Formula Weight Molar Mass Scientists work with chemicals








- Slides: 14
Formula Weight / Molar Mass

• Scientists work with chemicals every day. In order to do so, they have to be able to measure quantities that are easy to find the mass of on a scale or balance. • To make measurement easier, scientists created a UNIT OF MEASURE for chemicals called the MOLE. • A MOLE is the mass in grams of a substance that contains 602, 200, 000, 000 particles of that substance! That is a REALLY, REALLY BIG NUMBER!

• This number is called AVAGADRO’S NUMBER! It is a very important number in chemistry. • In fact, if everyone person on earth (about 7 BILLION people) were counting a pile of rocks that big and EVERYONE COUNTED ONE ROCK PER SECOND, it would still take them almost 4 YEARS to count them all! • That’s pretty impressive when you consider that a few paperclips contain about that many atoms!

• Scientists found that when they collect a sample of an element containing this number of atoms, the mass of the atoms will be the same in grams as the mass of one atom’s ATOMIC MASS NUMBER. • For instance: • - 1 atom of carbon has an atomic mass number of 12. 011 u. and • - 602, 200, 000, 000 atoms of carbon have a mass of 12. 011 grams. • So ONE MOLE of carbon atoms has a mass of 12. 011 grams. • As scientists, we use the MOLE all the time when we work with chemicals in the LAB or in INDUSTRIAL settings.

• PROBLEM • Suppose you were given a container of CALCIUM CARBONATE which has the chemical formula Ca. CO 3 and were asked to measure out ONE MOLE of the substance. How many grams would you need to measure out? • In order to answer this question, you would have to know the compound’s FORMULA WEIGHT which is also known as MOLAR MASS…. either name can be used. • To find an element’s FORMULA WEIGHT, you need a few tools: • 1. The compound’s CHEMICAL FORMULA. • 2. A PERIODIC TABLE. • 3. A CALCULATOR.

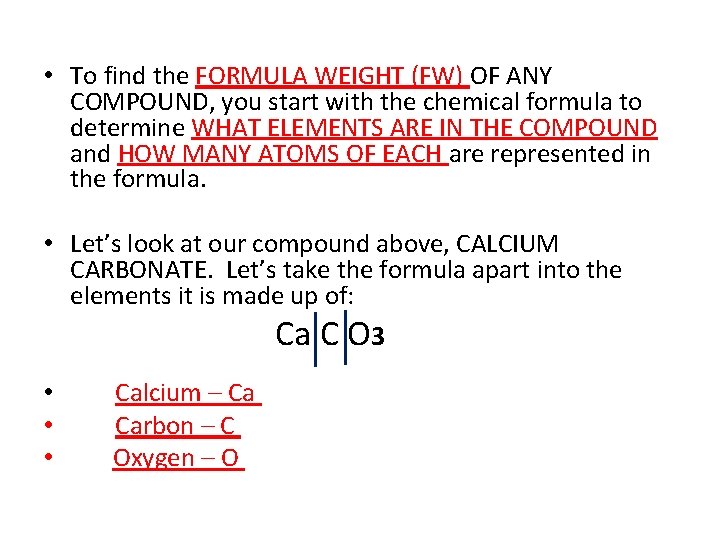
• To find the FORMULA WEIGHT (FW) OF ANY COMPOUND, you start with the chemical formula to determine WHAT ELEMENTS ARE IN THE COMPOUND and HOW MANY ATOMS OF EACH are represented in the formula. • Let’s look at our compound above, CALCIUM CARBONATE. Let’s take the formula apart into the elements it is made up of: Ca C O 3 • Calcium – Ca • Carbon – C • Oxygen – O

• Now let’s look at how many atoms of each element are represented by the formula. • In the formula Ca. CO 3, only oxygen has a SUBSCRIPT, a small number written slightly below the line following the element’s symbol. • The subscript indicates HOW MANY ATOMS are present in a formula. • If an atom DOES NOT have a subscript, then only ONE ATOM of that element is present. • So in our formula, Ca. CO 3, we have: • Calcium – Ca – 1 Atom • Carbon – C – 1 Atom • Oxygen – O – 3 Atoms

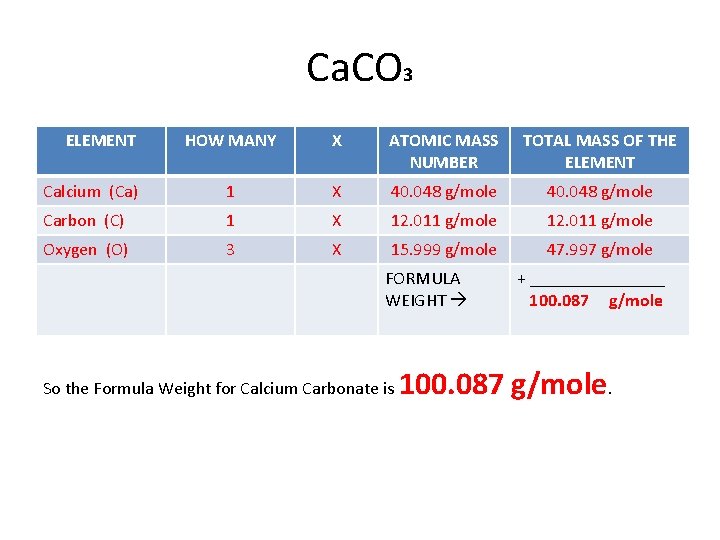
• Now all we need to calculate the Formula Weight for the compound Calcium Carbonate is the ATOMIC MASS NUMBER OF EACH ELEMENT from the periodic table: • • • Calcium – Ca – 40. 048 g/mole Carbon – C – 12. 011 g/mole Oxygen – O – 15. 999 g/mole • To find the formula weight, fill in the information in the table below. To get the FORMULA WEIGHT, ADD THE FINAL RESULTS! • The unit is GRAMS/MOLE, or just G/MOLE.

Ca. CO 3 ELEMENT HOW MANY X ATOMIC MASS NUMBER TOTAL MASS OF THE ELEMENT Calcium (Ca) 1 X 40. 048 g/mole Carbon (C) 1 X 12. 011 g/mole Oxygen (O) 3 X 15. 999 g/mole 47. 997 g/mole FORMULA WEIGHT So the Formula Weight for Calcium Carbonate is + ________ 100. 087 g/mole.

• Let’s try another one! What would be the FORMULA WEIGHT of the compound MAGNESIUM SULFATE, which has the formula Mg. SO 4? • First, decide what elements make up the compound and how many atoms of each. Here we have: • Magnesium – Mg – 1 Atom • Sulfur – S – 1 Atom • Oxygen – O – 4 Atoms

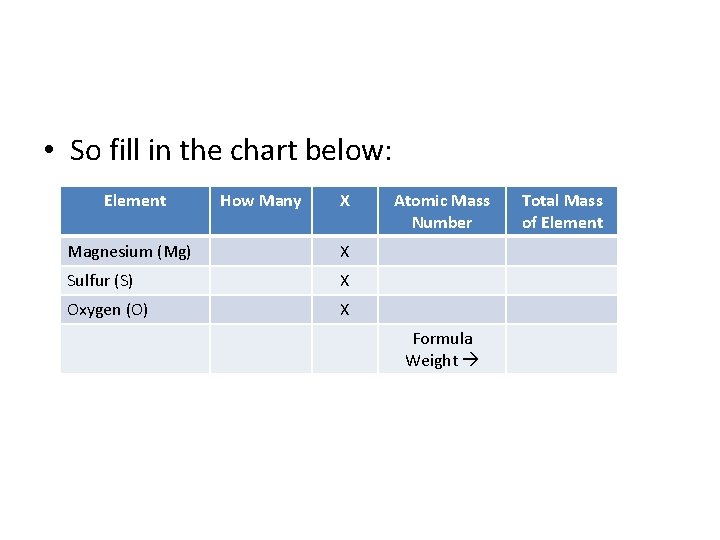
• So fill in the chart below: Element How Many X Magnesium (Mg) X Sulfur (S) X Oxygen (O) X Atomic Mass Number Formula Weight Total Mass of Element

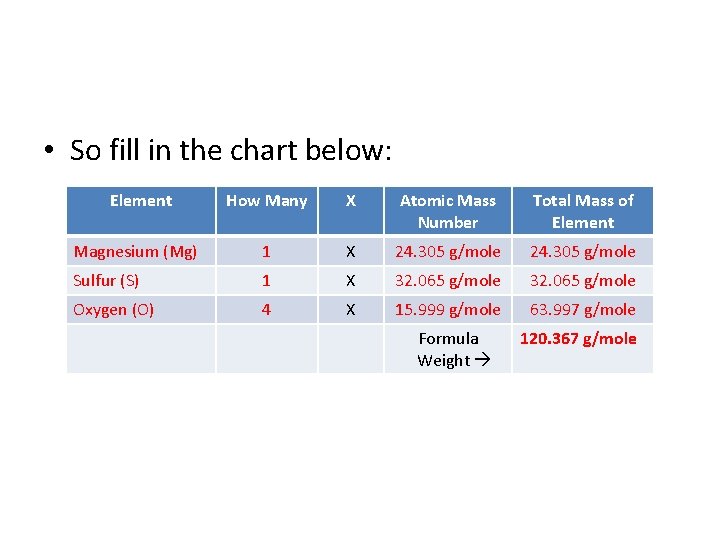
• So fill in the chart below: Element How Many X Atomic Mass Number Total Mass of Element Magnesium (Mg) 1 X 24. 305 g/mole Sulfur (S) 1 X 32. 065 g/mole Oxygen (O) 4 X 15. 999 g/mole 63. 997 g/mole Formula Weight 120. 367 g/mole

• One more on your own! Find the FORMULA WEIGHT of Aluminum Phosphate, Al. PO 4. • What elements are present? How many of each? • Aluminum 1 • Phosphorus 1 • Oxygen 4 • Now use the periodic table to find the atomic mass number of each element and complete the table.

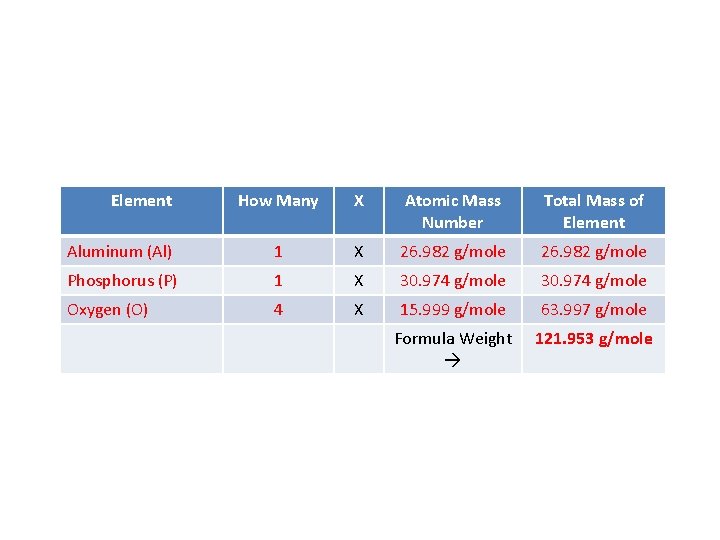
Element How Many X Atomic Mass Number Total Mass of Element Aluminum (Al) 1 X 26. 982 g/mole Phosphorus (P) 1 X 30. 974 g/mole Oxygen (O) 4 X 15. 999 g/mole 63. 997 g/mole Formula Weight 121. 953 g/mole