Gram Formula Mass or Molar Mass sum of





- Slides: 12

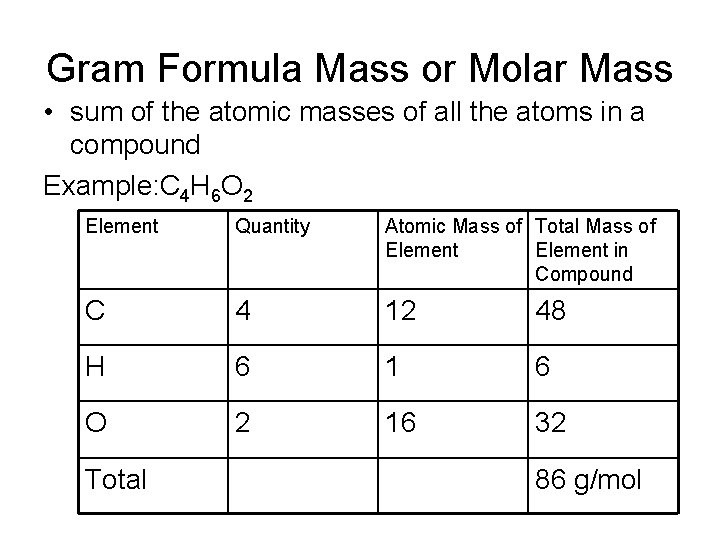
Gram Formula Mass or Molar Mass • sum of the atomic masses of all the atoms in a compound Example: C 4 H 6 O 2 Element Quantity Atomic Mass of Total Mass of Element in Compound C 4 12 48 H 6 1 6 O 2 16 32 Total 86 g/mol

Steps 1. Determine the number of atoms of every element present in the formula 2. Look up the atomic mass of each element on the periodic table 3. Multiply step one by step two for each element 4. Add the products of step three and round to the appropriate number of significant digits

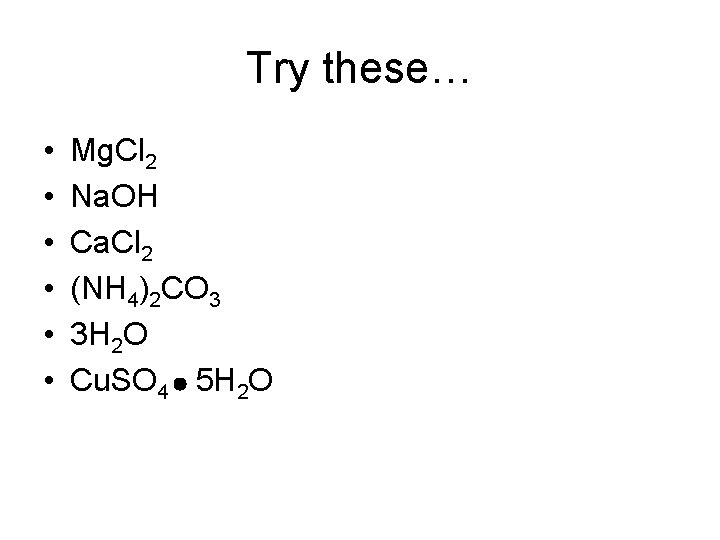
Try these… • • • Mg. Cl 2 Na. OH Ca. Cl 2 (NH 4)2 CO 3 3 H 2 O Cu. SO 4 5 H 2 O

AMU vs. gram • Atomic Mass Unit • 1. 00 amu = 1. 66 x 10 -24 g • 1/12 of the mass of an atom of carbon-12

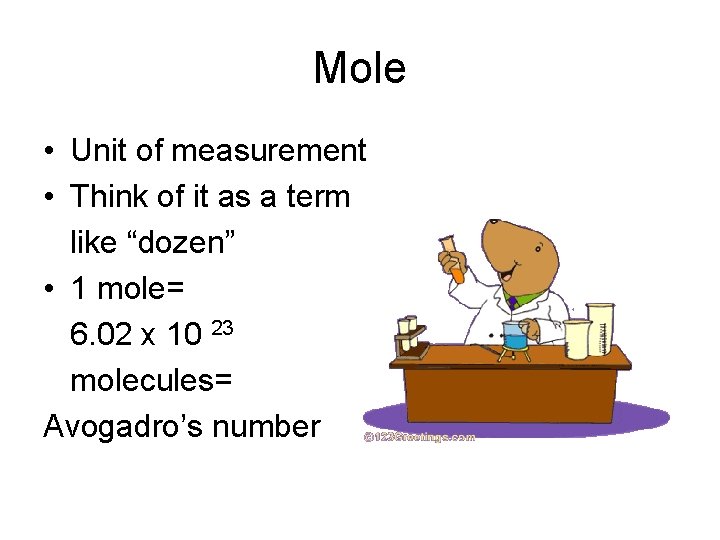
Mole • Unit of measurement • Think of it as a term like “dozen” • 1 mole= 6. 02 x 10 23 molecules= Avogadro’s number

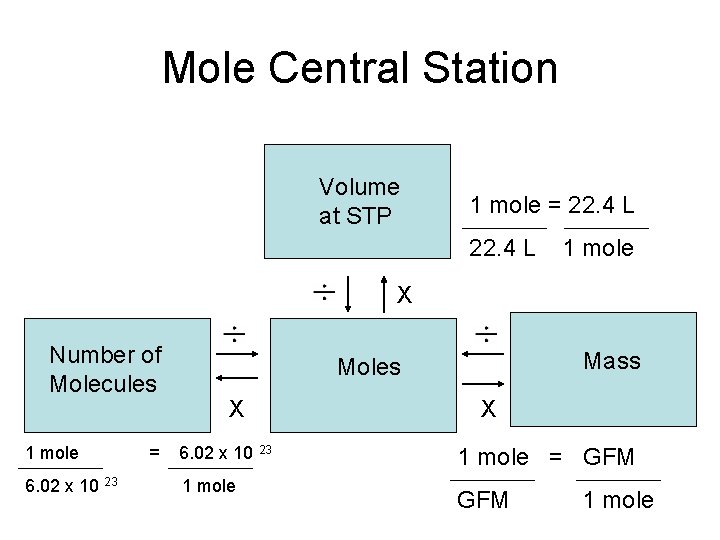
Mole Central Station Volume at STP 1 mole = 22. 4 L 1 mole X Number of Molecules 1 mole 6. 02 x 10 23 = Mass Moles X 6. 02 x 10 23 1 mole X 1 mole = GFM 1 mole

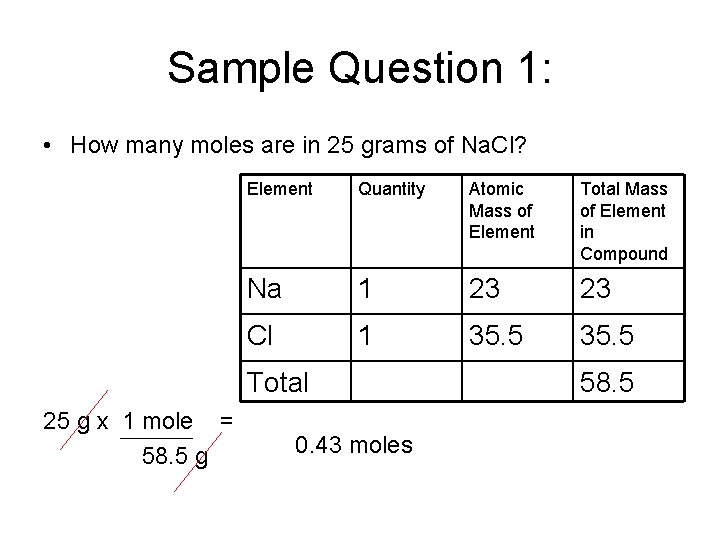
Sample Question 1: • How many moles are in 25 grams of Na. Cl? Element Quantity Atomic Mass of Element Total Mass of Element in Compound Na 1 23 23 Cl 1 35. 5 Total 25 g x 1 mole = 58. 5 g 0. 43 moles 58. 5

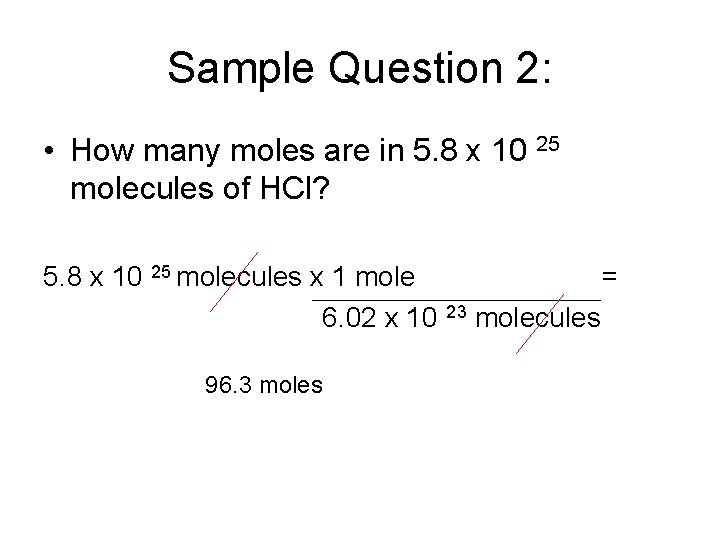
Sample Question 2: • How many moles are in 5. 8 x 10 25 molecules of HCl? 5. 8 x 10 25 molecules x 1 mole 6. 02 x 10 96. 3 moles = 23 molecules

Sample Question 3: • What volume does 2. 0 moles of Mg. Br 2 occupy? 2. 0 moles x 22. 4 L = 1 mole 44. 8 L

2 step problems • What volume does 33 grams of H 2 O occupy? Step 1: Convert 33 grams to moles 33 g x 1 mole = 1. 83 moles 18 g Step 2: Convert moles to volume 1. 83 moles x 22. 4 L = 41. 1 L 1 mole

2 Step • How many molecules are in 45 grams of H 2 SO 4? 45 g x 1 mole x 6. 02 x 10 23 molecules = 98 g 1 mole 2. 76 x 10 23 molecules How many atoms? 2. 76 x 10 23 x 7= 1. 9 x 1024

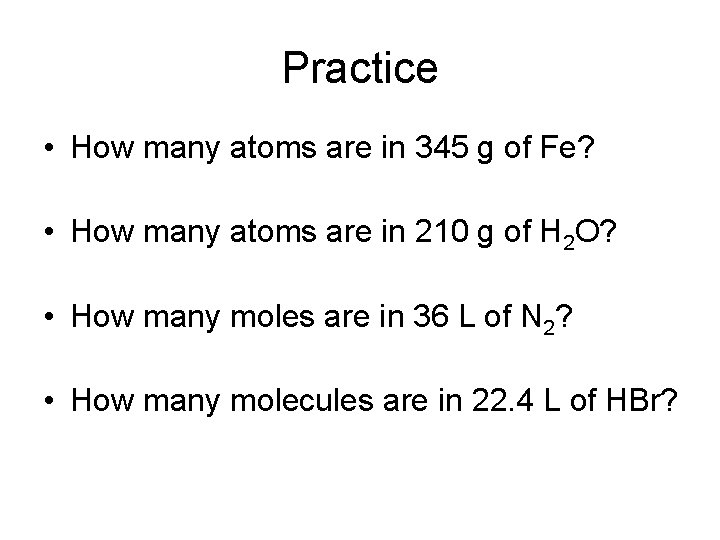
Practice • How many atoms are in 345 g of Fe? • How many atoms are in 210 g of H 2 O? • How many moles are in 36 L of N 2? • How many molecules are in 22. 4 L of HBr?