EMPIRICAL FORMULA VS MOLECULAR FORMULA EMPIRICAL FORMULA VS



- Slides: 16

EMPIRICAL FORMULA VS. MOLECULAR FORMULA

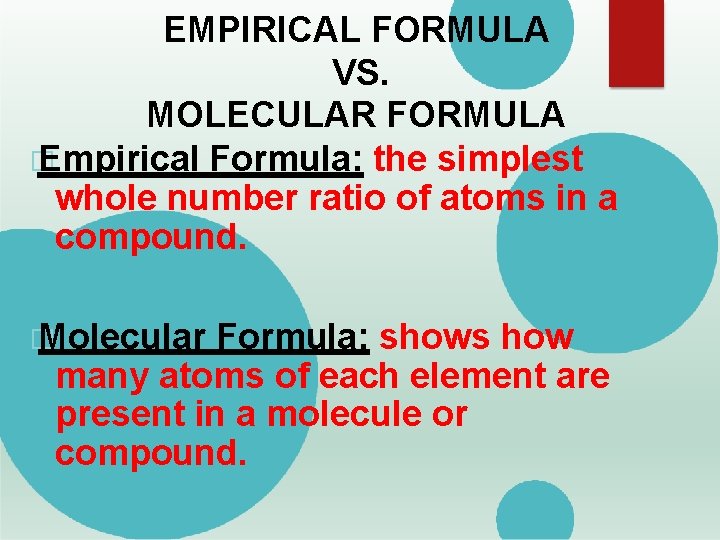
EMPIRICAL FORMULA VS. MOLECULAR FORMULA � Empirical Formula: the simplest whole number ratio of atoms in a compound. � Molecular Formula: shows how many atoms of each element are present in a molecule or compound.

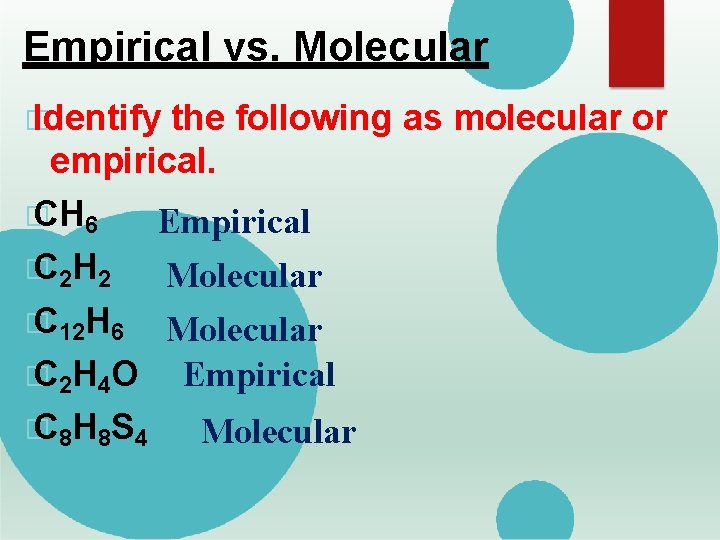
Empirical vs. Molecular � Identify the following as molecular or empirical. � CH 6 Empirical � C 2 H 2 Molecular � C 12 H 6 Molecular � C 2 H 4 O Empirical � C 8 H 8 S 4 Molecular

Calculating Empirical Formulas � Use the following poem to remember the steps: "Percent to mass, Mass to moles, Divide by small, Multiply ‘til whole"

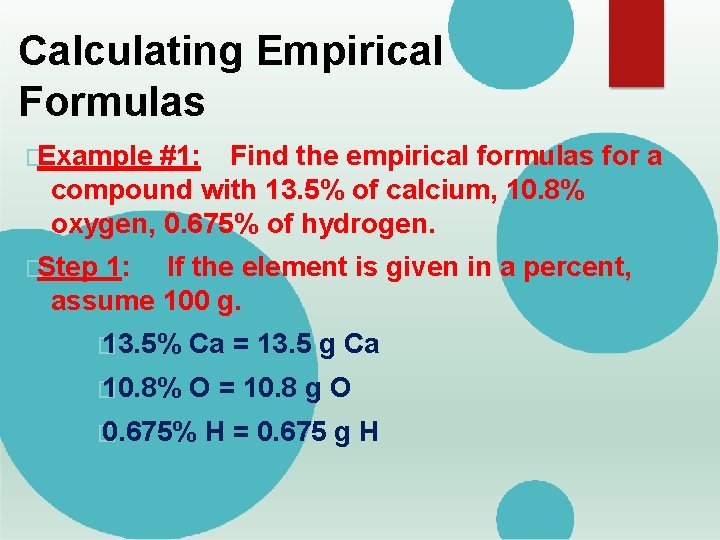
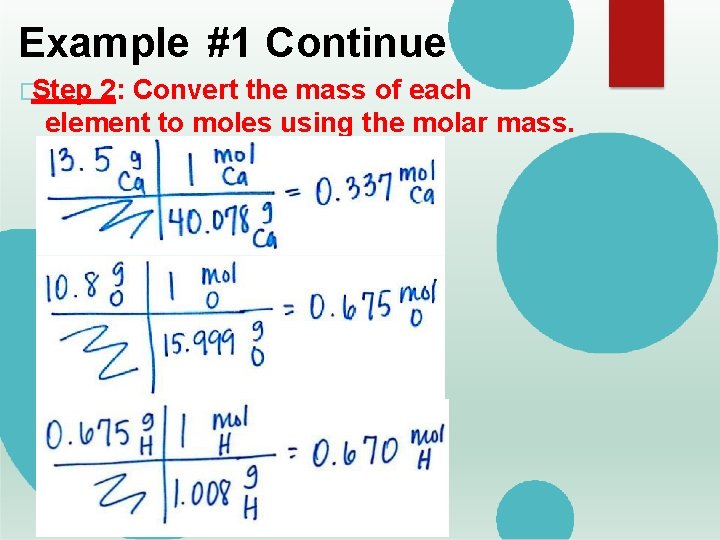
Calculating Empirical Formulas �Example #1: Find the empirical formulas for a compound with 13. 5% of calcium, 10. 8% oxygen, 0. 675% of hydrogen. �Step 1: If the element is given in a percent, assume 100 g. � 13. 5% Ca = 13. 5 g Ca � 10. 8% O = 10. 8 g O � 0. 675% H = 0. 675 g H

Example #1 Continue �Step 2: Convert the mass of each element to moles using the molar mass.

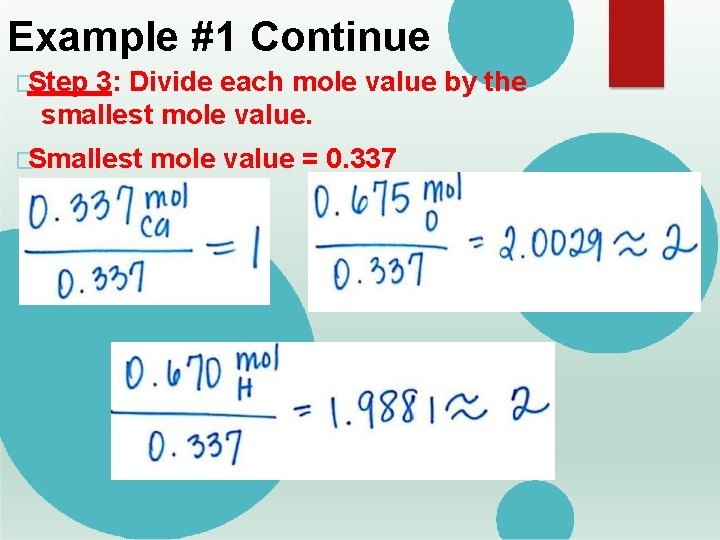
Example #1 Continue �Step 3: Divide each mole value by the smallest mole value. �Smallest mole value = 0. 337

Example #1 Continue �Step 4: If CLOSE, Round to the nearest whole number. �If answer is NOT close to a whole number, you will need to multiply by a factor of 2 or 3. � If answer ends with. 5, multiply by 2. � If answer ends with. 3 or. 6, multiply by 3. �You MUST multiply EACH element by the factor! �Example �Ca #1 Continue =1 �O = 2. 0029 = 2 �H = 1. 9881 = 2

Example #1 Continue � Step 5: Write Empirical Formula using answers as the subscripts. � Ca. O 2 H 2 = Ca(OH)2

Calculating Empirical Formulas � Example #2: Determine the empirical formula for a compound composed of 40. 00% C, 6. 72% H, and 53. 29% O.

Calculating Empirical Formulas � Example � 57. 4% #3 Carbon � 6. 16% Hydrogen � 9. 52% Nitrogen � 27. 18% Oxygen

Calculating Molecular Formulas � Step 1: Find the molar mass of the empirical formula. � Step 2: Divide the molecular mass by the empirical mass (big number by small number) � Step 3: Multiply answer by each subscript in the empirical formula to get molecular formula.

Calculating Molecular Formulas � Example #1: What is the molecular formula of a compound whose molar mass is 60. 0 g/mol and empirical formula is CH 4 N?

Calculating Molecular Formulas � Example #2: What is the molecular formula of CH 3 O if its molar mass is 62 g/mol?

Calculating Molecular Formulas � Example #3: Find the molecular formula for a compound with an empirical formula of C 2 H 8 N and a molecular mass of 46 grams per mole.

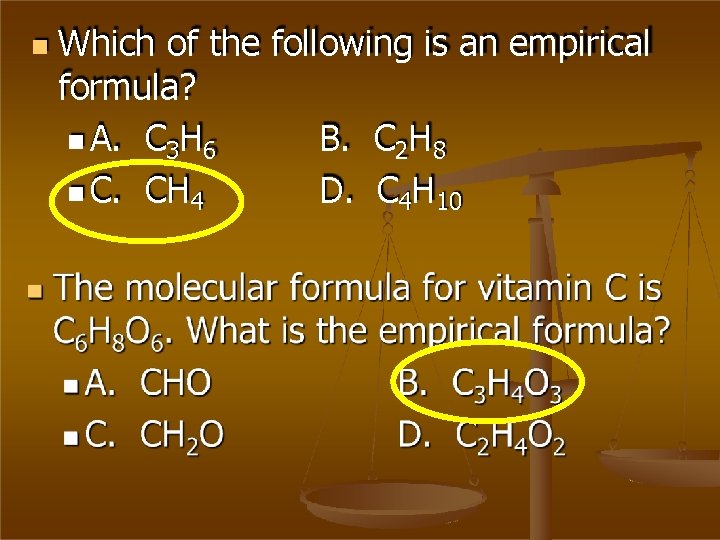
Which of the following is an empirical formula? A. C 3 H 6 B. C 2 H 8 C. CH 4 D. C 4 H 10