Mole Calculations 2 Molar Mass The atomic mass




- Slides: 17

Mole Calculations 2

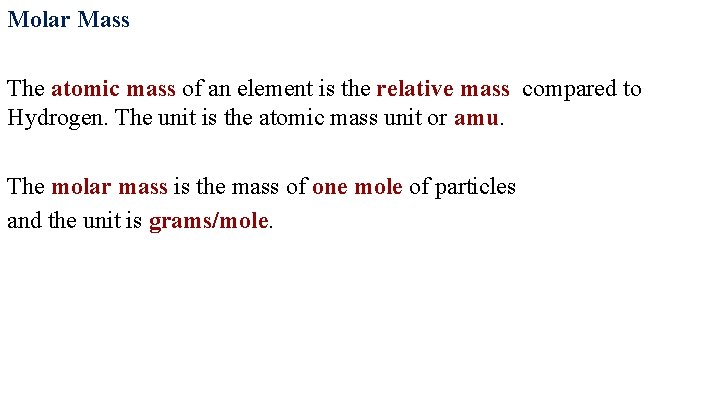
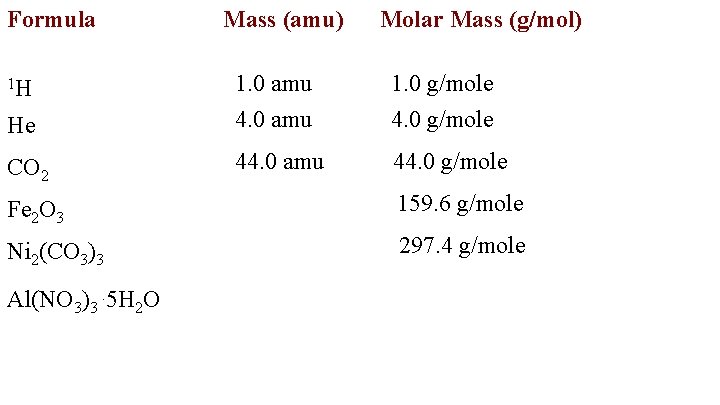
Molar Mass The atomic mass of an element is the relative mass compared to Hydrogen. The unit is the atomic mass unit or amu. The molar mass is the mass of one mole of particles and the unit is grams/mole.

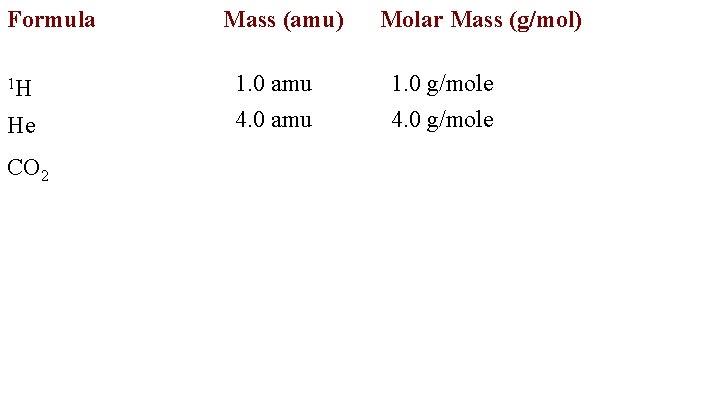
Formula 1 H He CO 2 Mass (amu) 1. 0 amu 4. 0 amu Molar Mass (g/mol) 1. 0 g/mole 4. 0 g/mole

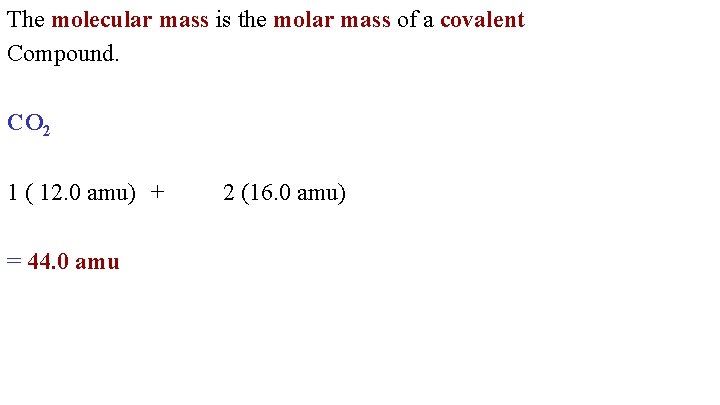
The molecular mass is the molar mass of a covalent Compound. CO 2 1 ( 12. 0 amu) + = 44. 0 amu 2 (16. 0 amu)

Formula Mass (amu) Molar Mass (g/mol) He 1. 0 amu 4. 0 amu 1. 0 g/mole 4. 0 g/mole CO 2 44. 0 amu 44. 0 g/mole 1 H Fe 2 O 3

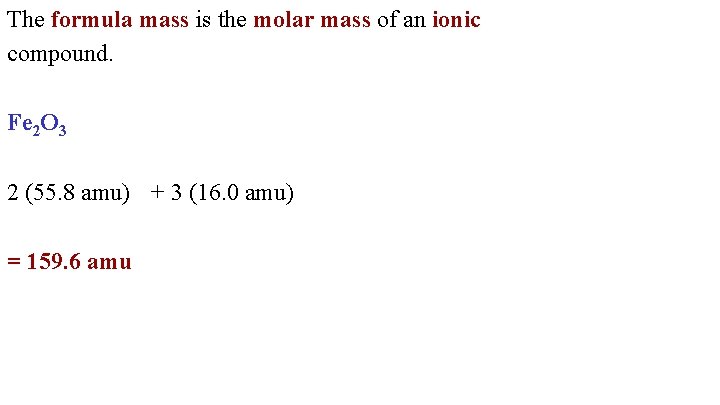
The formula mass is the molar mass of an ionic compound. Fe 2 O 3 2 (55. 8 amu) + 3 (16. 0 amu) = 159. 6 amu

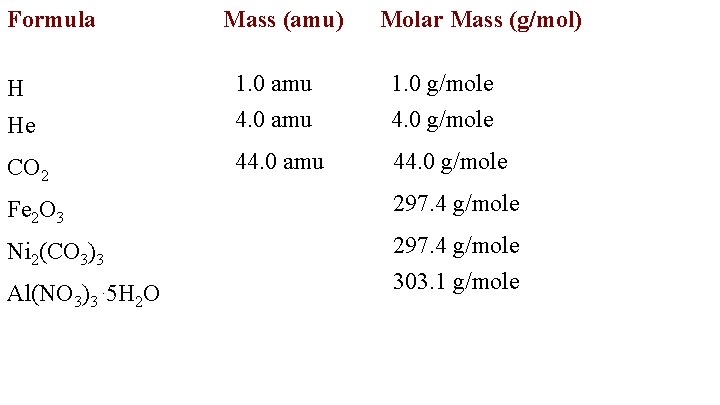
Formula Mass (amu) Molar Mass (g/mol) He 1. 0 amu 4. 0 amu 1. 0 g/mole 4. 0 g/mole CO 2 44. 0 amu 44. 0 g/mole 1 H Fe 2 O 3 159. 6 g/mole Ni 2(CO 3)3 297. 4 g/mole Al(NO 3)3. 5 H 2 O

Al(NO 3)3. 5 H 2 O 1 ( 27. 0) + 3 (14. 0) + 9 (16. 0) + 10 (1. 01) + 5 (16. 0) Al N O H O 303. 1 g/mole Note H is 1. 01 due to 1 H and 2 H

Formula Mass (amu) Molar Mass (g/mol) He 1. 0 amu 4. 0 amu 1. 0 g/mole 4. 0 g/mole CO 2 44. 0 amu 44. 0 g/mole H Fe 2 O 3 297. 4 g/mole Ni 2(CO 3)3 297. 4 g/mole Al(NO 3)3. 5 H 2 O 303. 1 g/mole

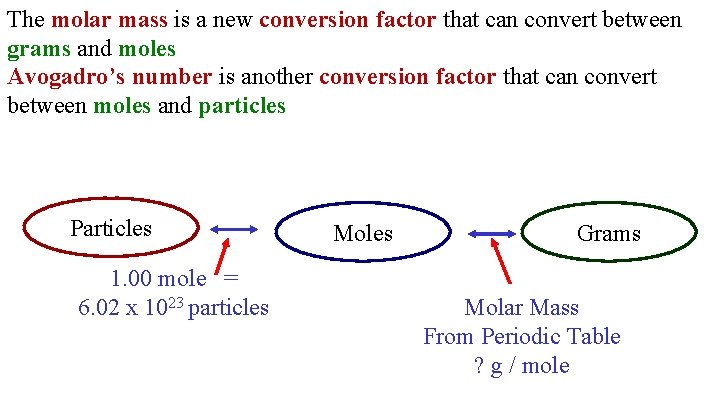
The molar mass is a new conversion factor that can convert between grams and moles Avogadro’s number is another conversion factor that can convert between moles and particles Particles 1. 00 mole = 6. 02 x 1023 particles Moles Grams Molar Mass From Periodic Table ? g / mole

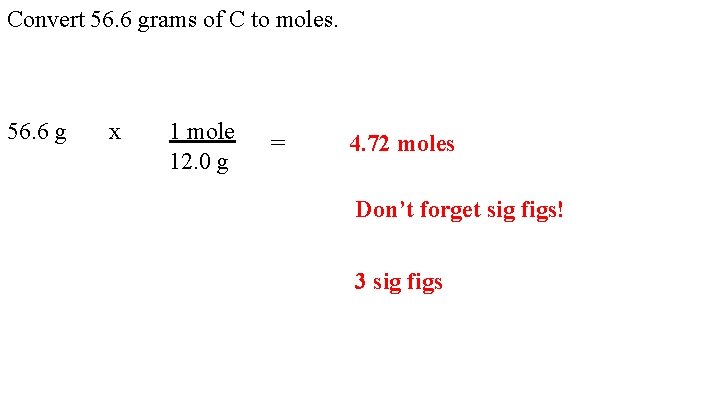
Convert 56. 6 grams of C to moles. 56. 6 g x 1 mole 12. 0 g = 4. 72 moles Don’t forget sig figs! 3 sig figs

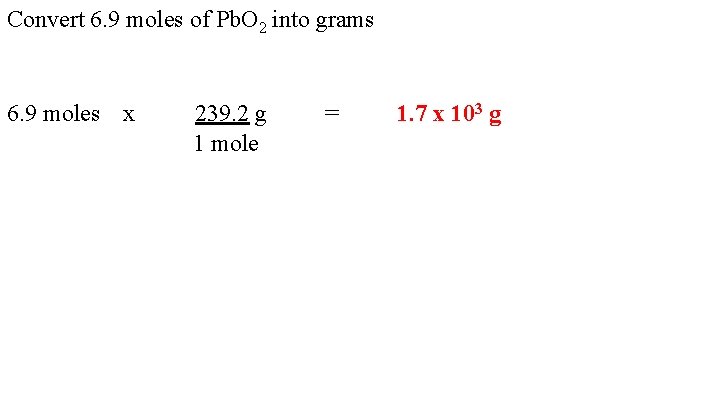
Convert 6. 9 moles of Pb. O 2 into grams 6. 9 moles x 239. 2 g 1 mole = 1. 7 x 103 g

Two Step Mole Calculations

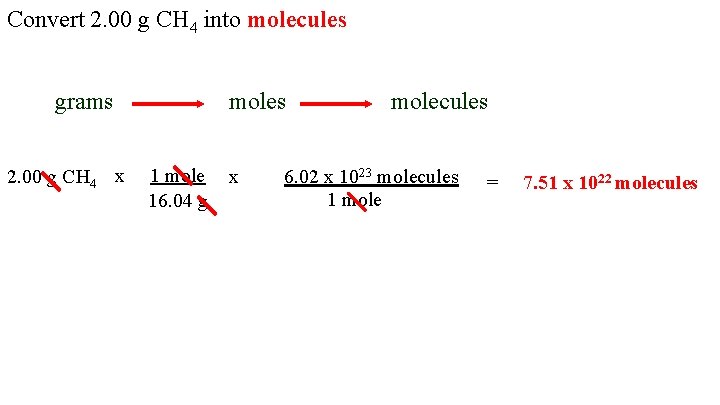
Convert 2. 00 g CH 4 into molecules grams 2. 00 g CH 4 x moles 1 mole 16. 04 g x molecules 6. 02 x 1023 molecules 1 mole = 7. 51 x 1022 molecules

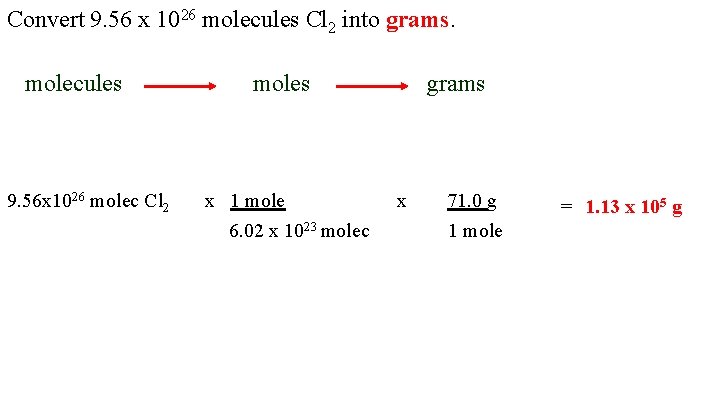
Convert 9. 56 x 1026 molecules Cl 2 into grams. molecules 9. 56 x 1026 molec Cl 2 moles x 1 mole 6. 02 x 1023 molec grams x 71. 0 g 1 mole = 1. 13 x 105 g

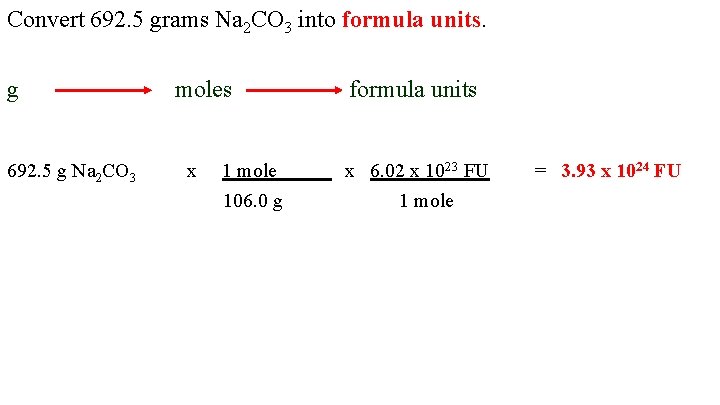
Convert 692. 5 grams Na 2 CO 3 into formula units. g 692. 5 g Na 2 CO 3 moles x 1 mole 106. 0 g formula units x 6. 02 x 1023 FU 1 mole = 3. 93 x 1024 FU

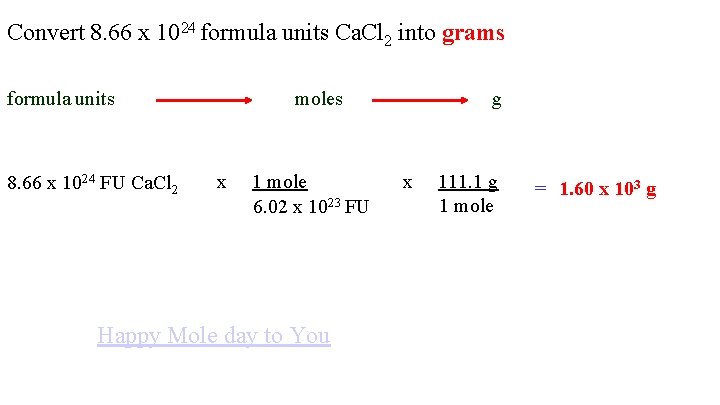
Convert 8. 66 x 1024 formula units Ca. Cl 2 into grams formula units 8. 66 x 1024 FU Ca. Cl 2 moles x 1 mole 6. 02 x 1023 FU Happy Mole day to You g x 111. 1 g 1 mole = 1. 60 x 103 g