Determining the Empirical Formula for a Compound The

- Slides: 8

Determining the Empirical Formula for a Compound • The empirical formula for a compound is the simplest _____ whole number _____ of the atoms in the compound. ratio Examples: H 2 O is the empirical formula for water. (can’t be reduced) C 1 H 2 O 1 Glucose, C H O , has the empirical formula ______. 6 12 6 Step 1: Change % to grams. Step 2: Convert grams to moles using the mole chart. Step 3: Divide each of these answers by the smallest number. Step 4: Your answers will be the subscripts for the empirical formula. Helpful Rhyme: % to mass, mass to mole, divide by small, times ’til whole.

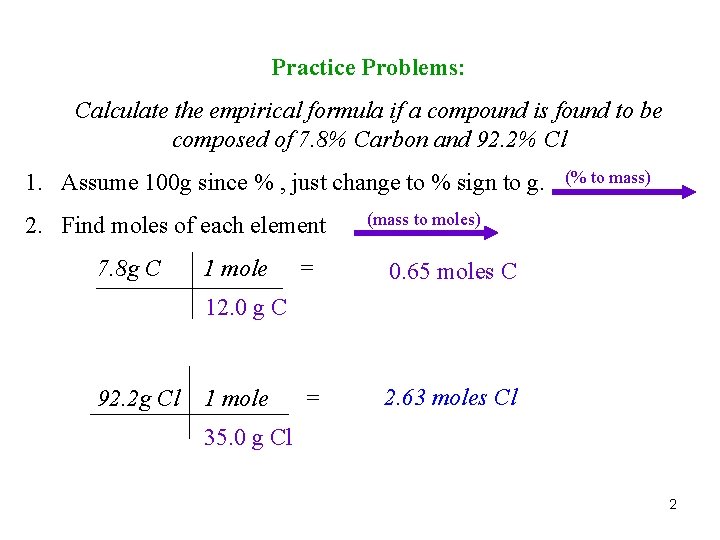
Practice Problems: Calculate the empirical formula if a compound is found to be composed of 7. 8% Carbon and 92. 2% Cl 1. Assume 100 g since % , just change to % sign to g. 2. Find moles of each element 7. 8 g C 1 mole (% to mass) (mass to moles) = 0. 65 moles C = 2. 63 moles Cl 12. 0 g C 92. 2 g Cl 1 mole 35. 0 g Cl 2

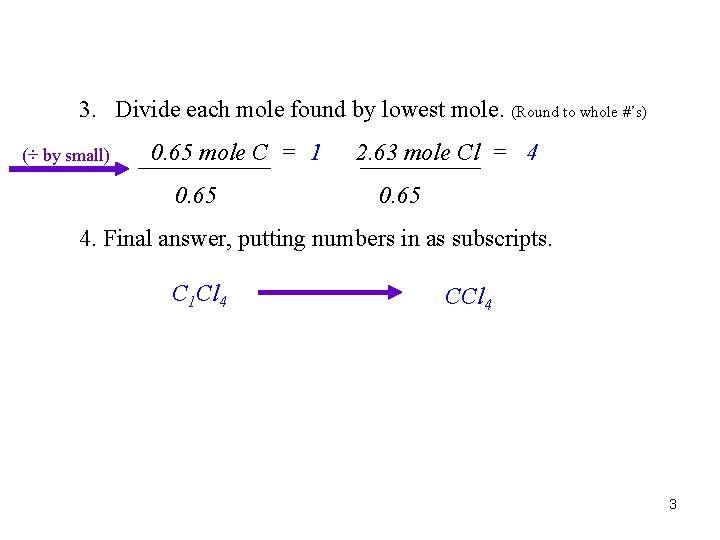
3. Divide each mole found by lowest mole. (Round to whole #’s) (÷ by small) 0. 65 mole C = 1 0. 65 2. 63 mole Cl = 4 0. 65 4. Final answer, putting numbers in as subscripts. C 1 Cl 4 CCl 4 3

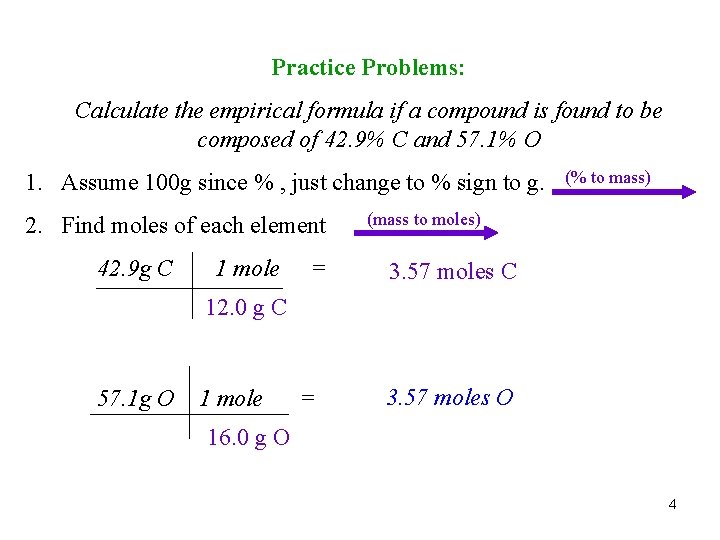
Practice Problems: Calculate the empirical formula if a compound is found to be composed of 42. 9% C and 57. 1% O 1. Assume 100 g since % , just change to % sign to g. 2. Find moles of each element 42. 9 g C 1 mole = (% to mass) (mass to moles) 3. 57 moles C 12. 0 g C 57. 1 g O 1 mole = 3. 57 moles O 16. 0 g O 4

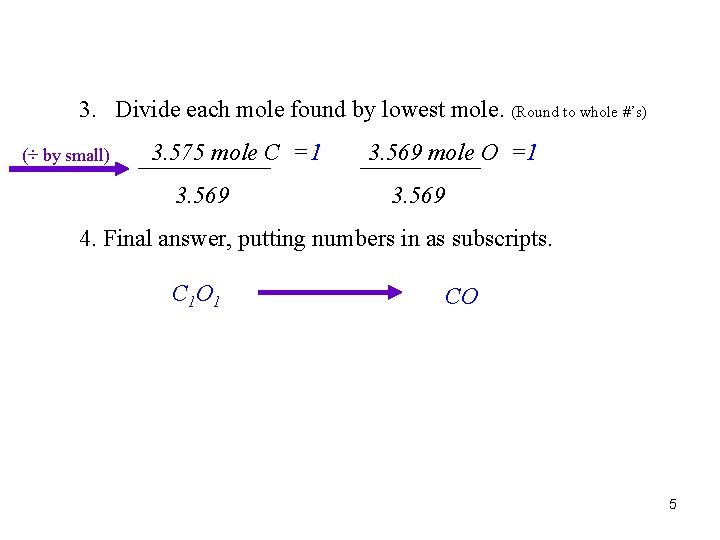
3. Divide each mole found by lowest mole. (Round to whole #’s) (÷ by small) 3. 575 mole C = 1 3. 569 mole O =1 3. 569 4. Final answer, putting numbers in as subscripts. C 1 O 1 CO 5

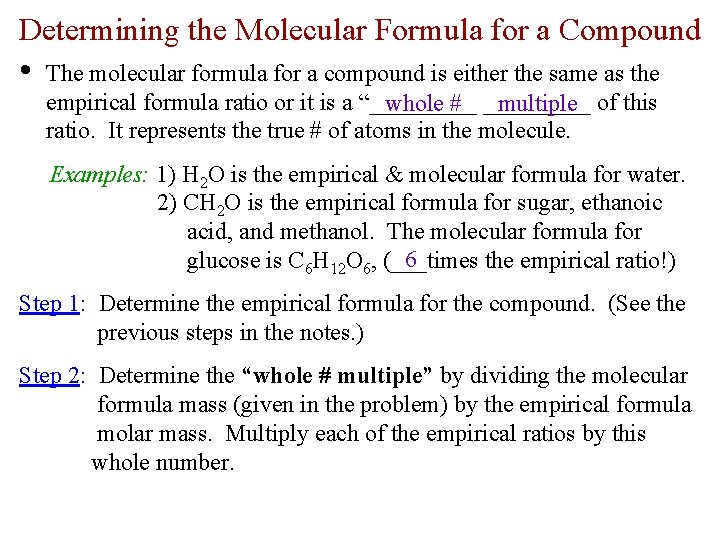
Determining the Molecular Formula for a Compound • The molecular formula for a compound is either the same as the empirical formula ratio or it is a “_____ whole # _____ multiple of this ratio. It represents the true # of atoms in the molecule. Examples: 1) H 2 O is the empirical & molecular formula for water. 2) CH 2 O is the empirical formula for sugar, ethanoic acid, and methanol. The molecular formula for 6 glucose is C 6 H 12 O 6, (___times the empirical ratio!) Step 1: Determine the empirical formula for the compound. (See the previous steps in the notes. ) Step 2: Determine the “whole # multiple” by dividing the molecular formula mass (given in the problem) by the empirical formula molar mass. Multiply each of the empirical ratios by this whole number.

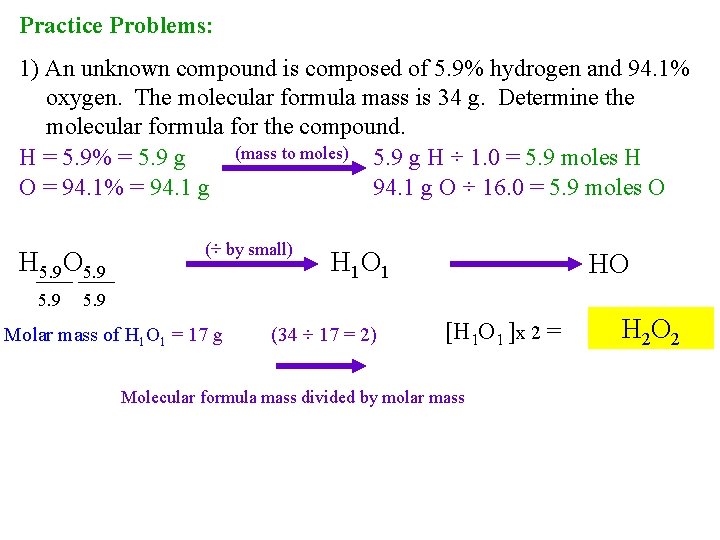
Practice Problems: 1) An unknown compound is composed of 5. 9% hydrogen and 94. 1% oxygen. The molecular formula mass is 34 g. Determine the molecular formula for the compound. (mass to moles) 5. 9 g H ÷ 1. 0 = 5. 9 moles H H = 5. 9% = 5. 9 g O = 94. 1% = 94. 1 g O ÷ 16. 0 = 5. 9 moles O H 5. 9 O 5. 9 (÷ by small) H 1 O 1 HO 5. 9 Molar mass of H 1 O 1 = 17 g (34 ÷ 17 = 2) [H 1 O 1 ]x 2 = Molecular formula mass divided by molar mass H 2 O 2

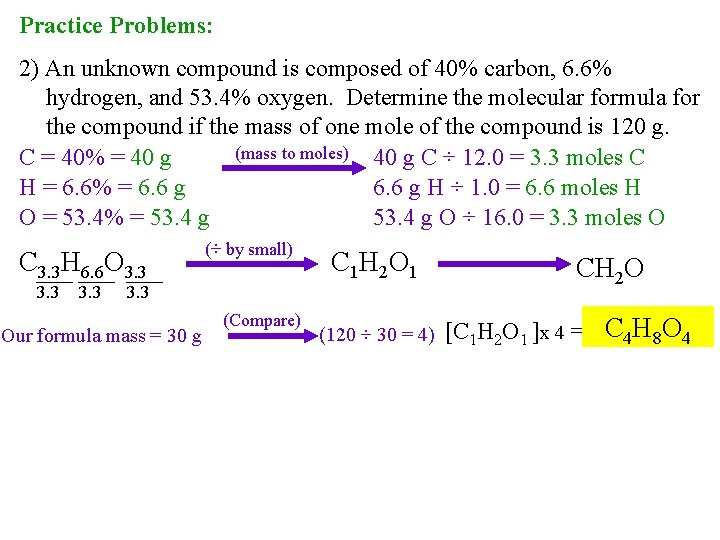
Practice Problems: 2) An unknown compound is composed of 40% carbon, 6. 6% hydrogen, and 53. 4% oxygen. Determine the molecular formula for the compound if the mass of one mole of the compound is 120 g. (mass to moles) 40 g C ÷ 12. 0 = 3. 3 moles C C = 40% = 40 g H = 6. 6% = 6. 6 g H ÷ 1. 0 = 6. 6 moles H O = 53. 4% = 53. 4 g O ÷ 16. 0 = 3. 3 moles O C 3. 3 H 6. 6 O 3. 3 (÷ by small) C 1 H 2 O 1 3. 3 Our formula mass = 30 g (Compare) (120 ÷ 30 = 4) CH 2 O [C 1 H 2 O 1 ]x 4 = C 4 H 8 O 4