CHEMICAL QUANTITIES Composition Stoichiometry Calculating Molar Mass Avogadros




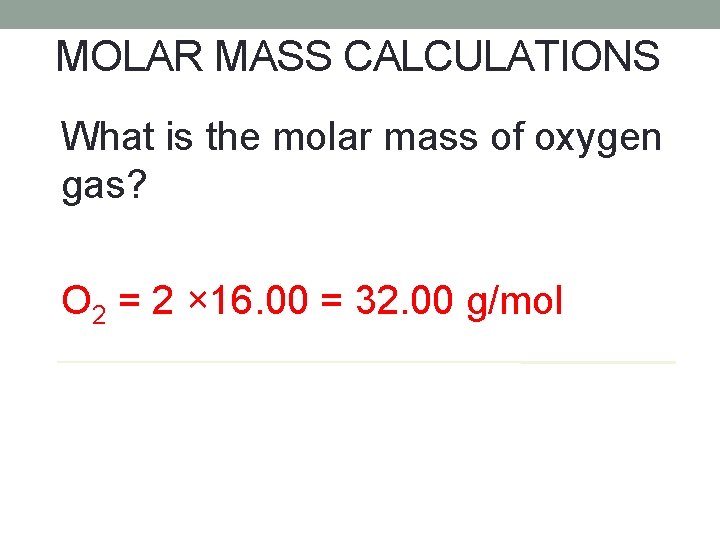





- Slides: 44

CHEMICAL QUANTITIES Composition Stoichiometry Calculating Molar Mass Avogadro’s Number and the Mole Percentage Composition and Empirical Formulas Molecular Formulas Reaction Stoichiometry

THE MOLE The mole (mol) is the SI unit for the amount of substance that contains as many particles as there are in exactly 12 g of carbon-12.

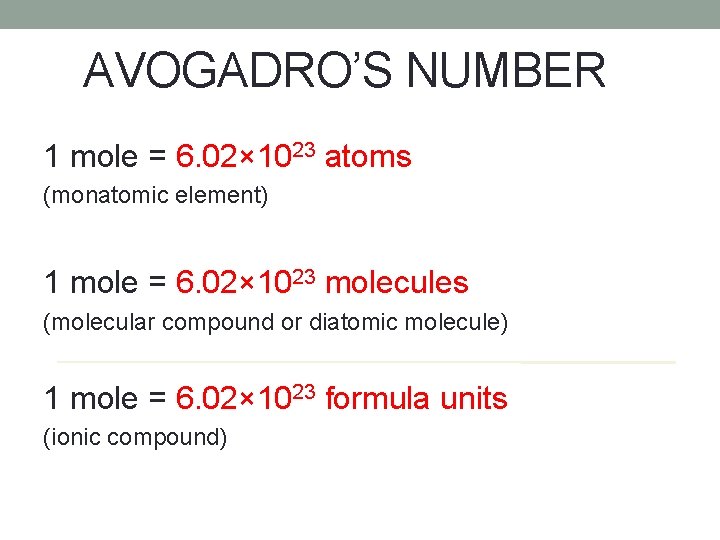
AVOGADRO’S NUMBER A mole of any substance contains 6. 02× 1023 representative particles of that substance. This is known as Avogadro’s Number.

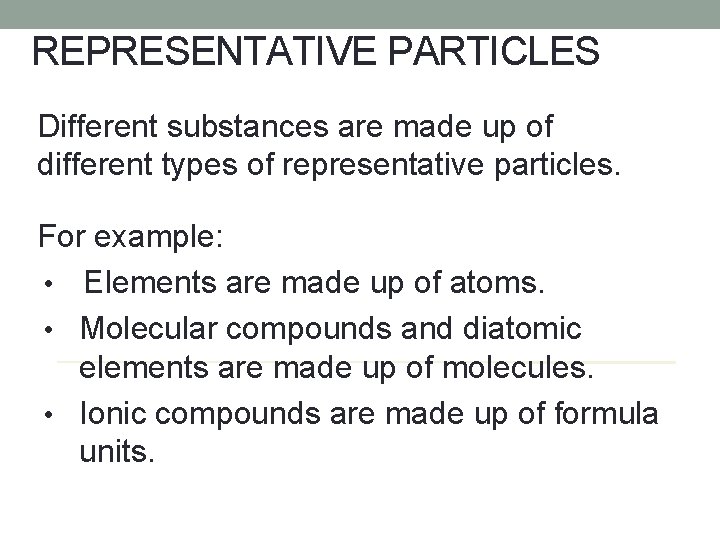
REPRESENTATIVE PARTICLES A representative particle is the smallest particle of a substance that has all of the properties of that substance.

REPRESENTATIVE PARTICLES Different substances are made up of different types of representative particles. For example: • Elements are made up of atoms. • Molecular compounds and diatomic elements are made up of molecules. • Ionic compounds are made up of formula units.

AVOGADRO’S NUMBER 1 mole = 6. 02× 1023 atoms (monatomic element) 1 mole = 6. 02× 1023 molecules (molecular compound or diatomic molecule) 1 mole = 6. 02× 1023 formula units (ionic compound)

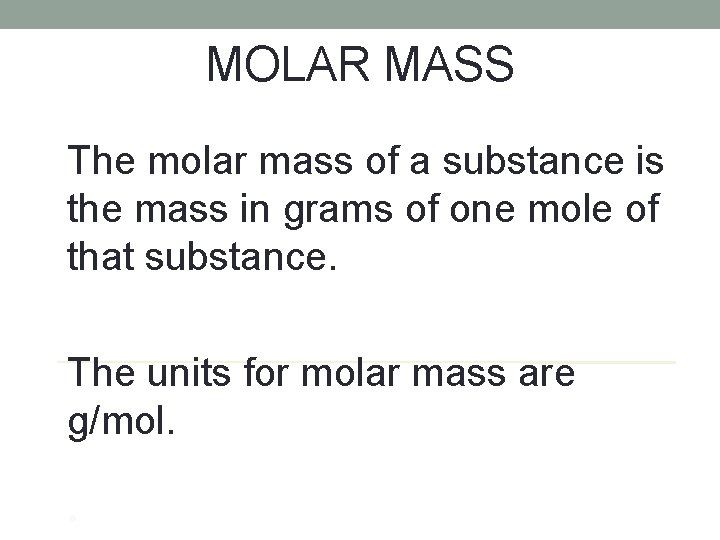
MOLAR MASS The molar mass of a substance is the mass in grams of one mole of that substance. The units for molar mass are g/mol. •

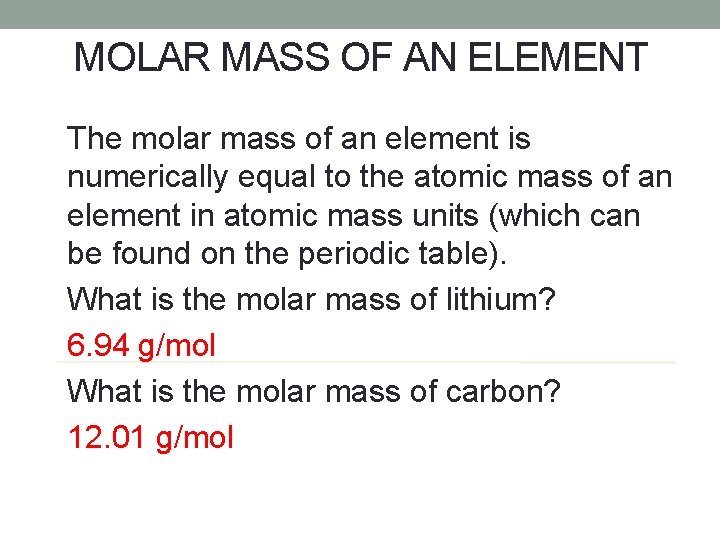
MOLAR MASS OF AN ELEMENT The molar mass of an element is numerically equal to the atomic mass of an element in atomic mass units (which can be found on the periodic table). What is the molar mass of lithium? 6. 94 g/mol What is the molar mass of carbon? 12. 01 g/mol

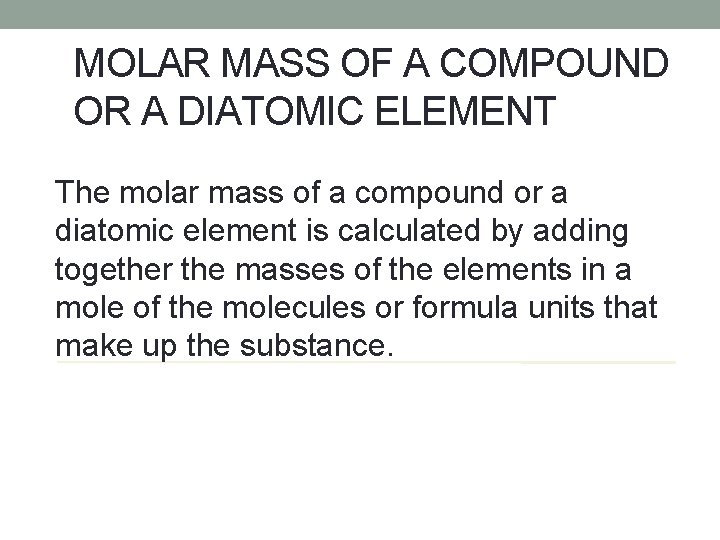
MOLAR MASS OF A COMPOUND OR A DIATOMIC ELEMENT The molar mass of a compound or a diatomic element is calculated by adding together the masses of the elements in a mole of the molecules or formula units that make up the substance.
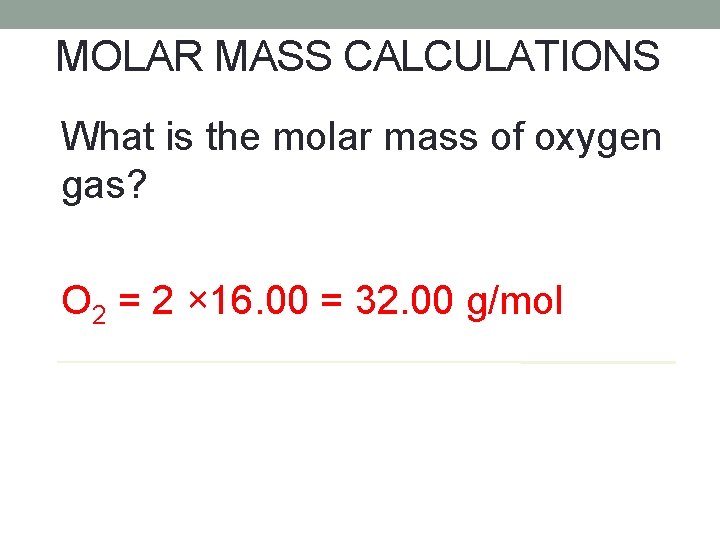
MOLAR MASS CALCULATIONS What is the molar mass of oxygen gas? O 2 = 2 × 16. 00 = 32. 00 g/mol

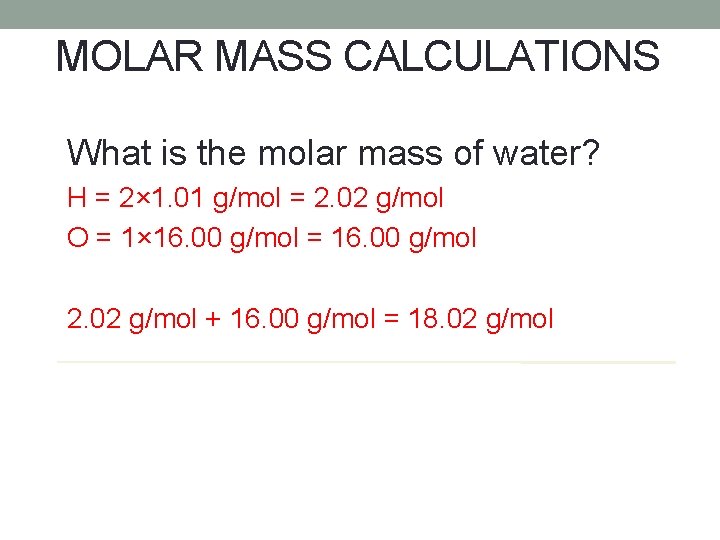
MOLAR MASS CALCULATIONS What is the molar mass of water? H = 2× 1. 01 g/mol = 2. 02 g/mol O = 1× 16. 00 g/mol = 16. 00 g/mol 2. 02 g/mol + 16. 00 g/mol = 18. 02 g/mol

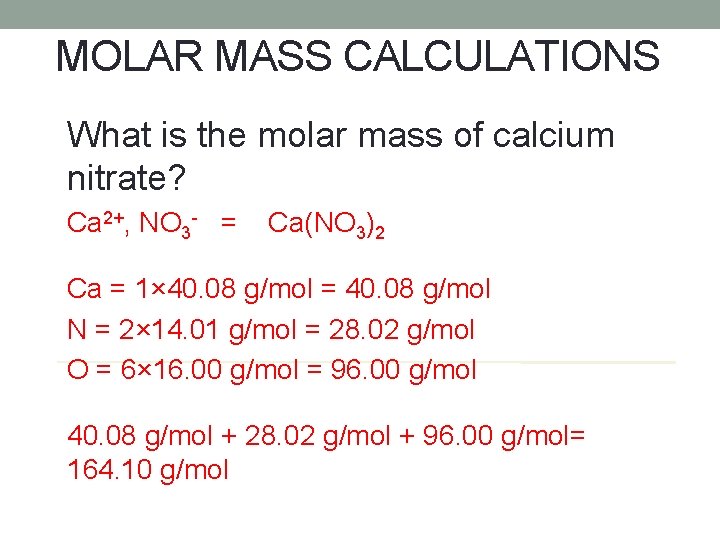
MOLAR MASS CALCULATIONS What is the molar mass of calcium nitrate? Ca 2+, NO 3 - = Ca(NO 3)2 Ca = 1× 40. 08 g/mol = 40. 08 g/mol N = 2× 14. 01 g/mol = 28. 02 g/mol O = 6× 16. 00 g/mol = 96. 00 g/mol 40. 08 g/mol + 28. 02 g/mol + 96. 00 g/mol= 164. 10 g/mol

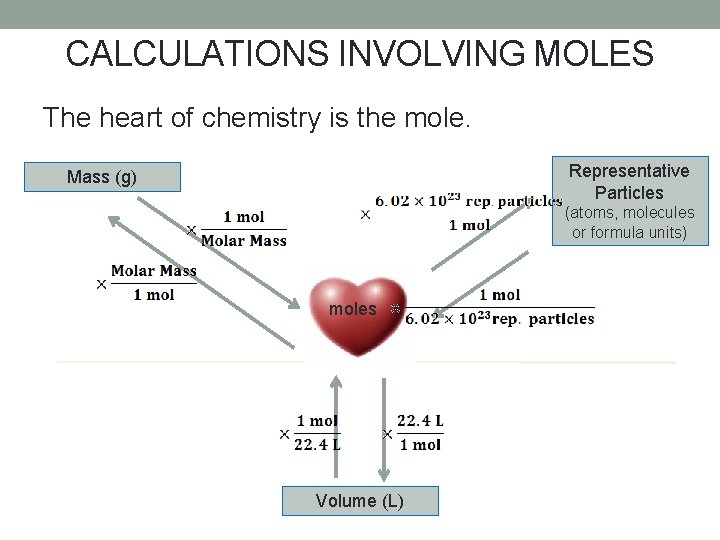
CALCULATIONS INVOLVING MOLES The heart of chemistry is the mole. Representative Particles Mass (g) (atoms, molecules or formula units) moles Volume (L)

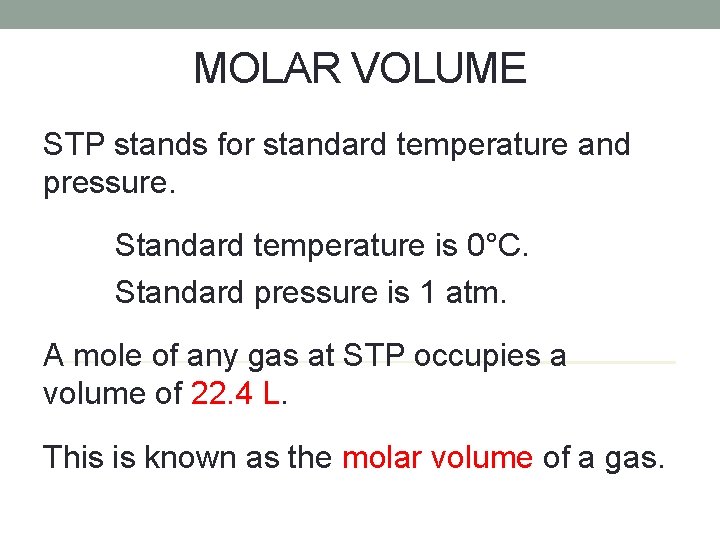
MOLAR VOLUME STP stands for standard temperature and pressure. Standard temperature is 0°C. Standard pressure is 1 atm. A mole of any gas at STP occupies a volume of 22. 4 L. This is known as the molar volume of a gas.

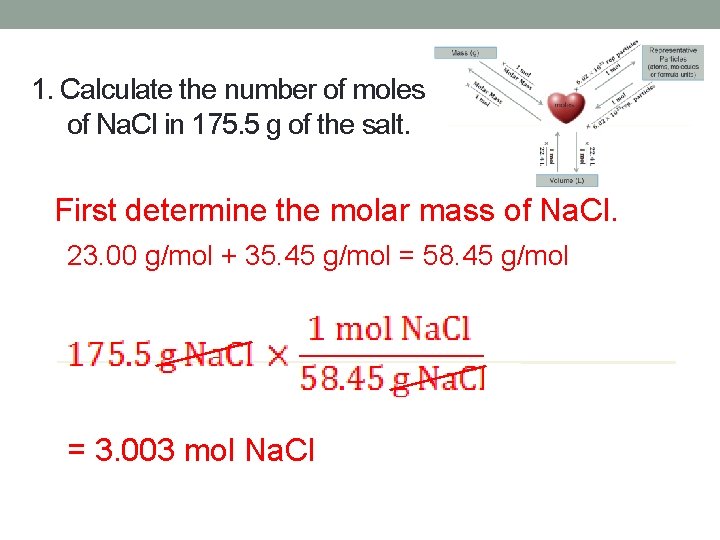
1. Calculate the number of moles of Na. Cl in 175. 5 g of the salt. First determine the molar mass of Na. Cl. 23. 00 g/mol + 35. 45 g/mol = 58. 45 g/mol = 3. 003 mol Na. Cl

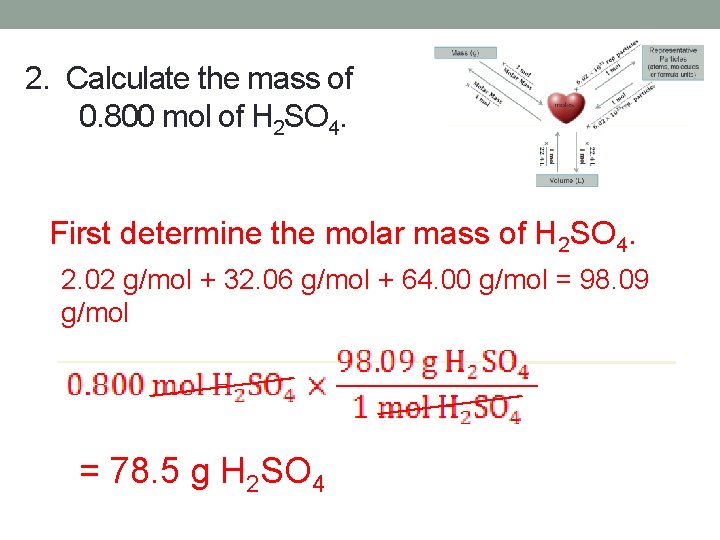
2. Calculate the mass of 0. 800 mol of H 2 SO 4. First determine the molar mass of H 2 SO 4. 2. 02 g/mol + 32. 06 g/mol + 64. 00 g/mol = 98. 09 g/mol = 78. 5 g H 2 SO 4

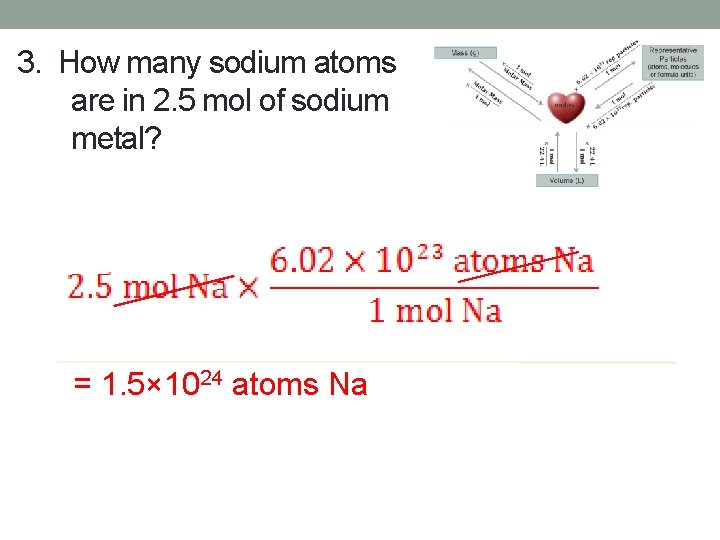
3. How many sodium atoms are in 2. 5 mol of sodium metal? = 1. 5× 1024 atoms Na

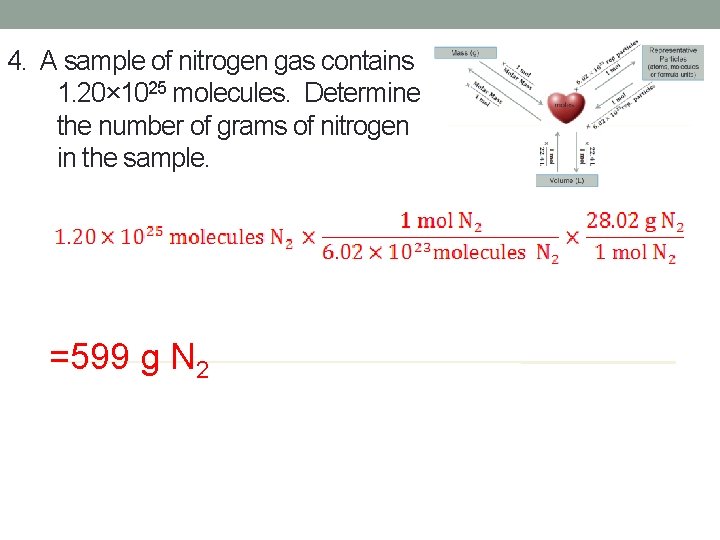
4. A sample of nitrogen gas contains 1. 20× 1025 molecules. Determine the number of grams of nitrogen in the sample. =599 g N 2

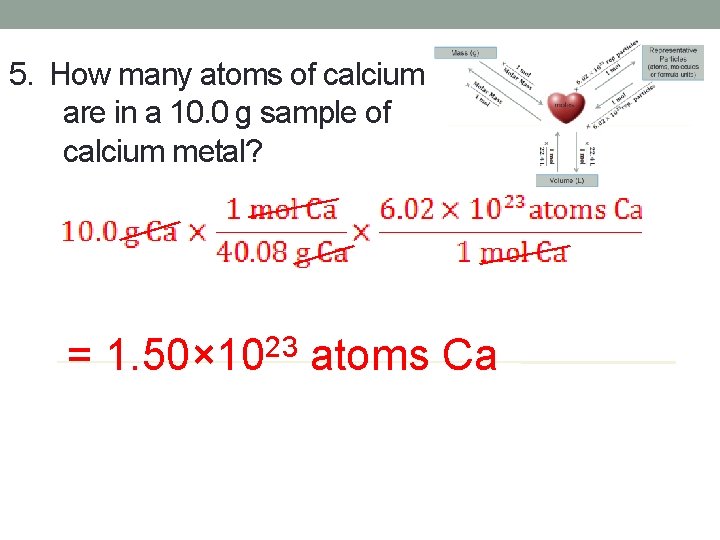
5. How many atoms of calcium are in a 10. 0 g sample of calcium metal? = 1. 50× 1023 atoms Ca

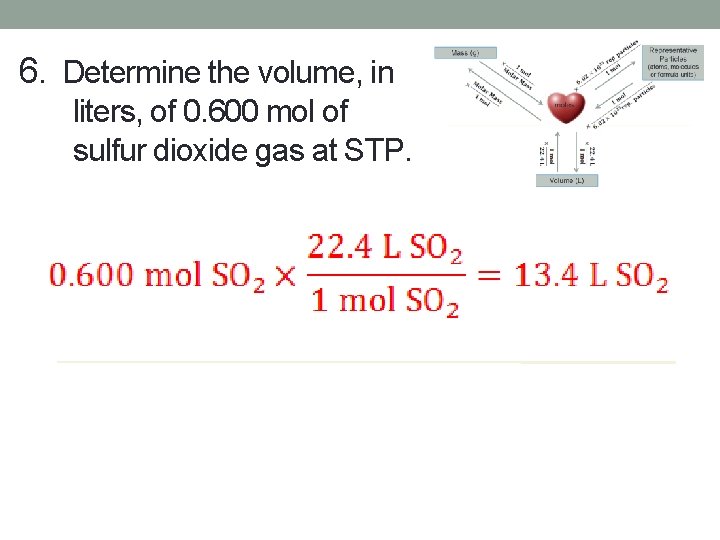
6. Determine the volume, in liters, of 0. 600 mol of sulfur dioxide gas at STP.

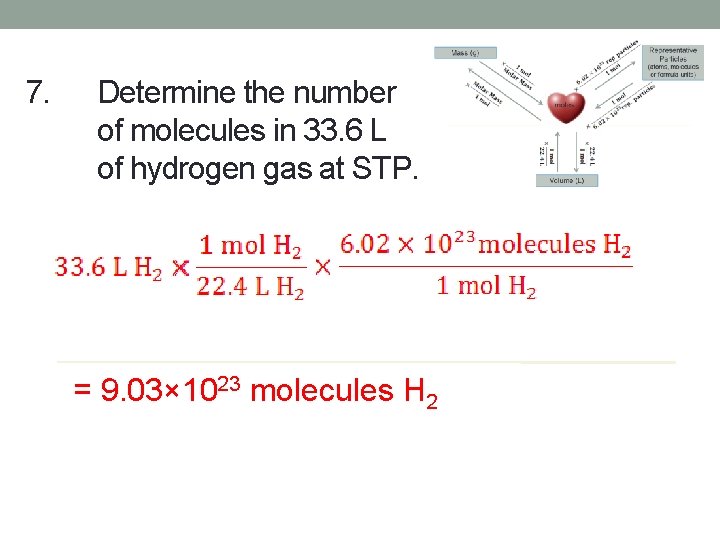
7. Determine the number of molecules in 33. 6 L of hydrogen gas at STP. = 9. 03× 1023 molecules H 2

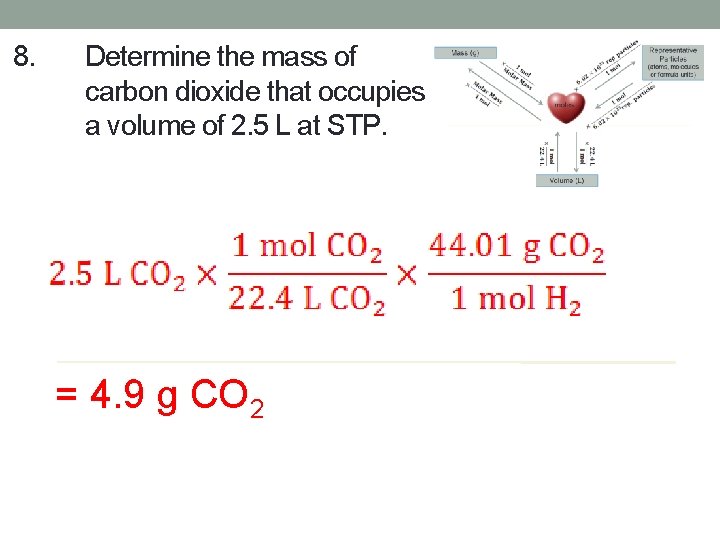
8. Determine the mass of carbon dioxide that occupies a volume of 2. 5 L at STP. = 4. 9 g CO 2

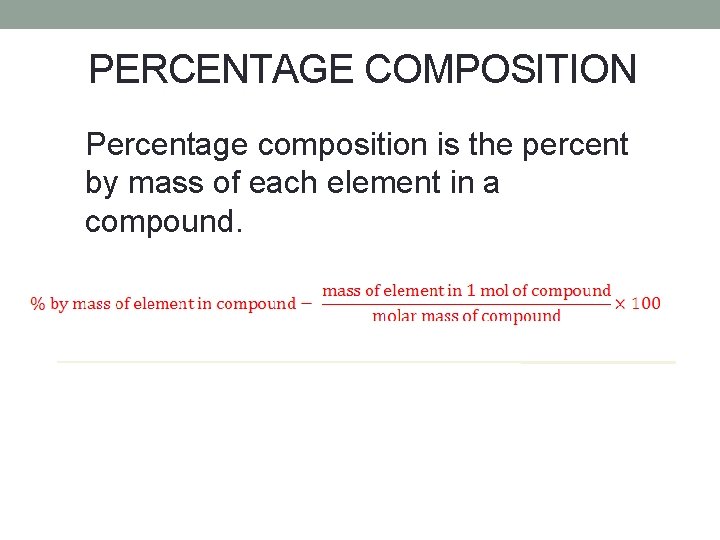
PERCENTAGE COMPOSITION Percentage composition is the percent by mass of each element in a compound.

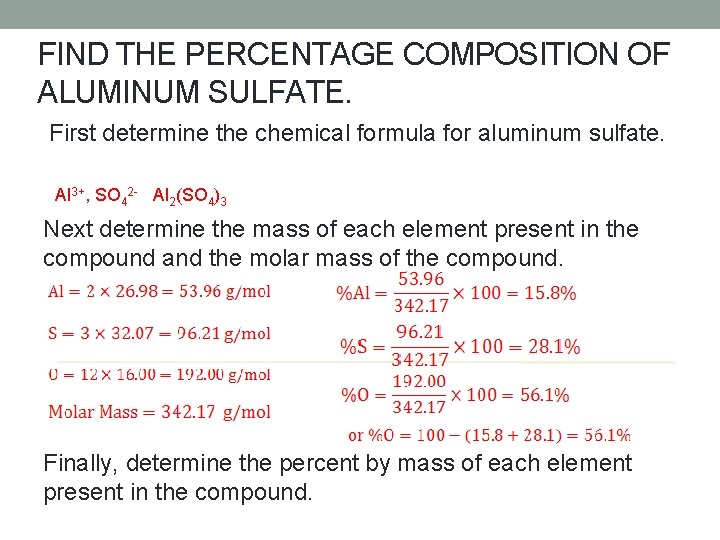
FIND THE PERCENTAGE COMPOSITION OF ALUMINUM SULFATE. First determine the chemical formula for aluminum sulfate. Al 3+, SO 42 - Al 2(SO 4)3 Next determine the mass of each element present in the compound and the molar mass of the compound. Finally, determine the percent by mass of each element present in the compound.

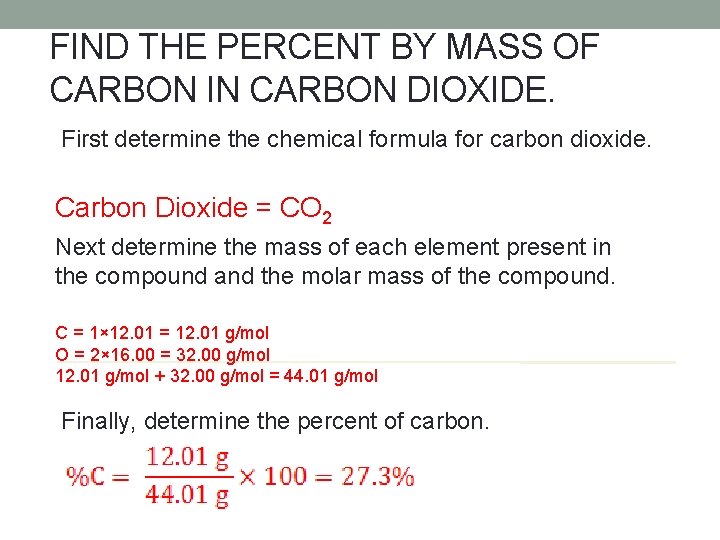
FIND THE PERCENT BY MASS OF CARBON IN CARBON DIOXIDE. First determine the chemical formula for carbon dioxide. Carbon Dioxide = CO 2 Next determine the mass of each element present in the compound and the molar mass of the compound. C = 1× 12. 01 = 12. 01 g/mol O = 2× 16. 00 = 32. 00 g/mol 12. 01 g/mol + 32. 00 g/mol = 44. 01 g/mol Finally, determine the percent of carbon.

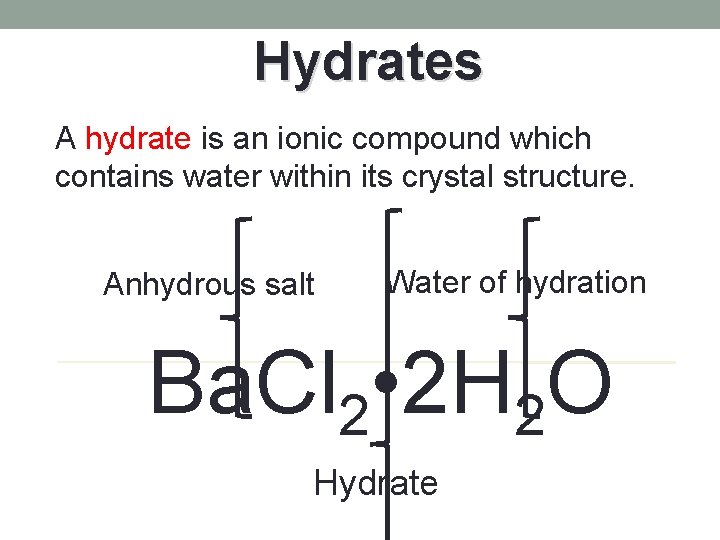
Hydrates A hydrate is an ionic compound which contains water within its crystal structure. Anhydrous salt Water of hydration Ba. Cl 2 • 2 H 2 O Hydrate

HYDRATES A hydrate can be heated to remove the water. The salt that is left after the water of hydration has been removed is called the anhydrous salt. •

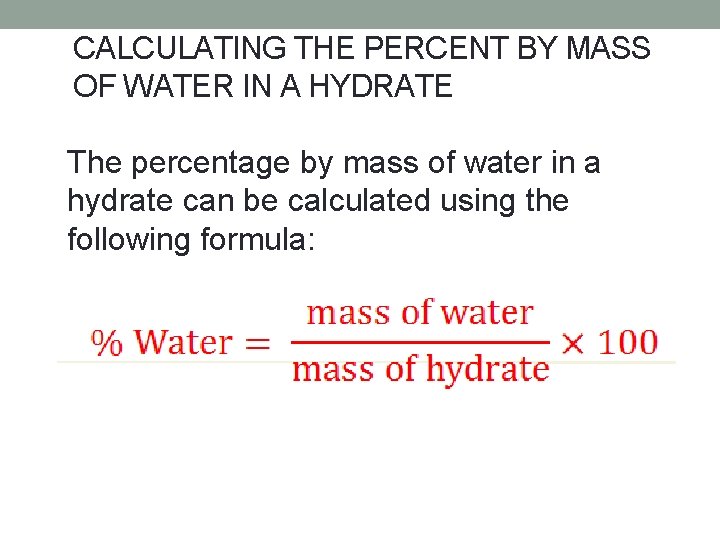
CALCULATING THE PERCENT BY MASS OF WATER IN A HYDRATE The percentage by mass of water in a hydrate can be calculated using the following formula:

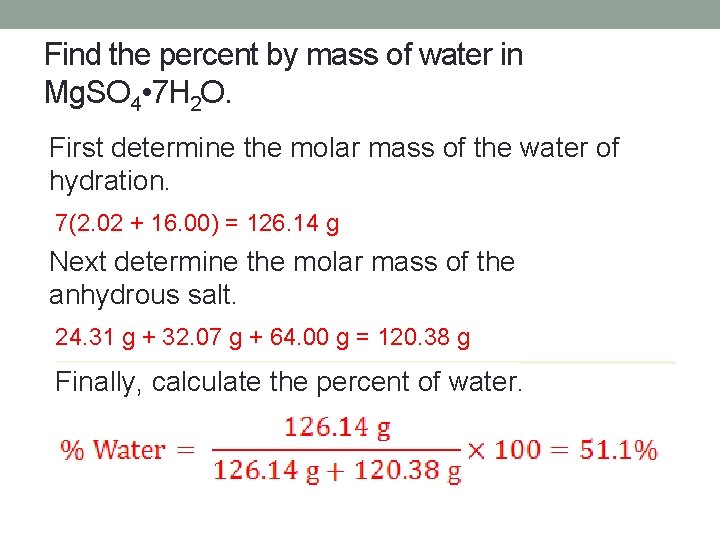
Find the percent by mass of water in Mg. SO 4 • 7 H 2 O. First determine the molar mass of the water of hydration. 7(2. 02 + 16. 00) = 126. 14 g Next determine the molar mass of the anhydrous salt. 24. 31 g + 32. 07 g + 64. 00 g = 120. 38 g Finally, calculate the percent of water.

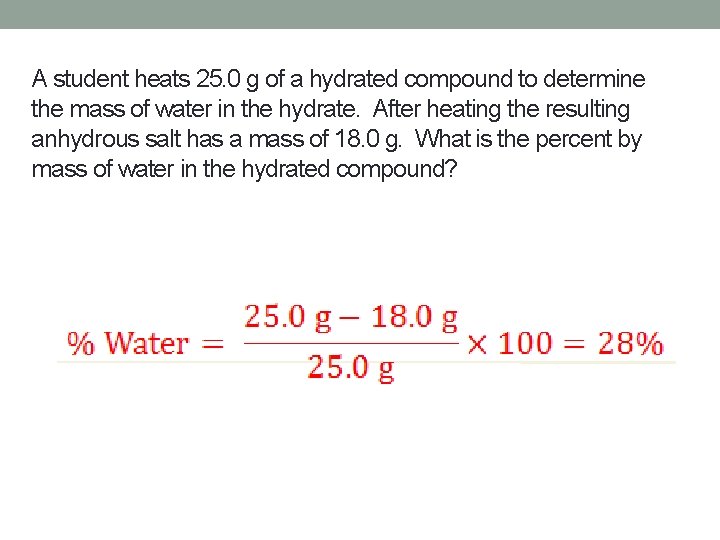
A student heats 25. 0 g of a hydrated compound to determine the mass of water in the hydrate. After heating the resulting anhydrous salt has a mass of 18. 0 g. What is the percent by mass of water in the hydrated compound?

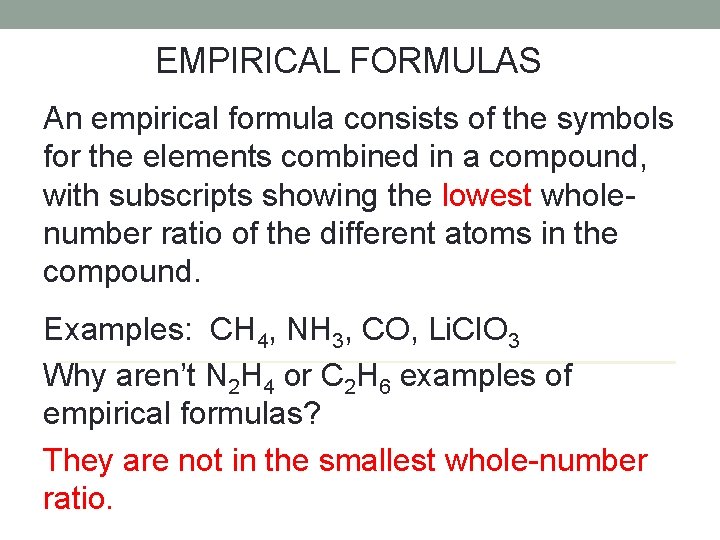
EMPIRICAL FORMULAS An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the lowest wholenumber ratio of the different atoms in the compound. Examples: CH 4, NH 3, CO, Li. Cl. O 3 Why aren’t N 2 H 4 or C 2 H 6 examples of empirical formulas? They are not in the smallest whole-number ratio.

STEPS FOR DETERMINING THE EMPIRICAL FORMULA 1. Determine moles of each element 2. Divide by the smallest value. If each resulting number is a whole number then they represent the subscripts. 3. If the numbers obtained in the previous step are not whole numbers, multiply each number by an integer in order to obtain a whole number.

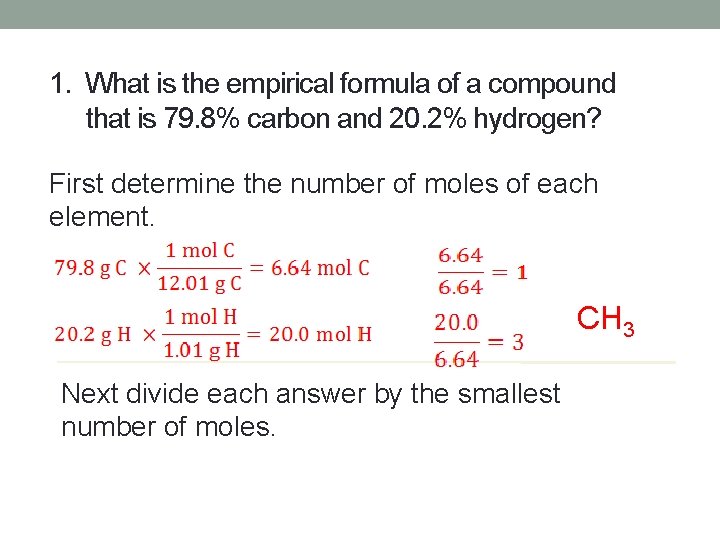
1. What is the empirical formula of a compound that is 79. 8% carbon and 20. 2% hydrogen? First determine the number of moles of each element. CH 3 Next divide each answer by the smallest number of moles.

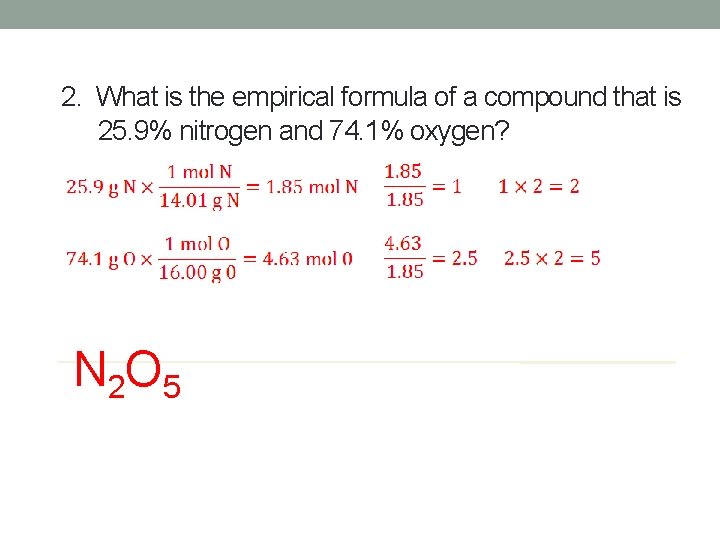
2. What is the empirical formula of a compound that is 25. 9% nitrogen and 74. 1% oxygen? N 2 O 5

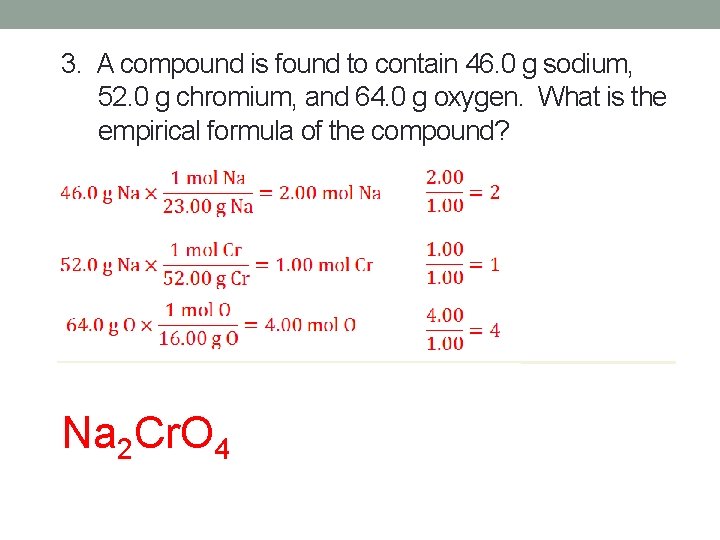
3. A compound is found to contain 46. 0 g sodium, 52. 0 g chromium, and 64. 0 g oxygen. What is the empirical formula of the compound? Na 2 Cr. O 4

4. A sample of a hydrated compound has a mass of 170. 0 grams. after drying the sample, a mass of 95. 3 grams is recorded. determine the formula for the hydrate, LINO 3 • x. H 2 O. First determine the mass of water driven off. Next determine the moles of anhydrous salt and water. Finally, determine the value of x. Li. NO 3 • 3 H 2 O

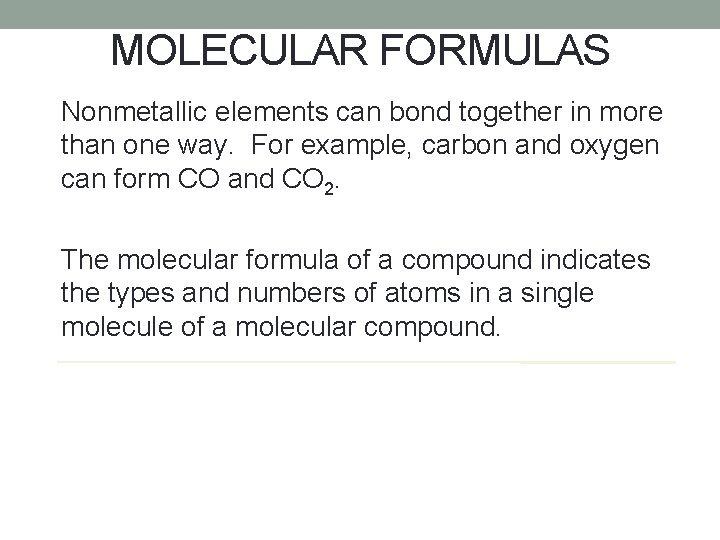
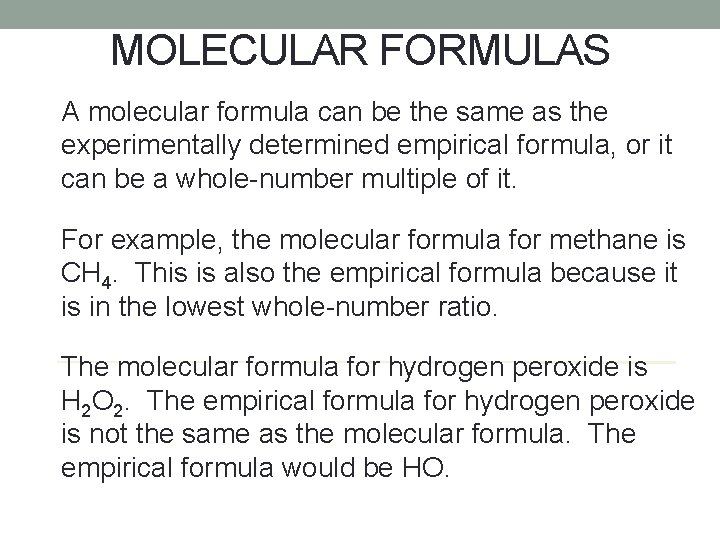
MOLECULAR FORMULAS Nonmetallic elements can bond together in more than one way. For example, carbon and oxygen can form CO and CO 2. The molecular formula of a compound indicates the types and numbers of atoms in a single molecule of a molecular compound.

MOLECULAR FORMULAS A molecular formula can be the same as the experimentally determined empirical formula, or it can be a whole-number multiple of it. For example, the molecular formula for methane is CH 4. This is also the empirical formula because it is in the lowest whole-number ratio. The molecular formula for hydrogen peroxide is H 2 O 2. The empirical formula for hydrogen peroxide is not the same as the molecular formula. The empirical formula would be HO.

CLASSIFY EACH OF THE FOLLOWING CHEMICAL FORMULAS AS EMPIRICAL, MOLECULAR OR BOTH. JUSTIFY YOUR ANSWER. Li. Cl Empirical Formula only Ionic Compound NH 3 Both, Smallest ratio and molecular compound C 2 H 2 Molecular Formula Only, It is a molecular compound but it is not in the smallest ratio

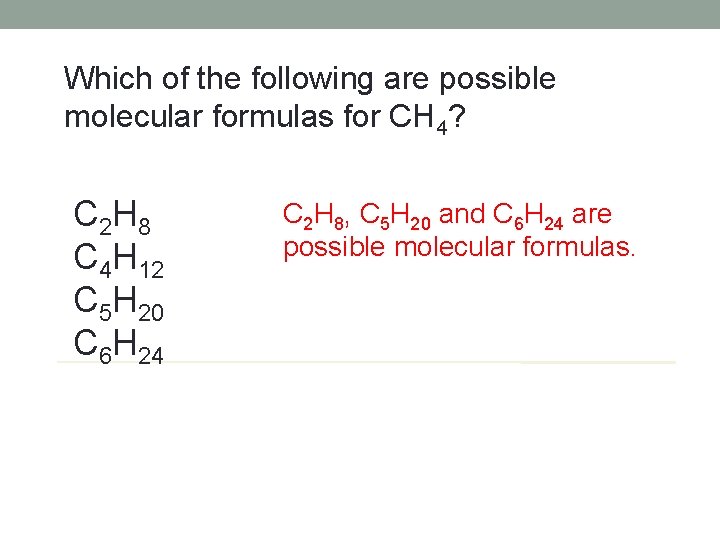
Which of the following are possible molecular formulas for CH 4? C 2 H 8 C 4 H 12 C 5 H 20 C 6 H 24 C 2 H 8, C 5 H 20 and C 6 H 24 are possible molecular formulas.

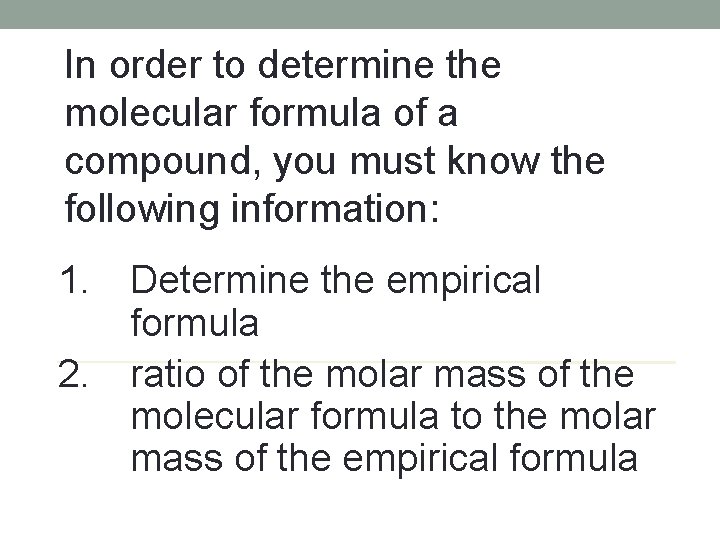
In order to determine the molecular formula of a compound, you must know the following information: 1. 2. Determine the empirical formula ratio of the molar mass of the molecular formula to the molar mass of the empirical formula

1. Determine the molecular formula of CH 4 N. The molar mass is 120. 0 g/mol. CH 4 N = 30. 0 g/mol The molecular formula is C 4 H 16 N 4.

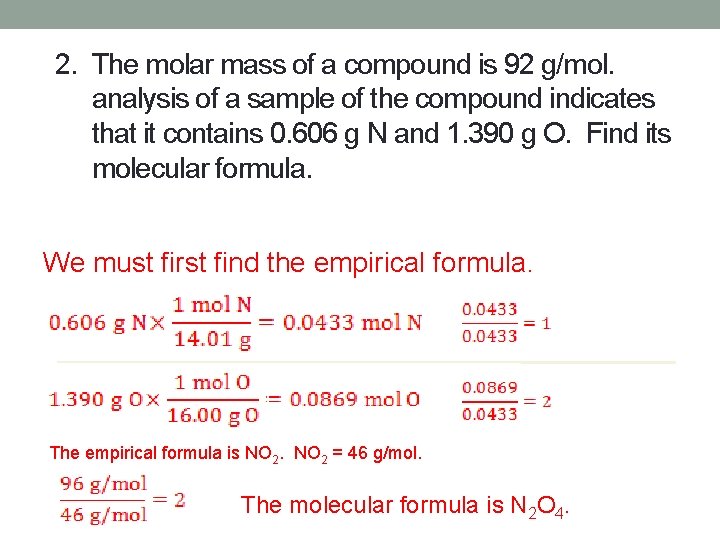
2. The molar mass of a compound is 92 g/mol. analysis of a sample of the compound indicates that it contains 0. 606 g N and 1. 390 g O. Find its molecular formula. We must first find the empirical formula. The empirical formula is NO 2 = 46 g/mol. The molecular formula is N 2 O 4.

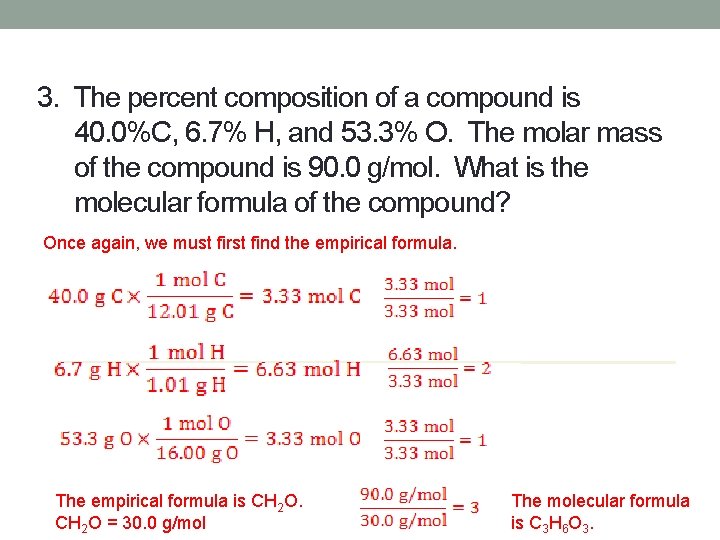
3. The percent composition of a compound is 40. 0%C, 6. 7% H, and 53. 3% O. The molar mass of the compound is 90. 0 g/mol. What is the molecular formula of the compound? Once again, we must first find the empirical formula. The empirical formula is CH 2 O = 30. 0 g/mol The molecular formula is C 3 H 6 O 3.
 Calculating molar mass using colligative properties
Calculating molar mass using colligative properties Molar mass from freezing point depression
Molar mass from freezing point depression Molar mass of chocolate chips
Molar mass of chocolate chips Calculating mass percent composition
Calculating mass percent composition Empirical formula from percent composition
Empirical formula from percent composition Percent composition
Percent composition How to find empirical formula with percentages
How to find empirical formula with percentages Mass to mass formula
Mass to mass formula Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Volume to mols
Volume to mols Mass to moles conversion factor
Mass to moles conversion factor Mass/molar mass
Mass/molar mass The mole bridge
The mole bridge Mass/molar mass
Mass/molar mass Mass number formula
Mass number formula Atomic mass vs molar mass
Atomic mass vs molar mass Relationship between linear and angular quantities
Relationship between linear and angular quantities Calculating molarity
Calculating molarity Define partial molar quantities
Define partial molar quantities Chemical potential
Chemical potential Partial molar quantities
Partial molar quantities Partial molar quantities
Partial molar quantities What is composition stoichiometry
What is composition stoichiometry Stoichiometry percent composition
Stoichiometry percent composition Stoichiometry deals with..,
Stoichiometry deals with.., Stoichiometry deals with.
Stoichiometry deals with. Steps for calculating average atomic mass
Steps for calculating average atomic mass Atomic mass of boron-10
Atomic mass of boron-10 Symbol for avogadro's constant
Symbol for avogadro's constant Percent by mass
Percent by mass Avogrados tal
Avogrados tal N = n/na
N = n/na The mole and avogadro's worksheet
The mole and avogadro's worksheet What is avogadros hypothesis
What is avogadros hypothesis Avogadro
Avogadro Define avogadros law
Define avogadros law Titrand
Titrand Avogadro's law example
Avogadro's law example Avogadros principle
Avogadros principle The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Stoichiometry review answers
Stoichiometry review answers Chemical quantities calculator
Chemical quantities calculator