Chapter 6 Section 4 Molar Mass Molar Mass

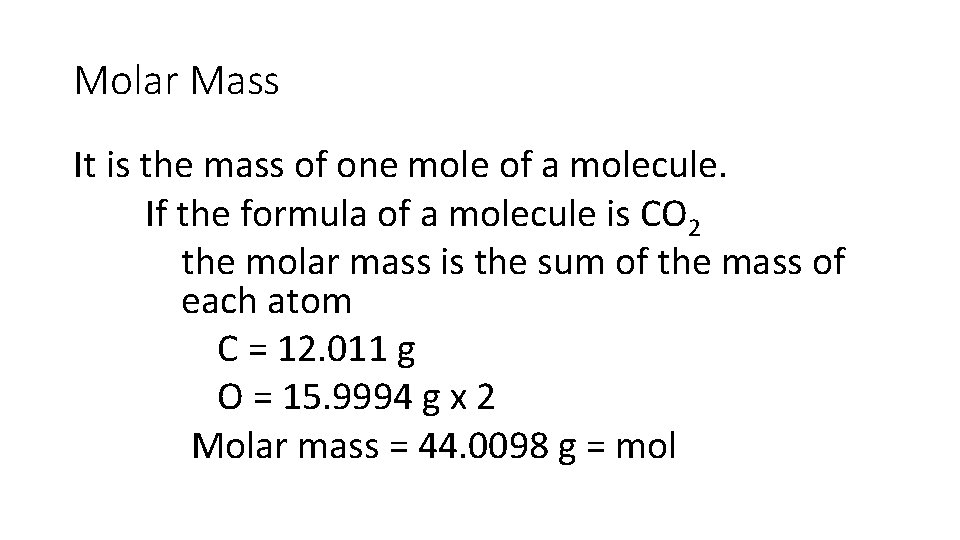
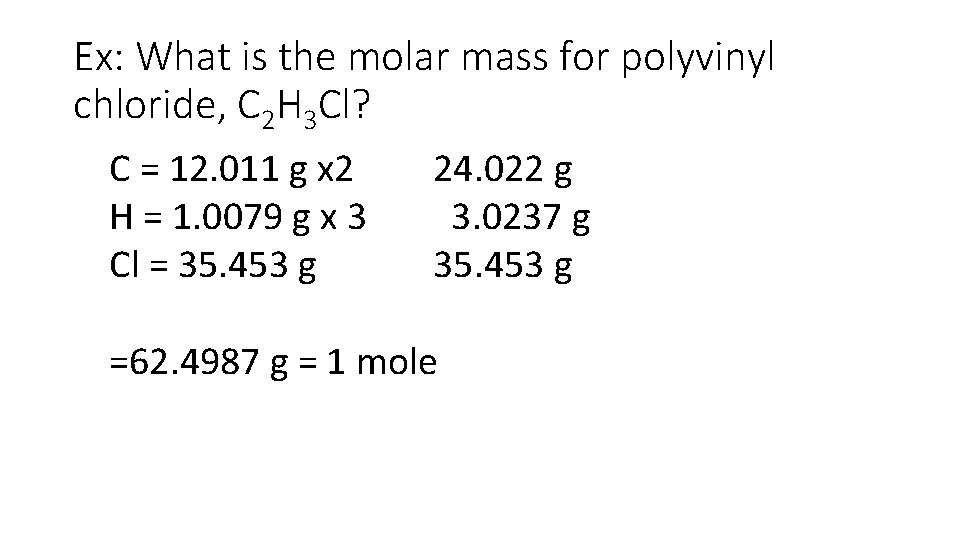

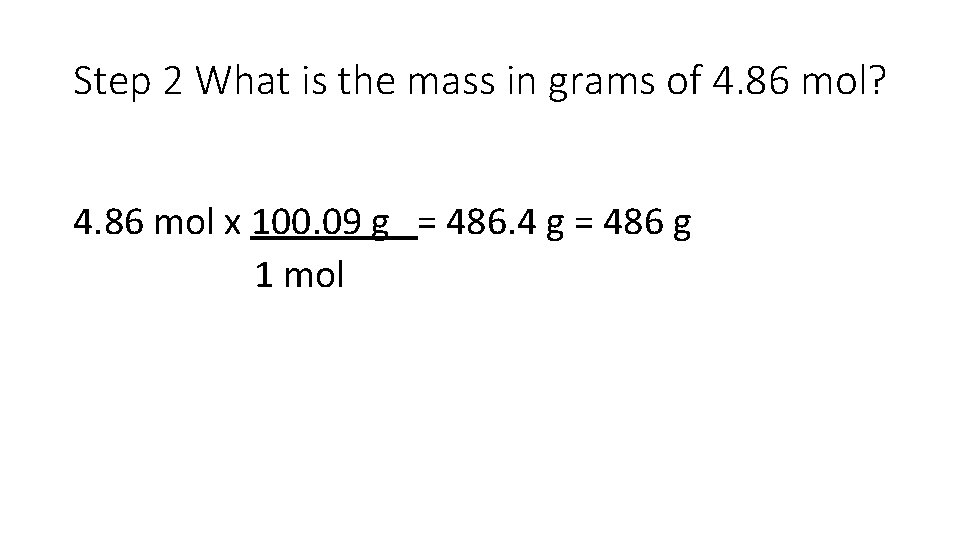




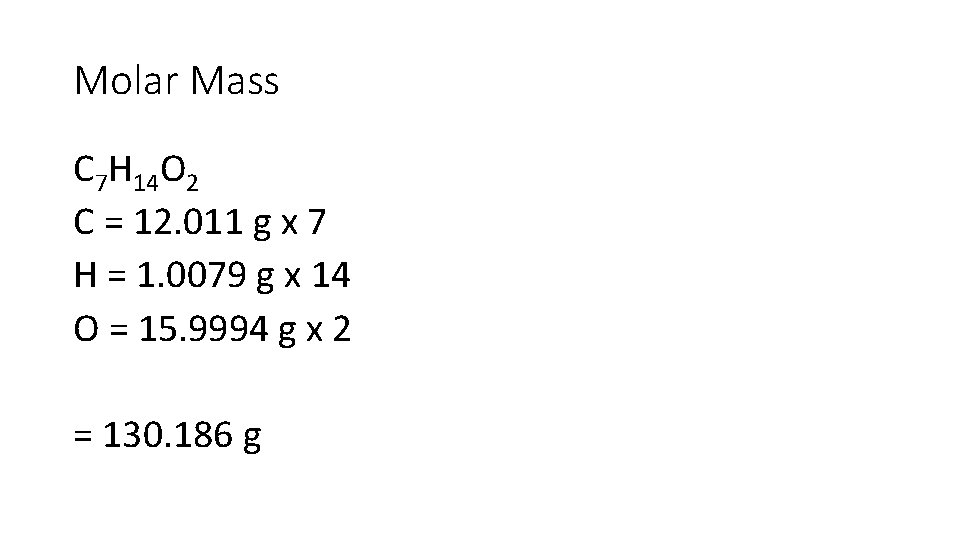

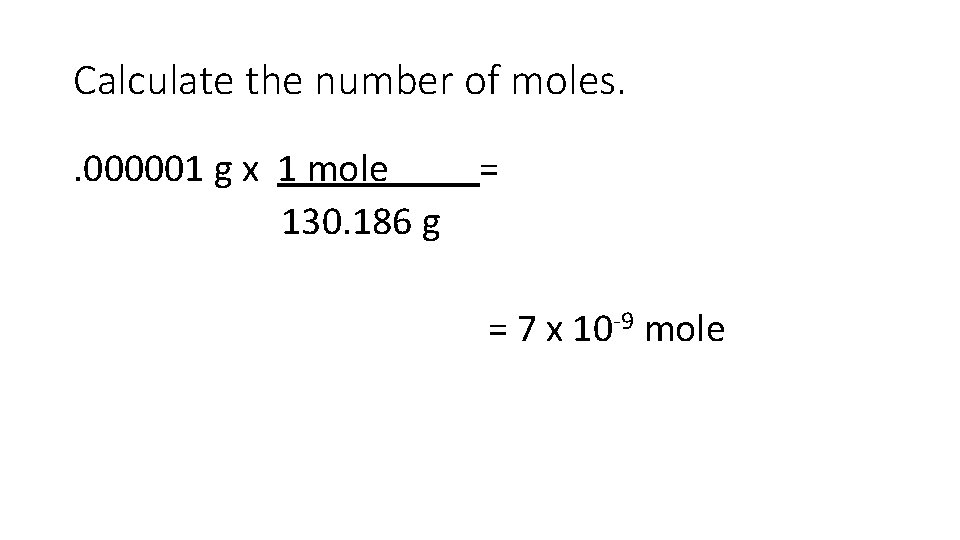
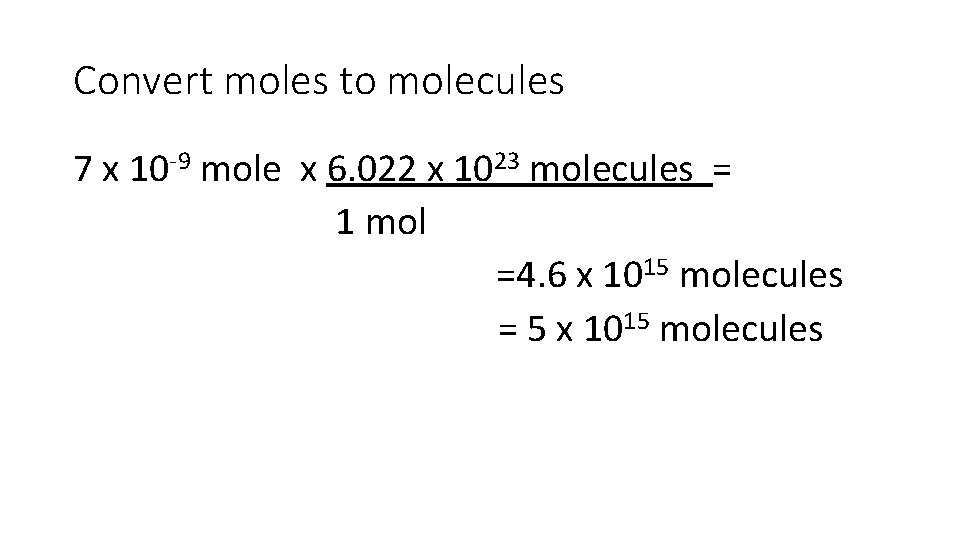

- Slides: 16

Chapter 6 Section 4 Molar Mass
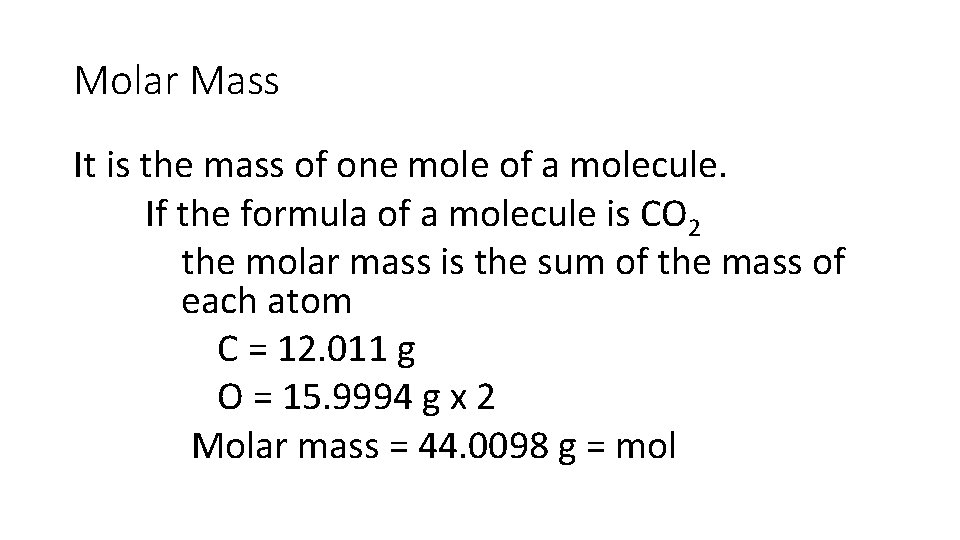
Molar Mass It is the mass of one mole of a molecule. If the formula of a molecule is CO 2 the molar mass is the sum of the mass of each atom C = 12. 011 g O = 15. 9994 g x 2 Molar mass = 44. 0098 g = mol

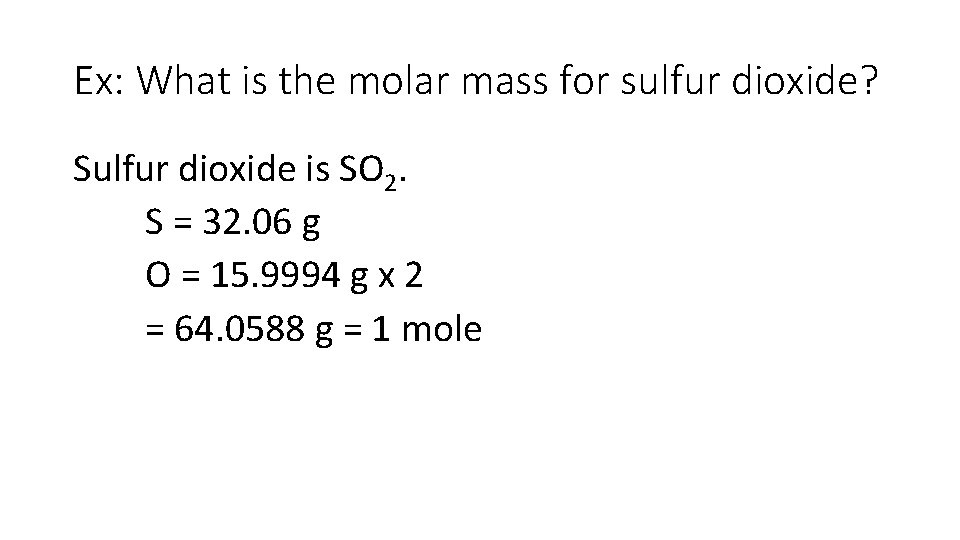
Ex: What is the molar mass for sulfur dioxide? Sulfur dioxide is SO 2. S = 32. 06 g O = 15. 9994 g x 2 = 64. 0588 g = 1 mole
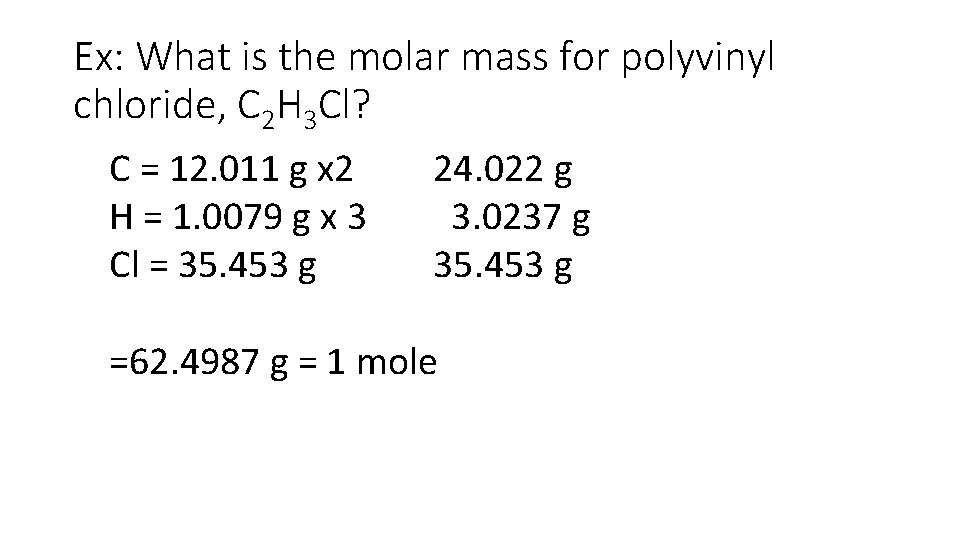
Ex: What is the molar mass for polyvinyl chloride, C 2 H 3 Cl? C = 12. 011 g x 2 H = 1. 0079 g x 3 Cl = 35. 453 g 24. 022 g 3. 0237 g 35. 453 g =62. 4987 g = 1 mole

Write down only the important information. Ex: Calcium carbonate, Ca. CO 3, is the principal mineral found in limestone, marble, chalk, pearls, and the shells of marine animals such as clams. Calculate the molar mass of calcium carbonate. A certain sample of calcium carbonate contains 4. 86 mol. What is the mass in grams of this sample?

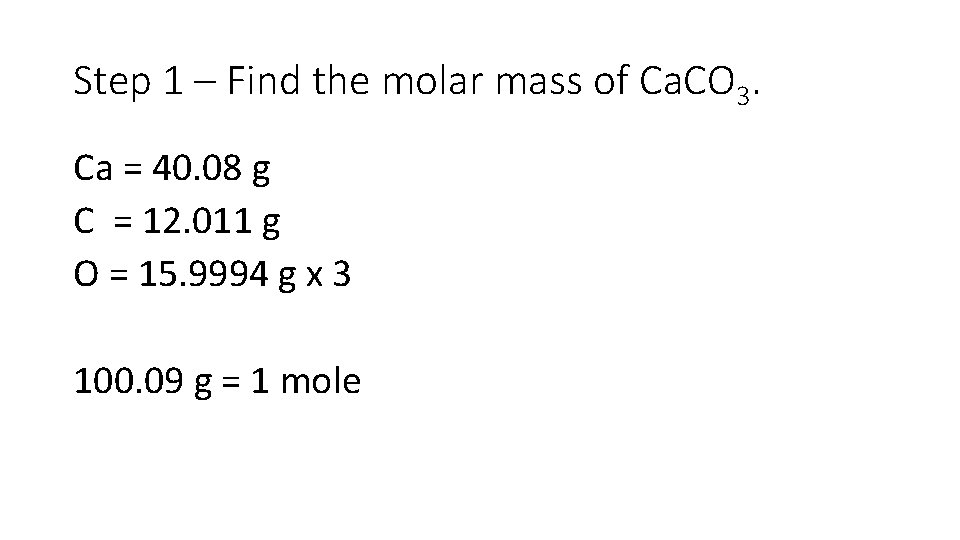
Step 1 – Find the molar mass of Ca. CO 3. Ca = 40. 08 g C = 12. 011 g O = 15. 9994 g x 3 100. 09 g = 1 mole
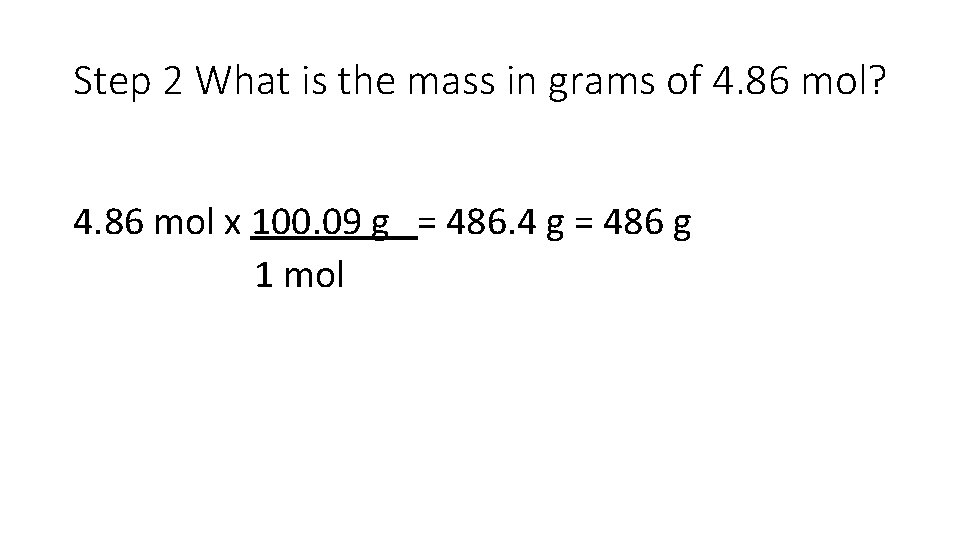
Step 2 What is the mass in grams of 4. 86 mol? 4. 86 mol x 100. 09 g = 486. 4 g = 486 g 1 mol

Calculating number of molecules. It is the same as going from moles to atoms. 6. 022 x 1023 molecules = 1 mole

Write down only the important information. Ex: Isopentyl acetate, C 7 H 14 O 2, the compound responsible for the scent of bananas, can be produced commercially. Interestingly, bees release about 1 g of this compound when they sting. This attracts other bees, which then join the attack. How many moles and how many molecules of isopentyl acetate are released in a typical bee sting?

Important information: C 7 H 14 O 2 1 g How many moles? How many molecules?

Steps Molar mass 1 g = ? g g to molecule
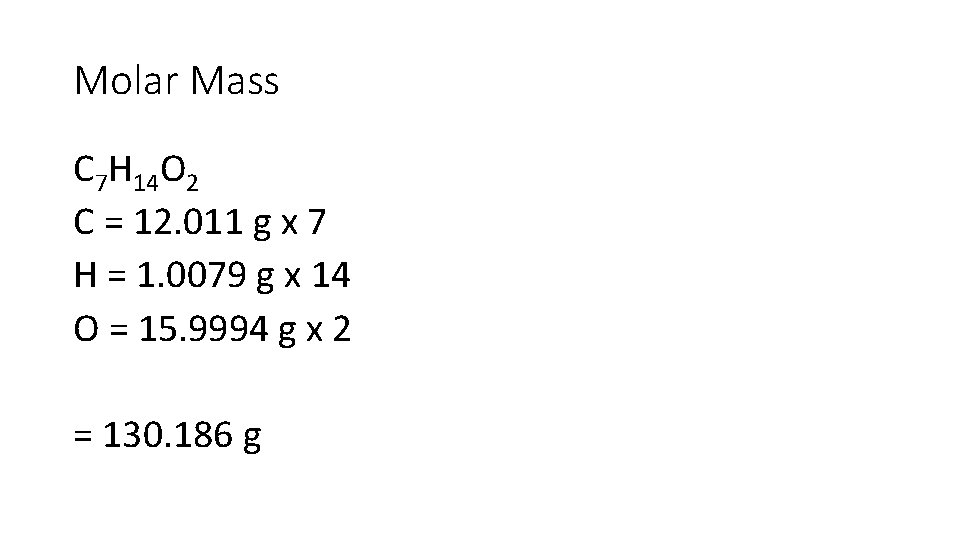
Molar Mass C 7 H 14 O 2 C = 12. 011 g x 7 H = 1. 0079 g x 14 O = 15. 9994 g x 2 = 130. 186 g

1 g to g M_ _ K H dk Base d c m _ _ n. 000001 g
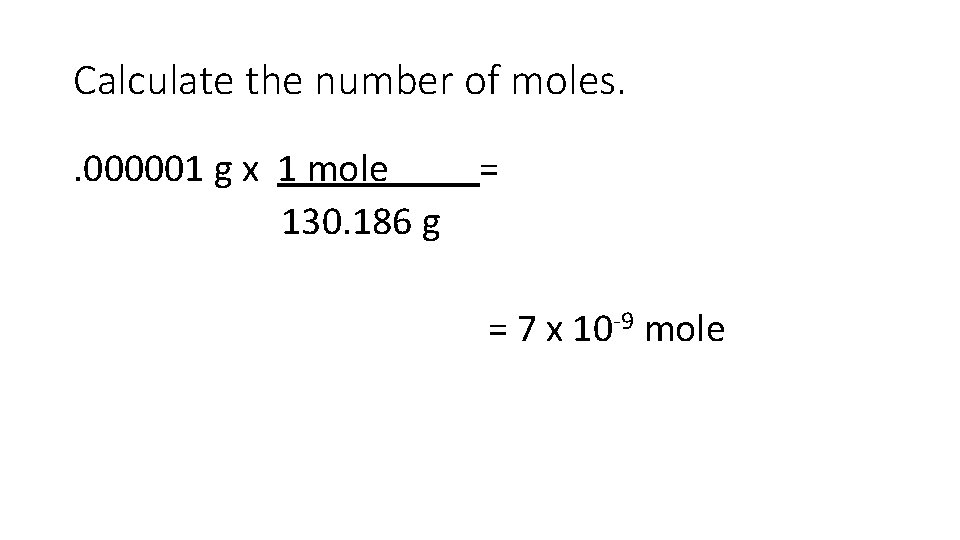
Calculate the number of moles. . 000001 g x 1 mole = 130. 186 g = 7 x 10 -9 mole
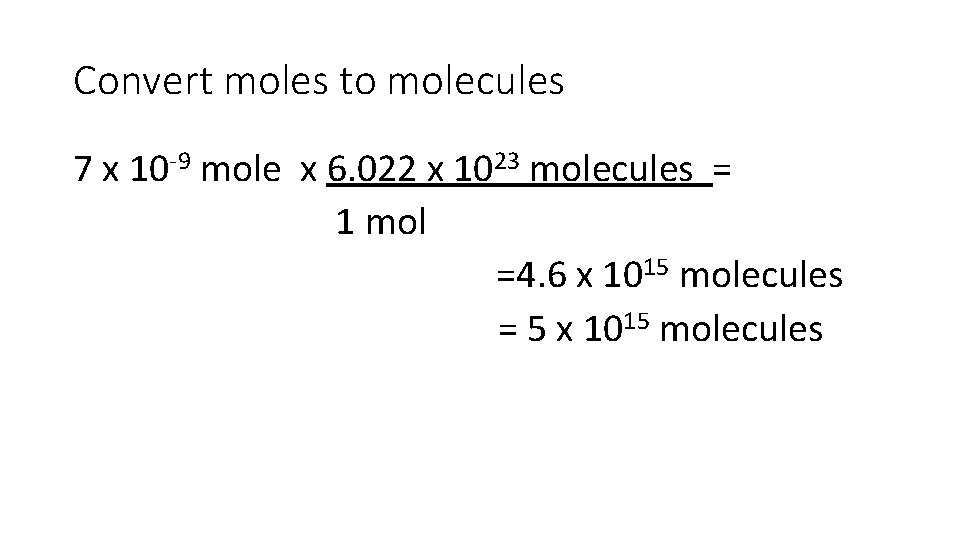
Convert moles to molecules 7 x 10 -9 mole x 6. 022 x 1023 molecules = 1 mol =4. 6 x 1015 molecules = 5 x 1015 molecules

Homework P 188: 19 -26 blue