Molar Mass Using Density Molar mass d RT






- Slides: 12

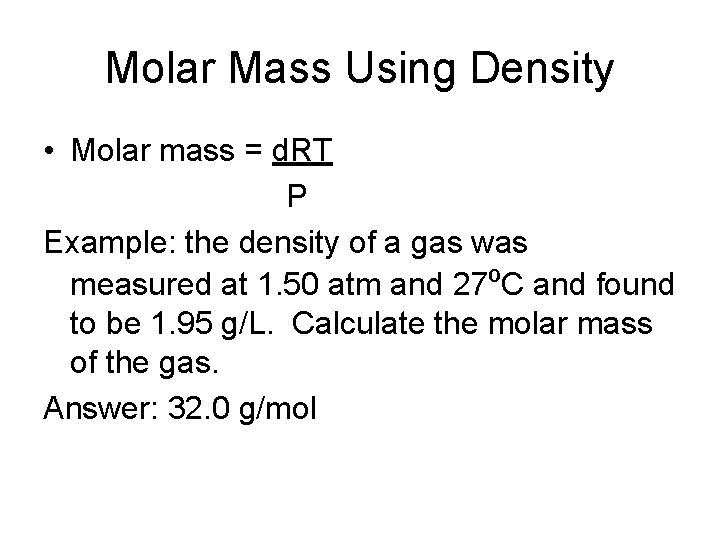
Molar Mass Using Density • Molar mass = d. RT P Example: the density of a gas was measured at 1. 50 atm and 27ºC and found to be 1. 95 g/L. Calculate the molar mass of the gas. Answer: 32. 0 g/mol

Dalton’s Law of Partial Pressures • The total pressure of a gas mixture is the sum of the partial pressures of the component gases. • Ptotal = P 1+P 2+P 3+P 4+… • For a mixture of ideal gases, the total number of moles of particles is important, not identity/composition

Simple Example • A gas mixture containing oxygen, nitrogen, and carbon dioxide has a total pressure of 32. 9 k. Pa. If PO 2=6. 6 k. Pa and PN 2=23. 0 k. Pa, what is PCO 2? • COMPLEX EXAMPLE pg. 200 in book!

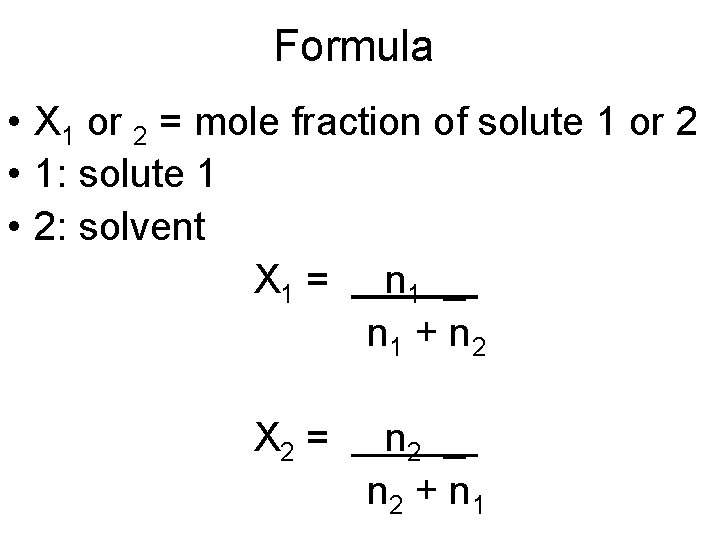
Mole Fraction • Ratio of moles of solute to total number of moles of solute(s) and solvent • No unit necessary • Watch out for significant figures

Formula • X 1 or 2 = mole fraction of solute 1 or 2 • 1: solute 1 • 2: solvent X 1 = n 1 _ n 1 + n 2 X 2 = n 2 _ n 2 + n 1

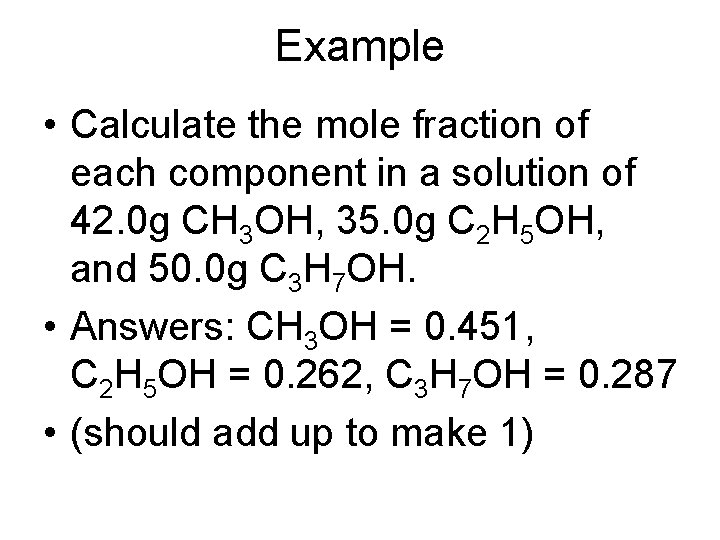
Example • Calculate the mole fraction of each component in a solution of 42. 0 g CH 3 OH, 35. 0 g C 2 H 5 OH, and 50. 0 g C 3 H 7 OH. • Answers: CH 3 OH = 0. 451, C 2 H 5 OH = 0. 262, C 3 H 7 OH = 0. 287 • (should add up to make 1)

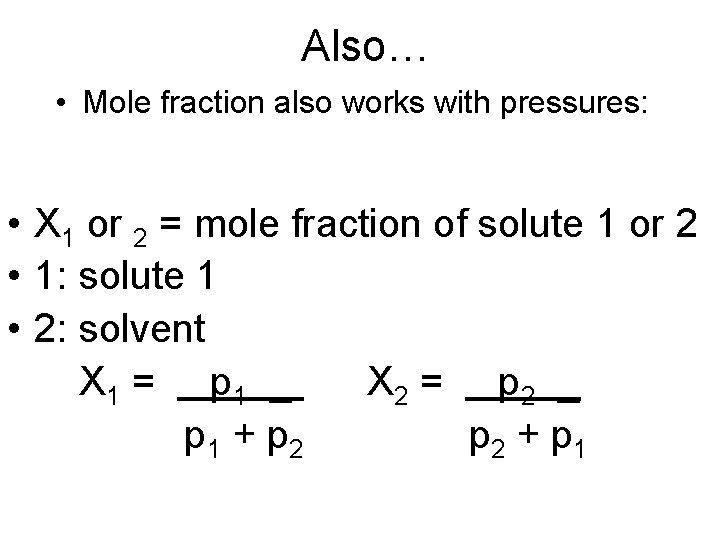
Also… • Mole fraction also works with pressures: • X 1 or 2 = mole fraction of solute 1 or 2 • 1: solute 1 • 2: solvent X 1 = p 1 _ X 2 = p 2 _ p 1 + p 2 + p 1

Collecting Gas Over Water • Vapor pressure • Go over example pg. 204

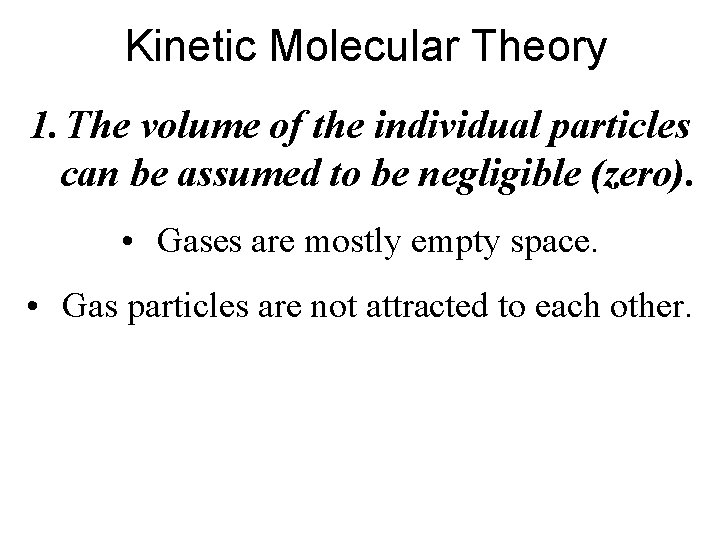
Kinetic Molecular Theory 1. The volume of the individual particles can be assumed to be negligible (zero). • Gases are mostly empty space. • Gas particles are not attracted to each other.

2. Gas particles are in constant motion. The collisions of the particles with the walls of the container are the cause of the pressure exerted by the gas. 3. The particles are assumed to exert no forces on each other; they are assumed neither to attract nor to repel each other. • Elastic means no loss of energy. • Like two billiard balls hitting each other – energy can be transferred but not lost.

4. The average kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas. Only explains properties of an ideal gas. A real gas does not conform to these… We can relate each of the gas laws to the KMT.

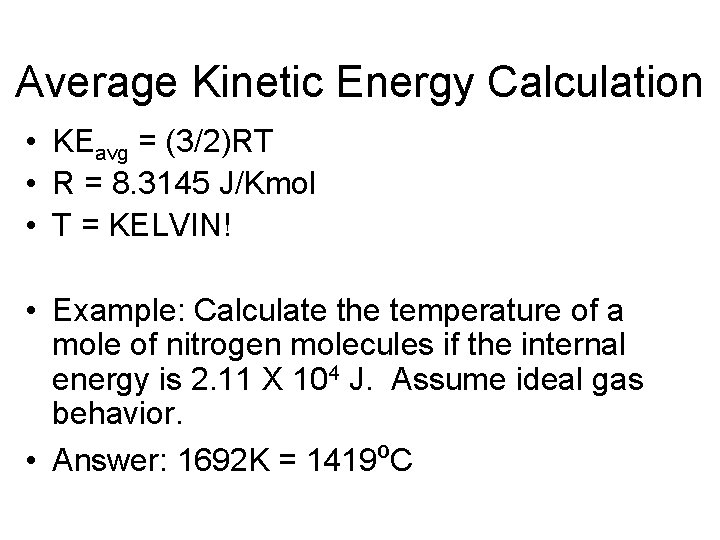
Average Kinetic Energy Calculation • KEavg = (3/2)RT • R = 8. 3145 J/Kmol • T = KELVIN! • Example: Calculate the temperature of a mole of nitrogen molecules if the internal energy is 2. 11 X 104 J. Assume ideal gas behavior. • Answer: 1692 K = 1419ºC