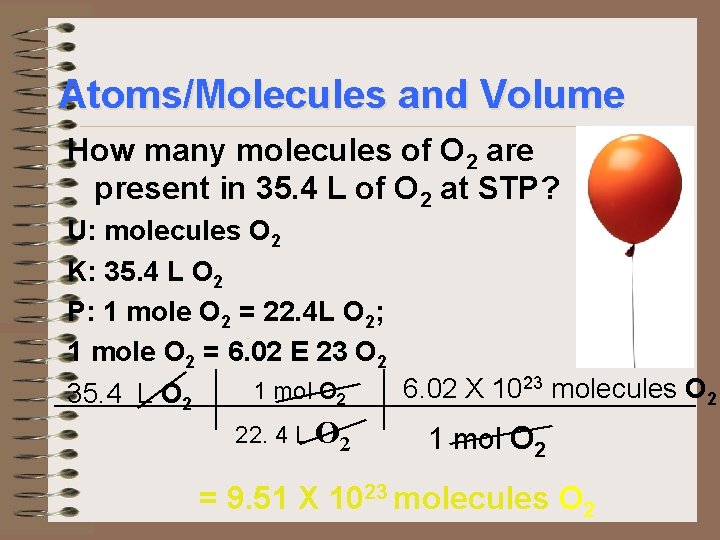
Atoms/Molecules and Volume How many molecules of O 2 are present in 35. 4 L of O 2 at STP? U: molecules O 2 K: 35. 4 L O 2 P: 1 mole O 2 = 22. 4 L O 2; 1 mole O 2 = 6. 02 E 23 O 2 6. 02 X 1023 molecules O 2 1 mol O 2 35. 4 L O 2 22. 4 L O 2 1 mol O 2 = 9. 51 X 1023 molecules O 2

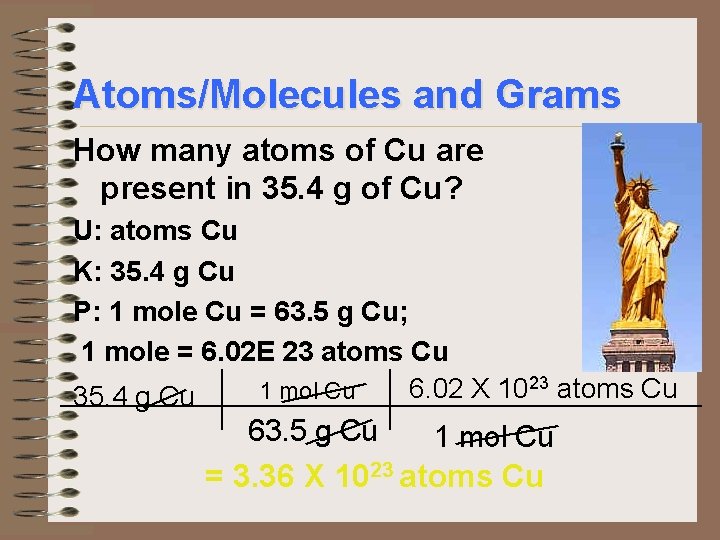
Atoms/Molecules and Grams How many atoms of Cu are present in 35. 4 g of Cu? U: atoms Cu K: 35. 4 g Cu P: 1 mole Cu = 63. 5 g Cu; 1 mole = 6. 02 E 23 atoms Cu 6. 02 X 10 1 mol Cu 35. 4 g Cu 63. 5 g Cu 1 mol Cu = 3. 36 X 1023 atoms Cu

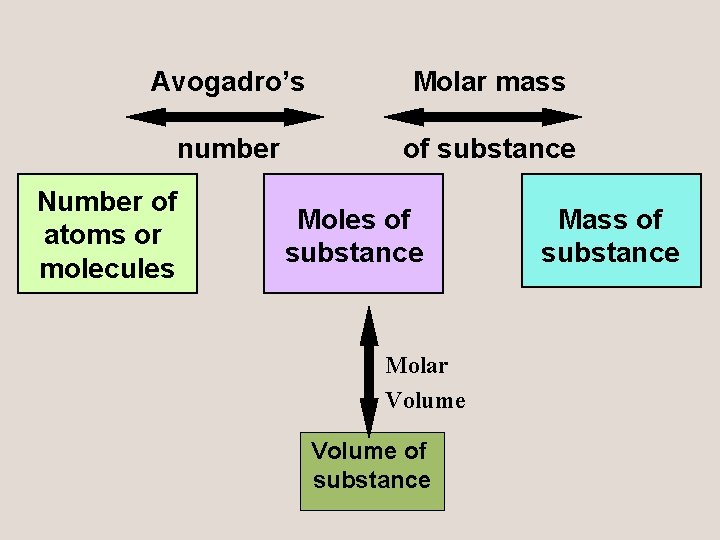
Avogadro’s Molar mass number of substance Number of atoms or molecules Moles of substance Molar Volume of substance Mass of substance

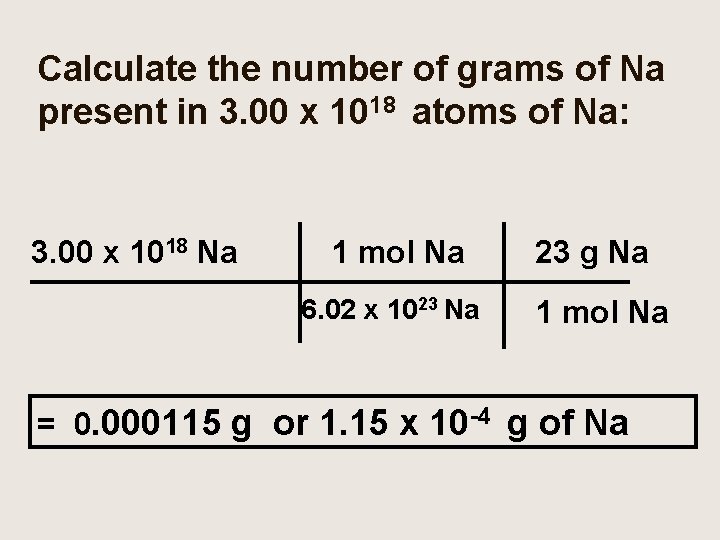
Calculate the number of grams of Na present in 3. 00 x 1018 atoms of Na: 3. 00 x 1018 Na 1 mol Na 6. 02 x 1023 Na 23 g Na 1 mol Na = 0. 000115 g or 1. 15 x 10 -4 g of Na

Calculate the number of atoms of Na in 160. 00 grams of Na: 160. 00 g Na 1 mol Na 23. 0 g Na 6. 02 x 1023 Na 1 mol Na = 4. 1878 x 1024 atoms of Na

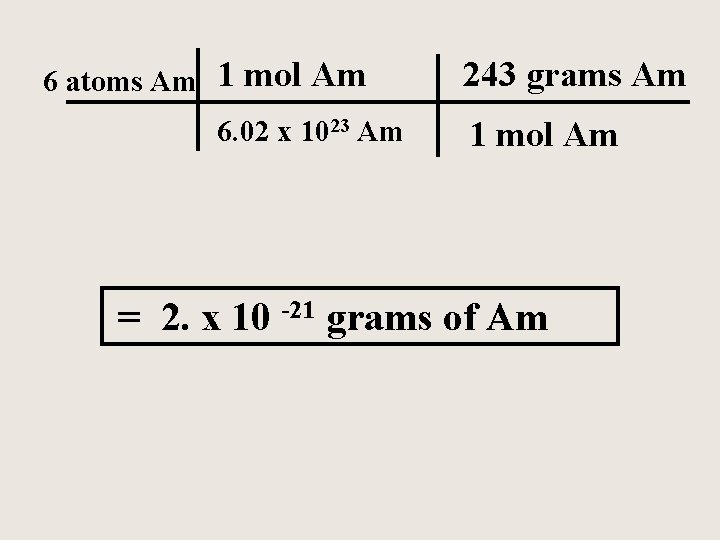
Americium is an element that does not occur naturally. It can be made in very small amounts in a particle accelerator. Calculate the mass in grams of a sample of americium containing only six atoms.

6 atoms Am 1 mol Am 243 grams Am 6. 02 x 1023 Am 1 mol Am = 2. x 10 -21 grams of Am

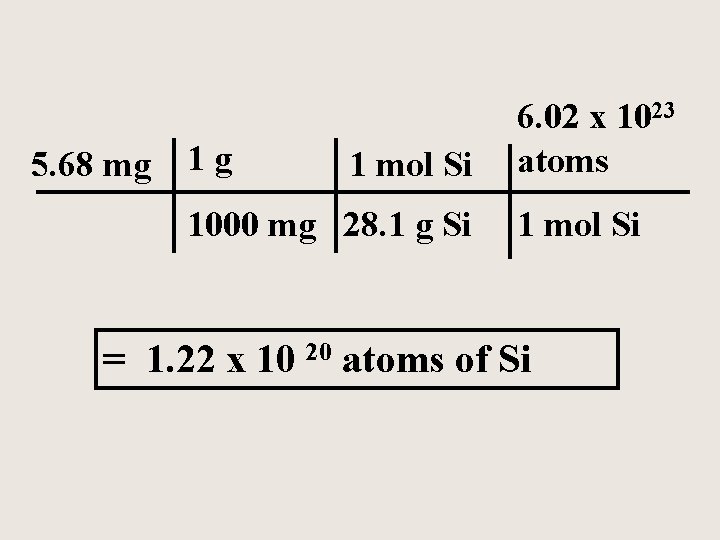
A silicon chip used in an integrated circuit of a microcomputer has a mass of 5. 68 mg. How many silicon (Si) atoms are present in the chip?

5. 68 mg 1 g 1 mol Si 1000 mg 28. 1 g Si 6. 02 x 1023 atoms 1 mol Si = 1. 22 x 10 20 atoms of Si

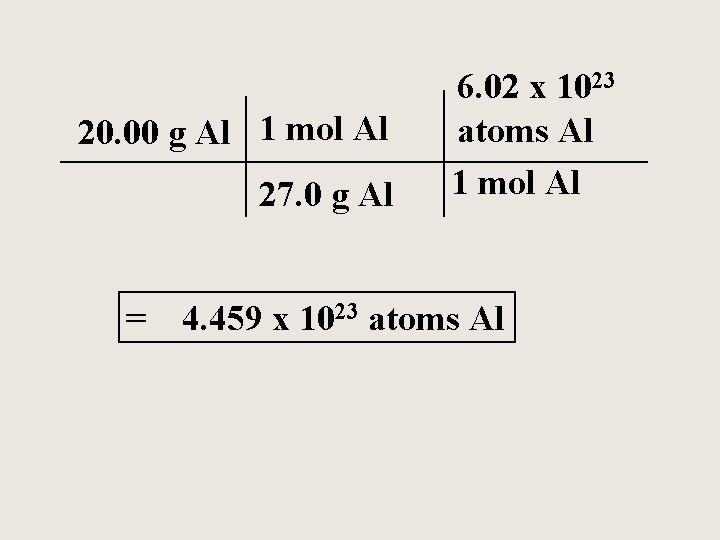
Aluminum is often used for the structure of light-weight bicycle frames. How many atoms of Al are in 20. 00 grams of Al?

20. 00 g Al 1 mol Al 27. 0 g Al = 6. 02 x 1023 atoms Al 1 mol Al 4. 459 x 1023 atoms Al

The artificial sweetener aspartame (Nutri-Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 mg of aspartame?
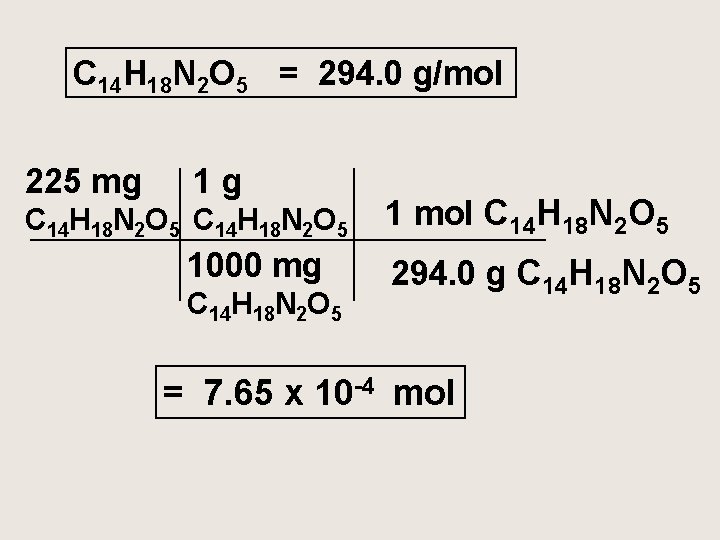
C 14 H 18 N 2 O 5 = 294. 0 g/mol 225 mg 1 g C 14 H 18 N 2 O 5 1000 mg C 14 H 18 N 2 O 5 1 mol C 14 H 18 N 2 O 5 294. 0 g C 14 H 18 N 2 O 5 = 7. 65 x 10 -4 mol


More Calculations with the Mole

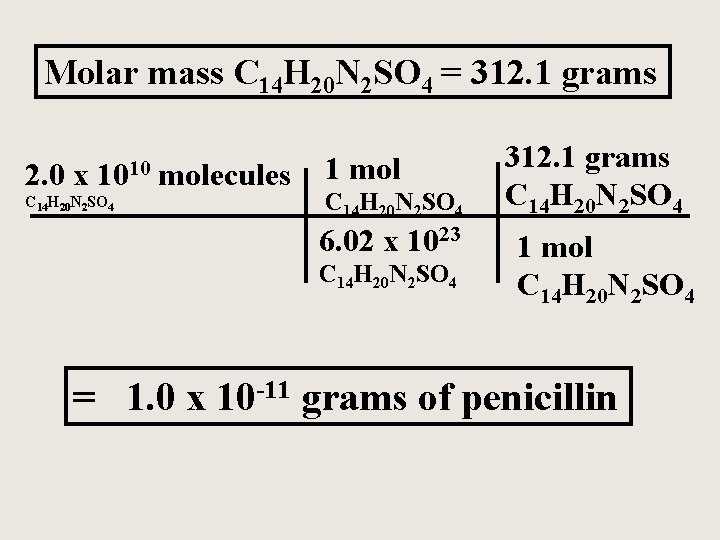
Penicillin, the first of a now large number of antibiotics, has the formula C 14 H 20 N 2 SO 4. Calculate the mass of 2. 0 x 1010 molecules of penicillin.

Molar mass C 14 H 20 N 2 SO 4 = 312. 1 grams 2. 0 x 1010 C 14 H 20 N 2 SO 4 molecules 1 mol C 14 H 20 N 2 SO 4 6. 02 x 1023 C 14 H 20 N 2 SO 4 312. 1 grams C 14 H 20 N 2 SO 4 1 mol C 14 H 20 N 2 SO 4 = 1. 0 x 10 -11 grams of penicillin

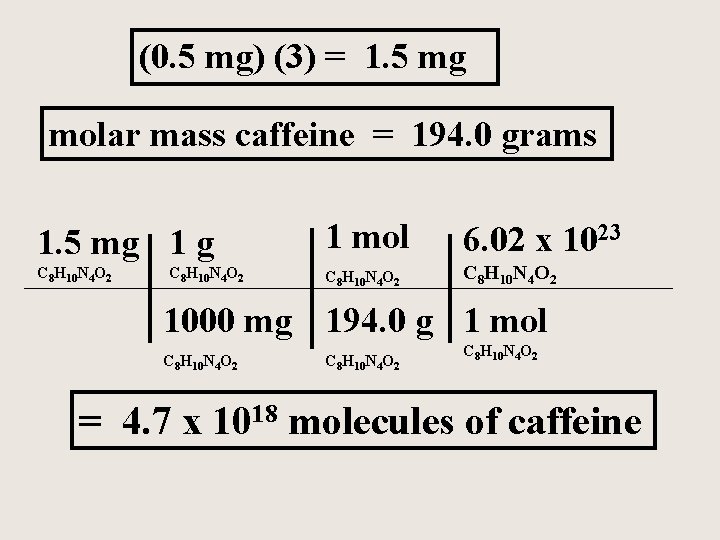
A student drinks 3 cups of coffee in order to stay awake and study. Coffee contains the compound caffeine, a stimulant , with the formula C 8 H 10 N 4 O 2 If the caffeine content in an average cup of coffee is 0. 5 mg. How many molecules of caffeine did the student ingest?

(0. 5 mg) (3) = 1. 5 mg molar mass caffeine = 194. 0 grams 1. 5 mg 1 mol 6. 02 x 1023 C 8 H 10 N 4 O 2 1000 mg 194. 0 g 1 mol C 8 H 10 N 4 O 2 = 4. 7 x 1018 molecules of caffeine

Isopentyl acetate (C 7 H 14 O 2) is the compound responsible for the scent of bananas. Bees release about 1 g of this compound when they sting. The resulting scent attracts other bees to join the attack.

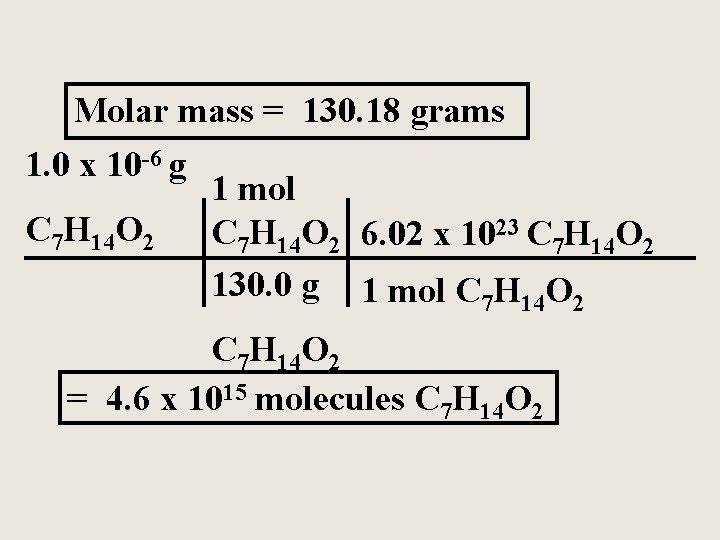
How many molecules of isopentyl acetate (C 7 H 14 O 2) are released in a typical bee sting of about 1 g (1 x 10 -6 g) ?

Molar mass = 130. 18 grams 1. 0 x 10 -6 g C 7 H 14 O 2 1 mol C 7 H 14 O 2 6. 02 x 1023 C 7 H 14 O 2 130. 0 g 1 mol C 7 H 14 O 2 = 4. 6 x 1015 molecules C 7 H 14 O 2
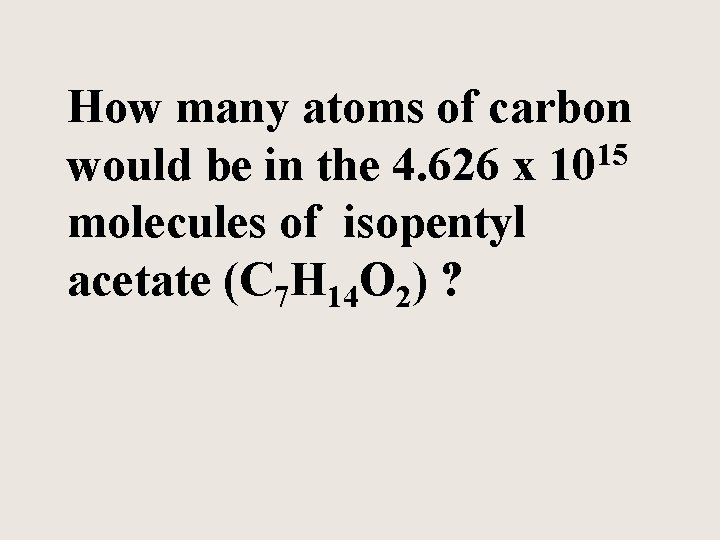
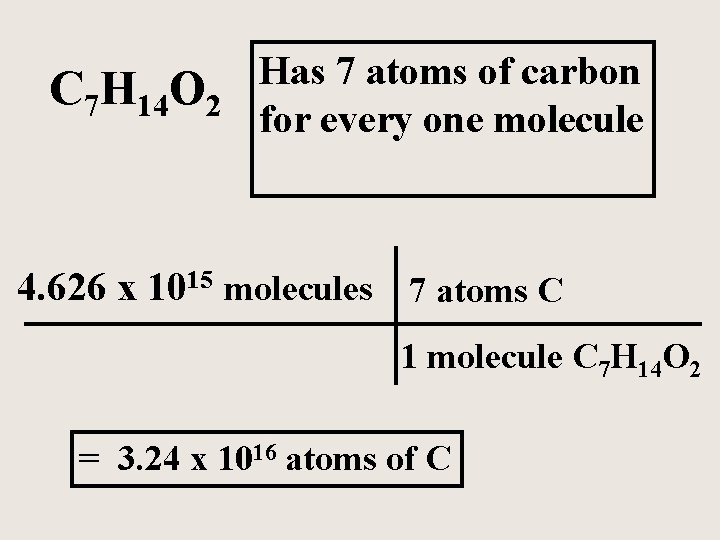
How many atoms of carbon would be in the 4. 626 x 1015 molecules of isopentyl acetate (C 7 H 14 O 2) ?

C 7 H 14 O 2 Has 7 atoms of carbon for every one molecule 4. 626 x 1015 molecules 7 atoms C 1 molecule C 7 H 14 O 2 = 3. 24 x 1016 atoms of C

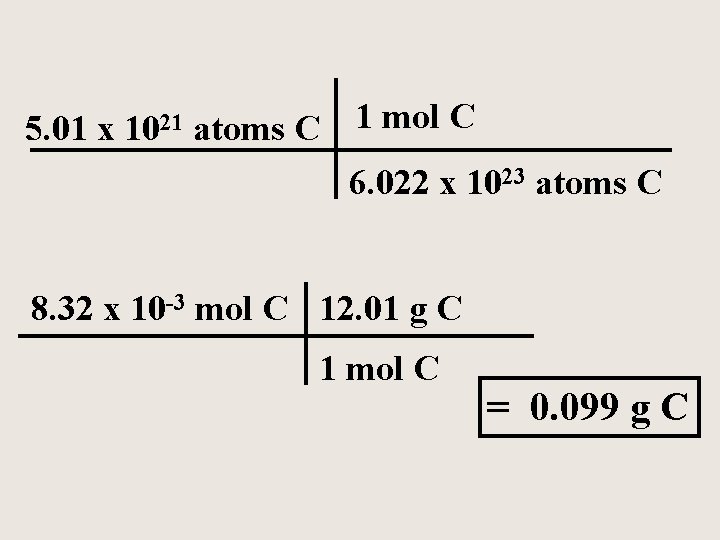
Diamond is a natural form of pure carbon A diamond contains 5. 0 x 1021 atoms of carbon. How many moles of carbon and how many grams of carbon are in this diamond?

5. 01 x 1021 atoms C 1 mol C 6. 022 x 1023 atoms C 8. 32 x 10 -3 mol C 12. 01 g C 1 mol C = 0. 099 g C

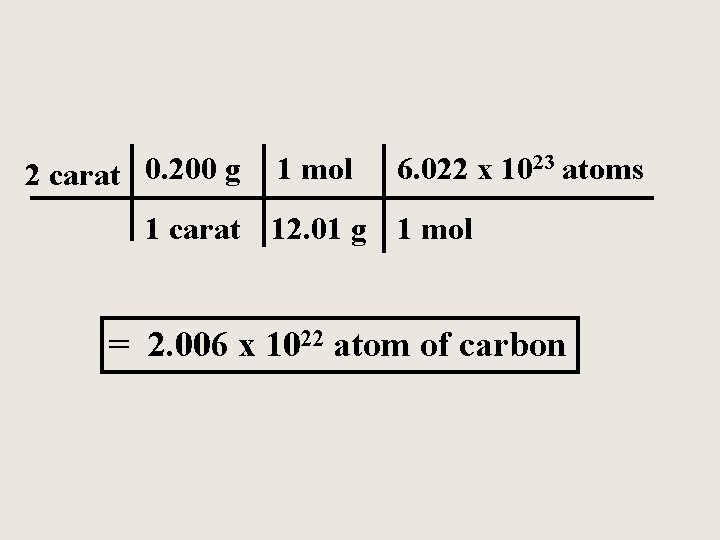
How many atoms of carbon are in a 2. 00 carat diamond? ( 1. 00 carat = 0. 200 grams )

2 carat 0. 200 g 1 mol 6. 022 x 1023 atoms 1 carat 12. 01 g 1 mol = 2. 006 x 1022 atom of carbon

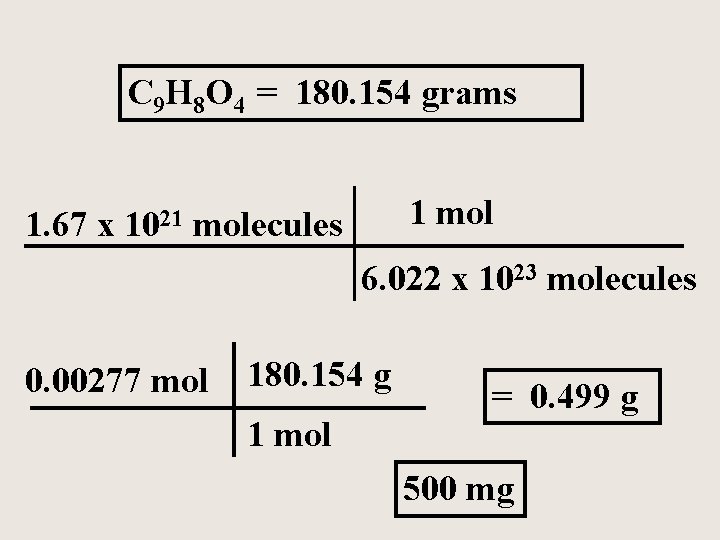
The formula for acetylsalicylic acid (aspirin) is C 9 H 8 O 4 A typical aspirin tablet contains 1. 67 x 1021 molecules of aspirin. How many moles and how many grams of aspirin are in a typical tablet?

C 9 H 8 O 4 = 180. 154 grams 1 mol 1. 67 x 1021 molecules 6. 022 x 1023 molecules 0. 00277 mol 180. 154 g 1 mol = 0. 499 g 500 mg

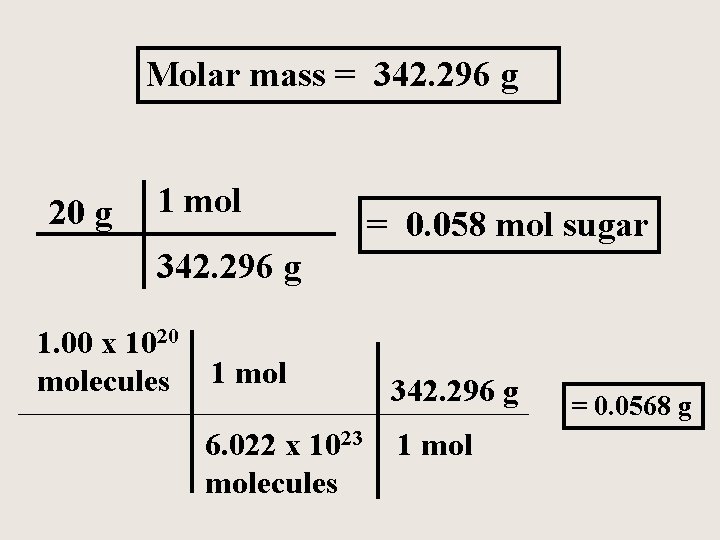
Sucrose or table sugar has the formula C 12 H 22 O 11 How many moles of sugar are in 20 grams of sugar? What is the mass of 1. 00 x 1020 molecules of sugar?

Molar mass = 342. 296 g 20 g 1 mol = 0. 058 mol sugar 342. 296 g 1. 00 x 1020 molecules 1 mol 6. 022 x 1023 molecules 342. 296 g 1 mol = 0. 0568 g

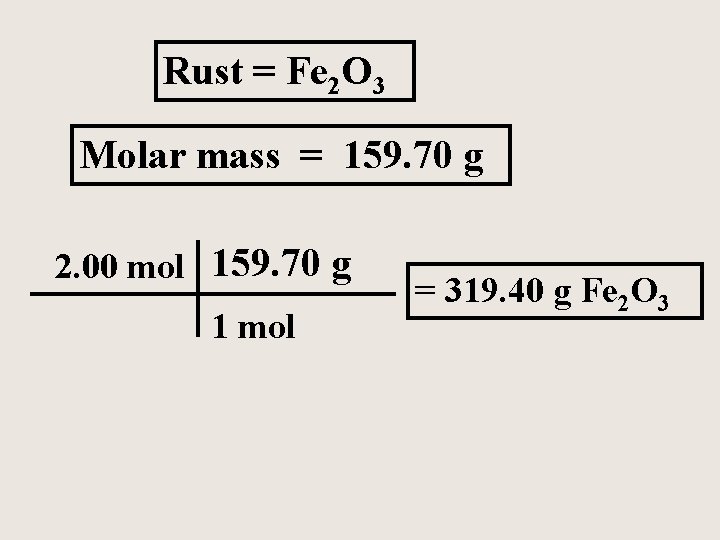
What is the mass of 2. 00 moles of Iron (III) oxide, commonly known as rust?

Rust = Fe 2 O 3 Molar mass = 159. 70 g 2. 00 mol 159. 70 g 1 mol = 319. 40 g Fe 2 O 3

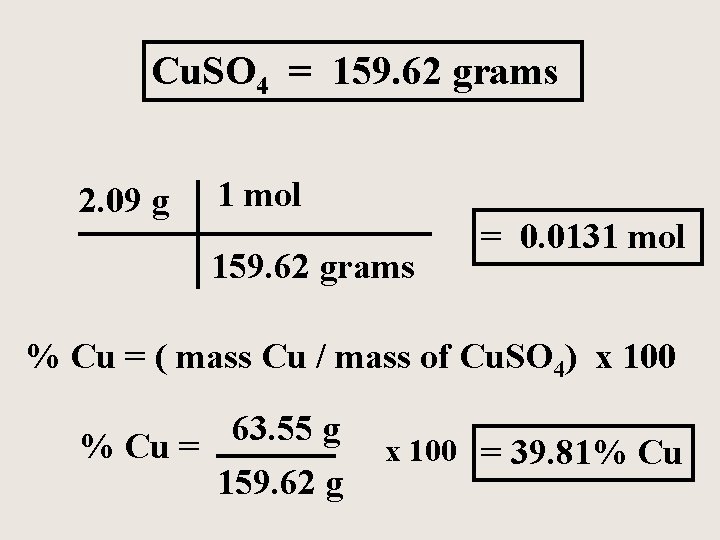
A chemistry student prepares 2. 09 grams of the compound copper (II) sulfate in the laboratory. How many moles of the compound does he make? What is the % copper in the compound?

Cu. SO 4 = 159. 62 grams 2. 09 g 1 mol 159. 62 grams = 0. 0131 mol % Cu = ( mass Cu / mass of Cu. SO 4) x 100 63. 55 g % Cu = 159. 62 g x 100 = 39. 81% Cu

Calcium carbonate is the principle compound found in pearls, marble, shells, and chalk.

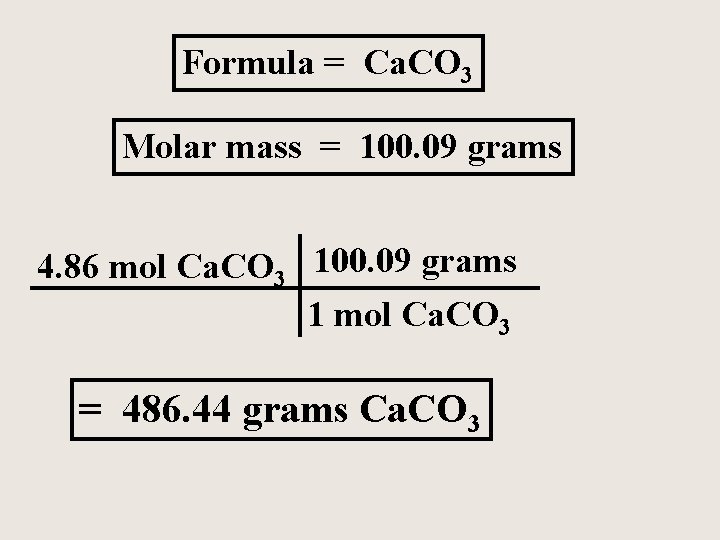
A certain sample of calcium carbonate contains 4. 86 moles. What is the mass of this sample and how many formula units of calcium carbonate are present in the sample? Ca. CO 3 is an ionic compound and is not a molecule, but a crystal lattice with the simplest ratio of ions.

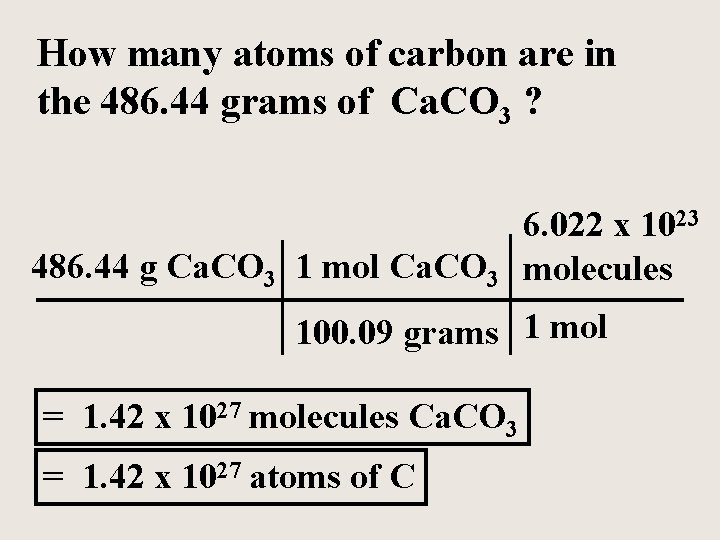
Formula = Ca. CO 3 Molar mass = 100. 09 grams 4. 86 mol Ca. CO 3 100. 09 grams 1 mol Ca. CO 3 = 486. 44 grams Ca. CO 3

How many atoms of carbon are in the 486. 44 grams of Ca. CO 3 ? 486. 44 g Ca. CO 3 1 mol Ca. CO 3 6. 022 x 1023 molecules 100. 09 grams 1 mol = 1. 42 x 1027 molecules Ca. CO 3 = 1. 42 x 1027 atoms of C

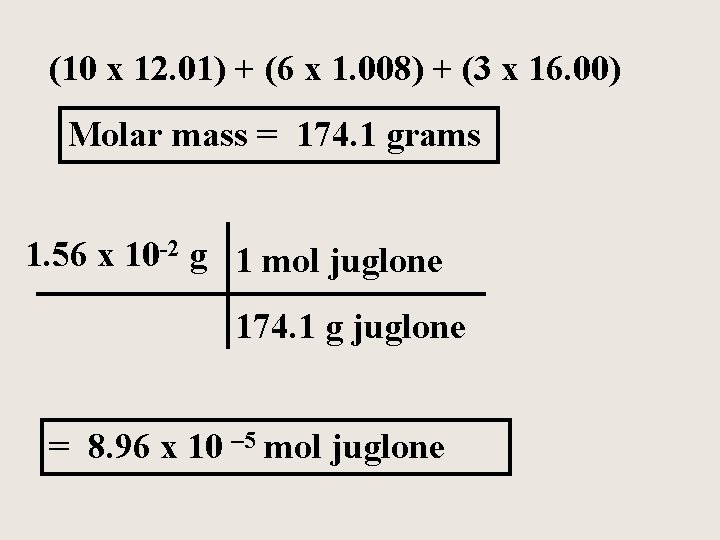
Juglone is a natural herbicide produced from the husks of black walnuts. The formula for juglone is C 10 H 6 O 3. Calculate the molar mass of juglone and how many moles of juglone would be in a 1. 56 x 10 -2 gram sample.

(10 x 12. 01) + (6 x 1. 008) + (3 x 16. 00) Molar mass = 174. 1 grams 1. 56 x 10 -2 g 1 mol juglone 174. 1 g juglone = 8. 96 x 10 – 5 mol juglone


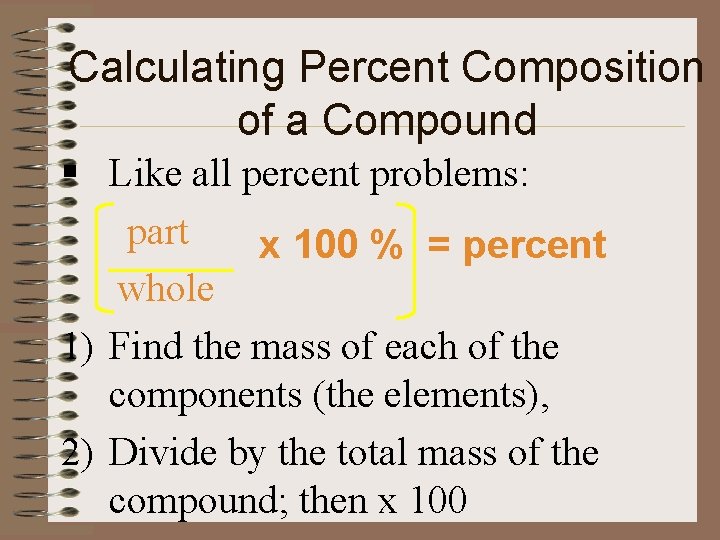
Calculating Percent Composition of a Compound § Like all percent problems: part x 100 % = percent whole 1) Find the mass of each of the components (the elements), 2) Divide by the total mass of the compound; then x 100

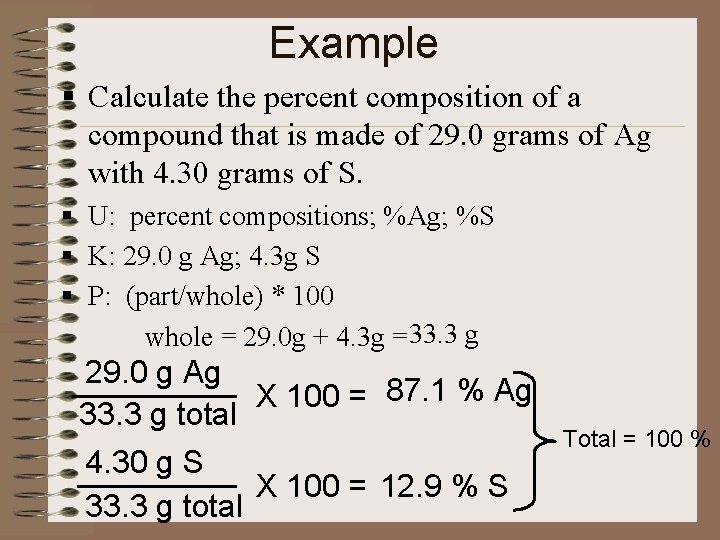
Example § Calculate the percent composition of a compound that is made of 29. 0 grams of Ag with 4. 30 grams of S. § U: percent compositions; %Ag; %S § K: 29. 0 g Ag; 4. 3 g S § P: (part/whole) * 100 whole = 29. 0 g + 4. 3 g = 33. 3 g 29. 0 g Ag X 100 = 87. 1 % Ag 33. 3 g total 4. 30 g S X 100 = 12. 9 % S 33. 3 g total Total = 100 %

Percent Composition from compound • Assume you have ONE MOLE!!! • Therefore the “part mass” is the mass of each element from the periodic table and the “whole mass” is the mass of the compound.

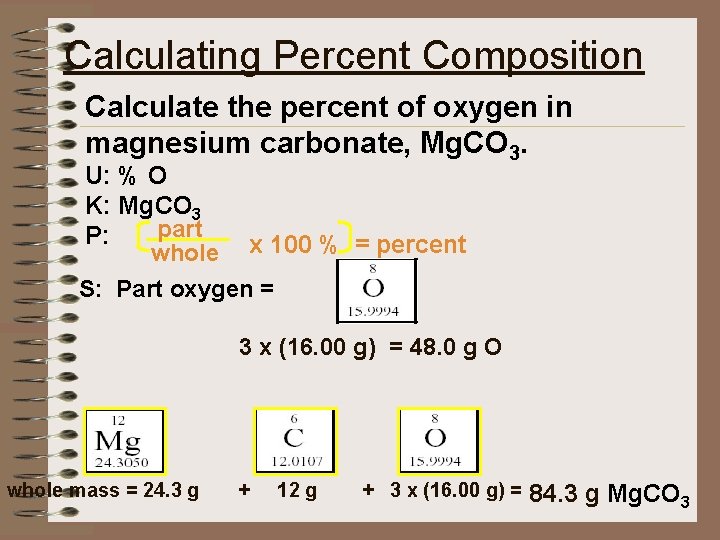
Calculating Percent Composition Calculate the percent of oxygen in magnesium carbonate, Mg. CO 3. U: % O K: Mg. CO 3 part P: whole x 100 % = percent S: Part oxygen = 3 x (16. 00 g) = 48. 0 g O whole mass = 24. 3 g + 12 g + 3 x (16. 00 g) = 84. 3 g Mg. CO 3

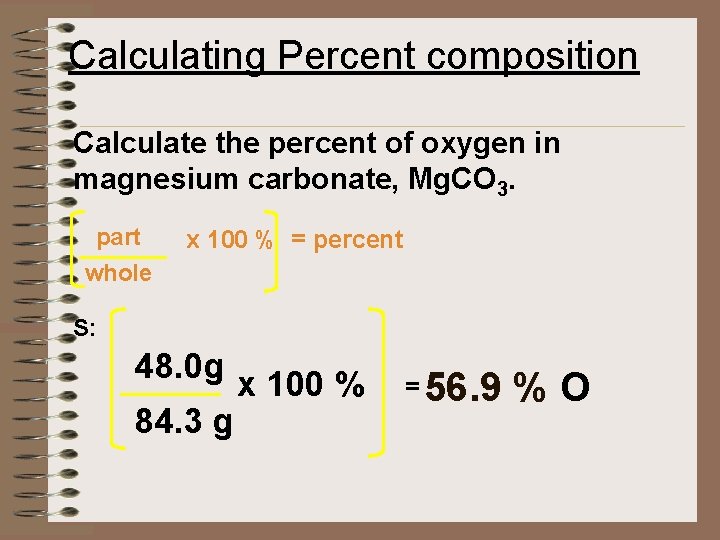
Calculating Percent composition Calculate the percent of oxygen in magnesium carbonate, Mg. CO 3. part x 100 % = percent whole S: 48. 0 g 84. 3 g x 100 % = 56. 9 % O

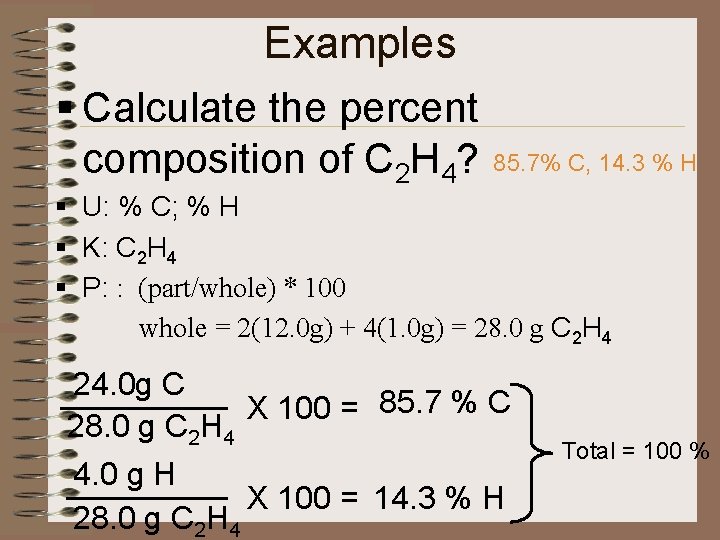
Examples § Calculate the percent composition of C 2 H 4? 85. 7% C, 14. 3 % H § U: % C; % H § K: C 2 H 4 § P: : (part/whole) * 100 whole = 2(12. 0 g) + 4(1. 0 g) = 28. 0 g C 2 H 4 24. 0 g C X 100 = 85. 7 % C 28. 0 g C 2 H 4 4. 0 g H X 100 = 14. 3 % H 28. 0 g C 2 H 4 Total = 100 %

Examples § How about Aluminum carbonate? 23. 1% Al, 15. 4% C, and 61. 5 % O

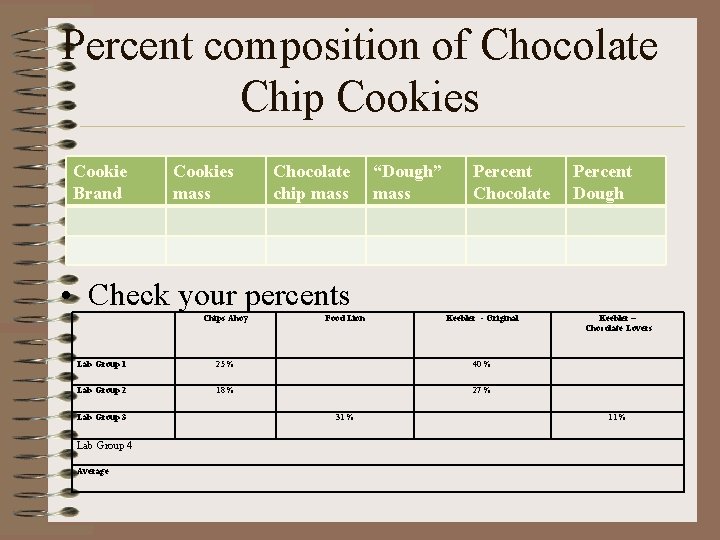
Percent composition of Chocolate Chip Cookies Cookie Brand Cookies mass Chocolate chip mass “Dough” mass Percent Chocolate Percent Dough • Check your percents Chips Ahoy Food Lion Keebler - Original Lab Group 1 25 % 40 % Lab Group 2 18 % 27 % Lab Group 3 Lab Group 4 Average 31 % Keebler – Chocolate Lovers 11 %

Group Average percentage Chips Ahoy Food Lion Keebler Original Keebler – Chocolate Lovers

• How could you determine the percent of sugar in a piece of bubble gum?

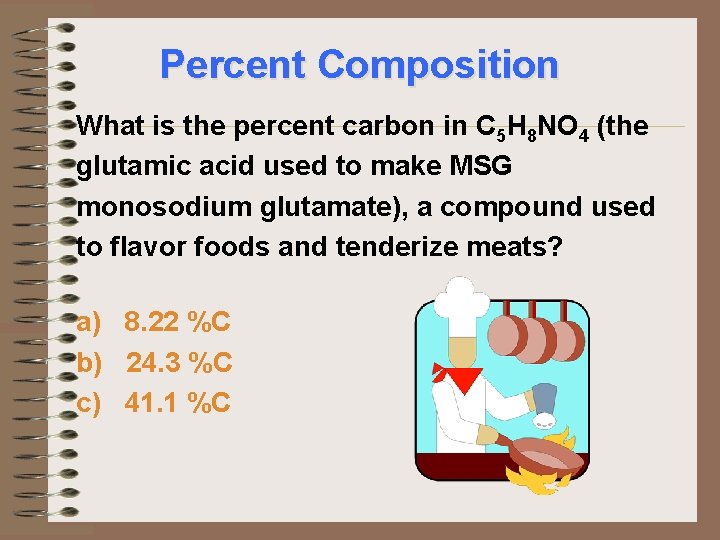
Percent Composition What is the percent carbon in C 5 H 8 NO 4 (the glutamic acid used to make MSG monosodium glutamate), a compound used to flavor foods and tenderize meats? a) 8. 22 %C b) 24. 3 %C c) 41. 1 %C

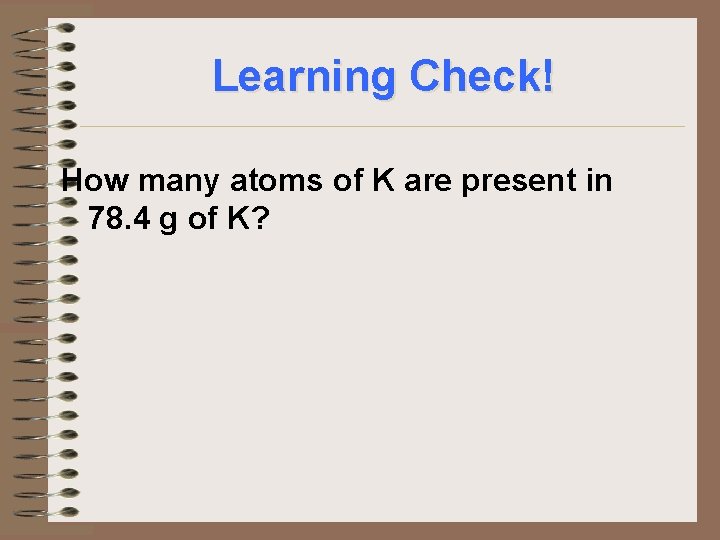
Learning Check! How many atoms of K are present in 78. 4 g of K?

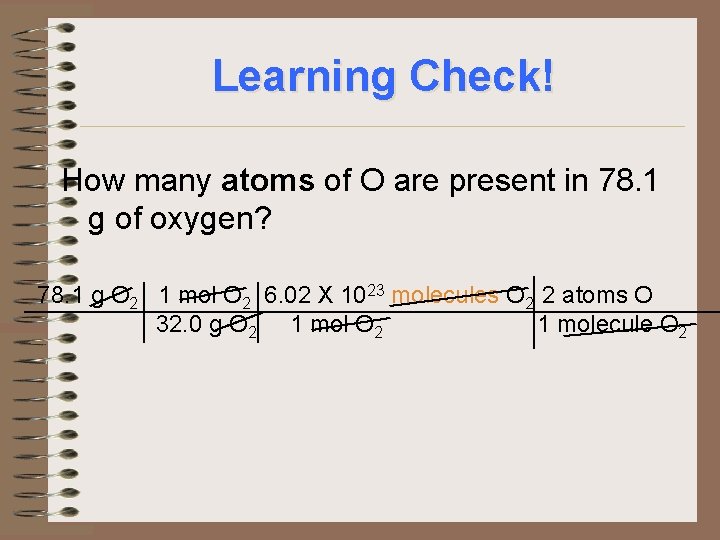
Learning Check! How many atoms of O are present in 78. 1 g of oxygen? 78. 1 g O 2 1 mol O 2 6. 02 X 1023 molecules O 2 2 atoms O 32. 0 g O 2 1 molecule O 2

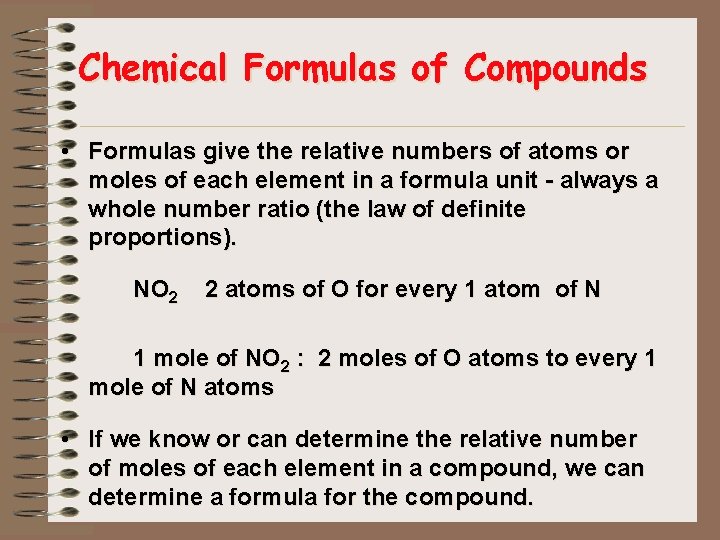
Chemical Formulas of Compounds • Formulas give the relative numbers of atoms or moles of each element in a formula unit - always a whole number ratio (the law of definite proportions). NO 2 2 atoms of O for every 1 atom of N 1 mole of NO 2 : 2 moles of O atoms to every 1 mole of N atoms • If we know or can determine the relative number of moles of each element in a compound, we can determine a formula for the compound.

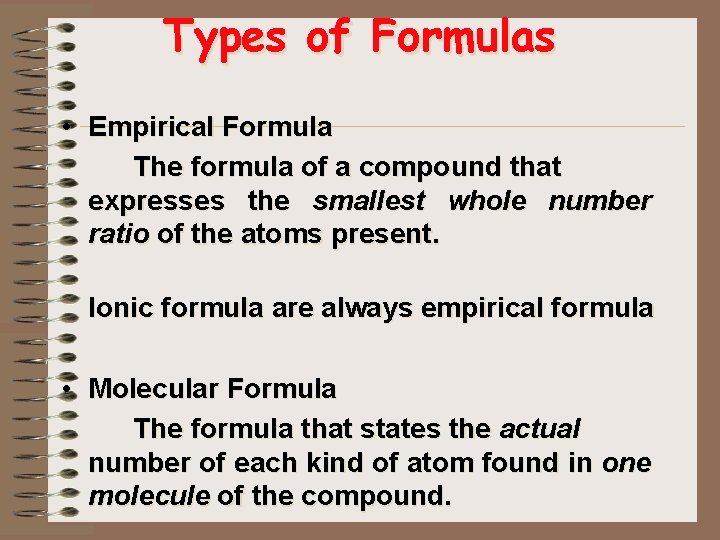
Types of Formulas • Empirical Formula The formula of a compound that expresses the smallest whole number ratio of the atoms present. Ionic formula are always empirical formula • Molecular Formula The formula that states the actual number of each kind of atom found in one molecule of the compound.

To obtain an Empirical Formula 1. Determine the mass in grams of each element present, if necessary. 2. Calculate the number of moles of each element. 3. Divide each by the smallest number of moles to obtain the simplest whole number ratio. 4. If whole numbers are not obtained* in step 3), multiply through by the smallest number that will give all whole numbers * Be careful! Do not round off numbers prematurely

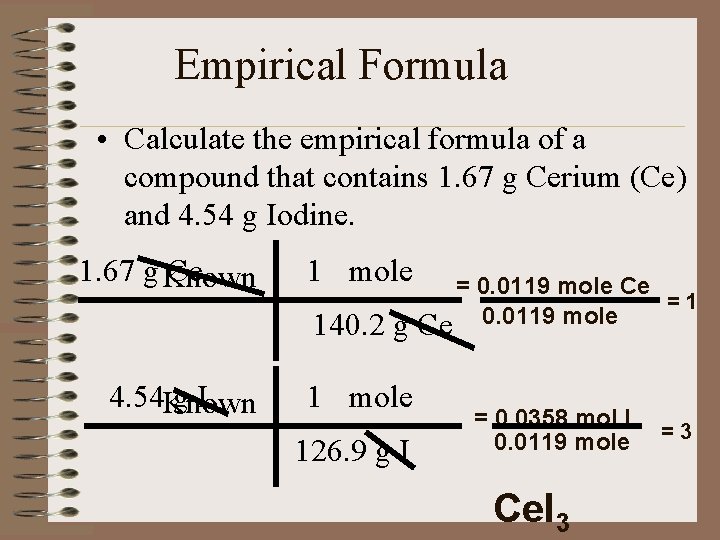
Empirical Formula • Calculate the empirical formula of a compound that contains 1. 67 g Cerium (Ce) and 4. 54 g Iodine. 1. 67 g Known Ce 1 mole 140. 2 g 4. 54 Known g. I 1 mole 126. 9 g I = 0. 0119 mole Ce =1 Ce 0. 0119 mole = 0. 0358 mol I 0. 0119 mole Ce. I 3 =3

Molecular formula • What if the actual mass 1041. 6 g Ce. I 3

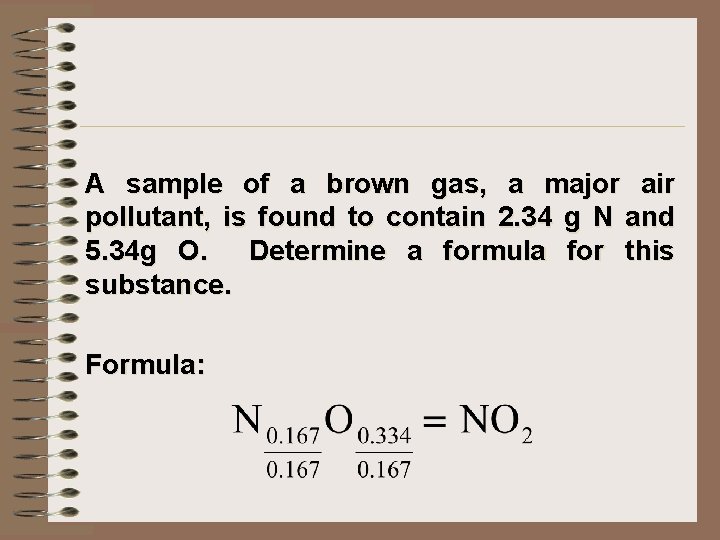
A sample of a brown gas, a major air pollutant, is found to contain 2. 34 g N and 5. 34 g O. Determine a formula for this substance. Formula:

Calculation of the Molecular Formula A compound has an empirical formula of NO 2. The colorless liquid, used in rocket engines has a molar mass of 92. 0 g/mole. What is the molecular formula of this substance?

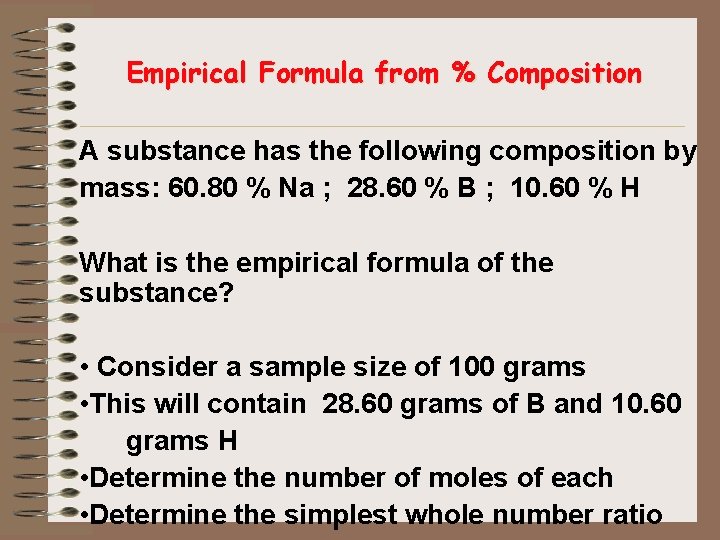
Empirical Formula from % Composition A substance has the following composition by mass: 60. 80 % Na ; 28. 60 % B ; 10. 60 % H What is the empirical formula of the substance? • Consider a sample size of 100 grams • This will contain 28. 60 grams of B and 10. 60 grams H • Determine the number of moles of each • Determine the simplest whole number ratio