Empirical Formula The steps in determining empirical formula


- Slides: 12

Empirical Formula The steps in determining empirical formula

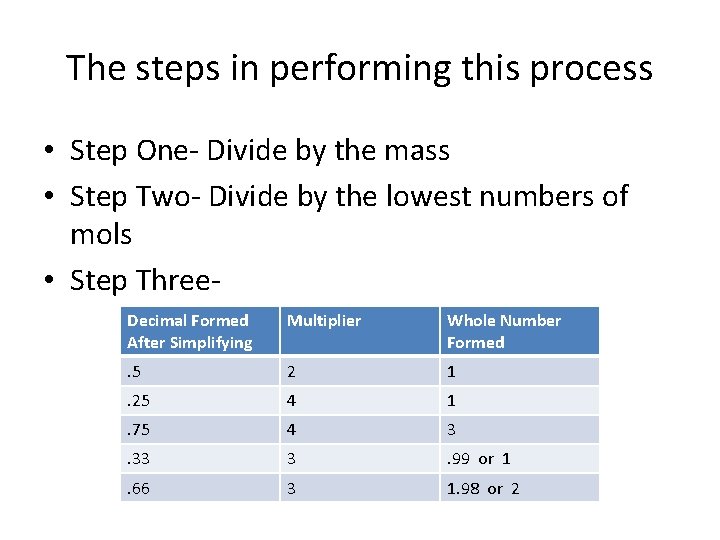
The steps in performing this process • Step One- Divide by the mass • Step Two- Divide by the lowest numbers of mols • Step Three. Decimal Formed After Simplifying Multiplier Whole Number Formed . 5 2 1 . 25 4 1 . 75 4 3 . 33 3 . 99 or 1 . 66 3 1. 98 or 2

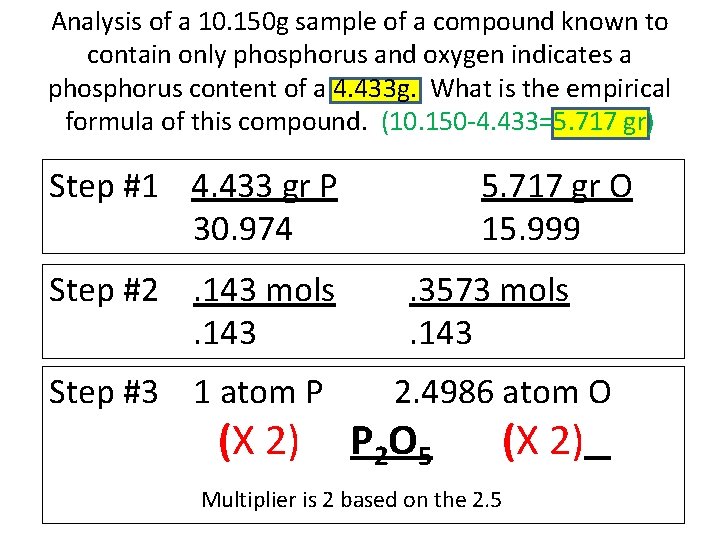
Analysis of a 10. 150 g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of a 4. 433 g. What is the empirical formula of this compound. (10. 150 -4. 433=5. 717 gr) Step #1 4. 433 gr P 30. 974 Step #2. 143 mols. 143 Step #3 1 atom P (X 2) 5. 717 gr O 15. 999. 3573 mols. 143 2. 4986 atom O P 2 O 5 (X 2) Multiplier is 2 based on the 2. 5

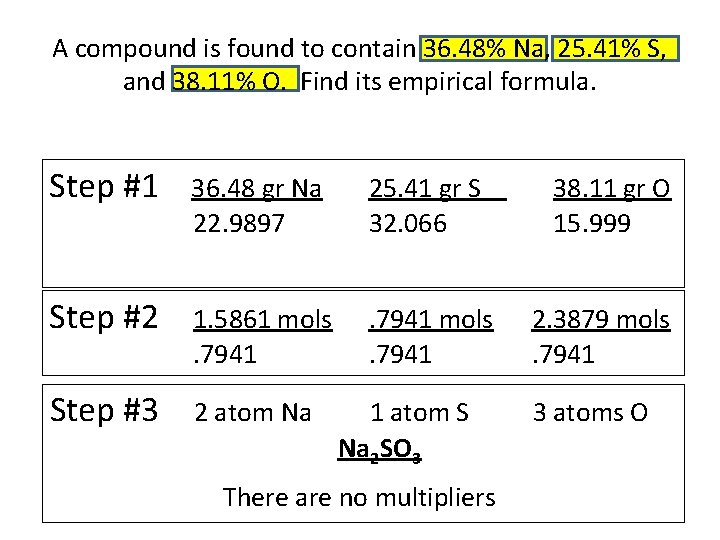
A compound is found to contain 36. 48% Na, 25. 41% S, and 38. 11% O. Find its empirical formula. Step #1 36. 48 gr Na 22. 9897 25. 41 gr S 32. 066 Step #2 1. 5861 mols. 7941 Step #3 2 atom Na 1 atom S Na 2 SO 3 There are no multipliers 38. 11 gr O 15. 999 2. 3879 mols. 7941 3 atoms O

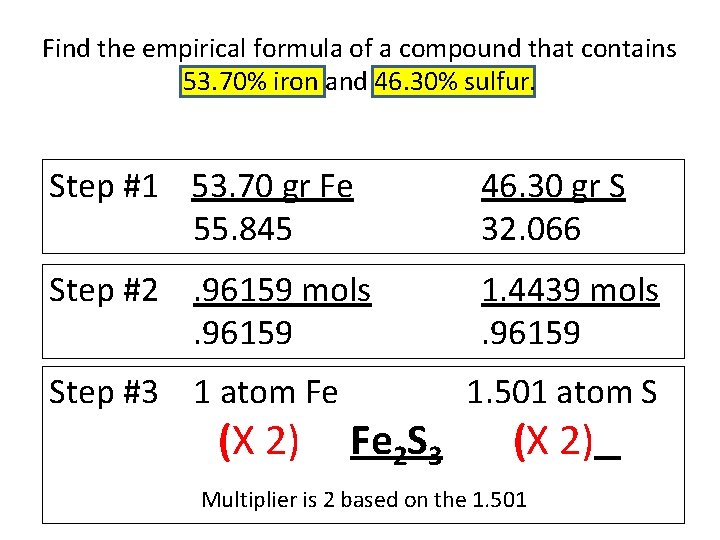
Find the empirical formula of a compound that contains 53. 70% iron and 46. 30% sulfur. Step #1 53. 70 gr Fe 55. 845 46. 30 gr S 32. 066 Step #2. 96159 mols. 96159 1. 4439 mols. 96159 Step #3 1 atom Fe (X 2) Fe 2 S 3 1. 501 atom S (X 2) Multiplier is 2 based on the 1. 501

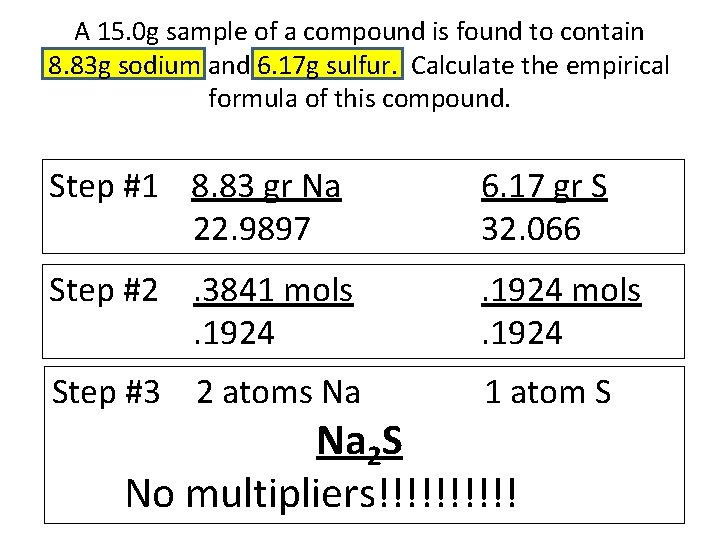
A 15. 0 g sample of a compound is found to contain 8. 83 g sodium and 6. 17 g sulfur. Calculate the empirical formula of this compound. Step #1 8. 83 gr Na 22. 9897 6. 17 gr S 32. 066 Step #2. 3841 mols. 1924 Step #3 2 atoms Na 1 atom S Na 2 S No multipliers!!!!!

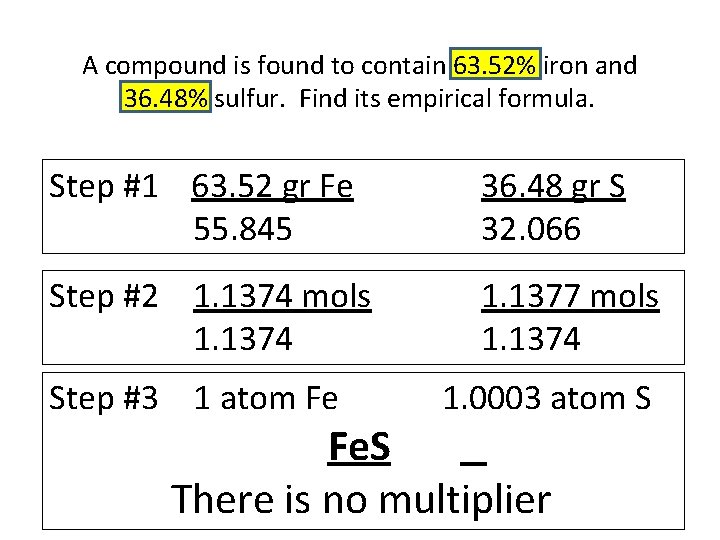
A compound is found to contain 63. 52% iron and 36. 48% sulfur. Find its empirical formula. Step #1 63. 52 gr Fe 55. 845 36. 48 gr S 32. 066 Step #2 1. 1374 mols 1. 1374 1. 1377 mols 1. 1374 Step #3 1 atom Fe 1. 0003 atom S Fe. S There is no multiplier

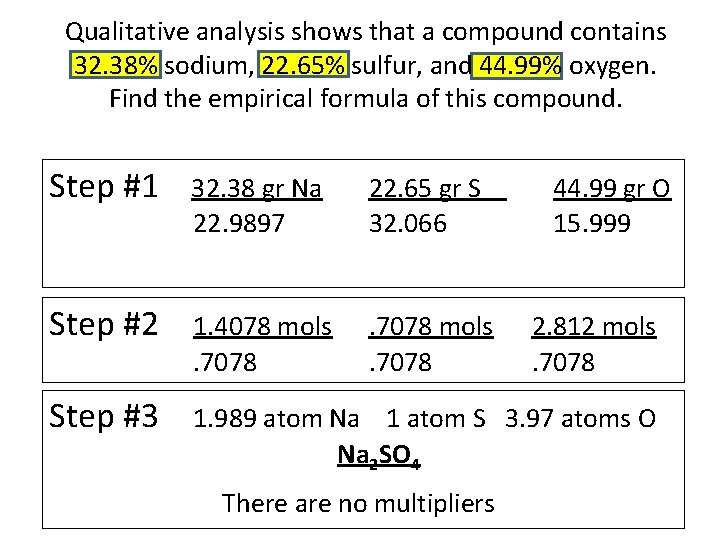
Qualitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. Find the empirical formula of this compound. Step #1 32. 38 gr Na 22. 9897 22. 65 gr S 32. 066 Step #2 1. 4078 mols. 7078 Step #3 1. 989 atom Na 1 atom S 3. 97 atoms O Na 2 SO 4 There are no multipliers 44. 99 gr O 15. 999 2. 812 mols. 7078

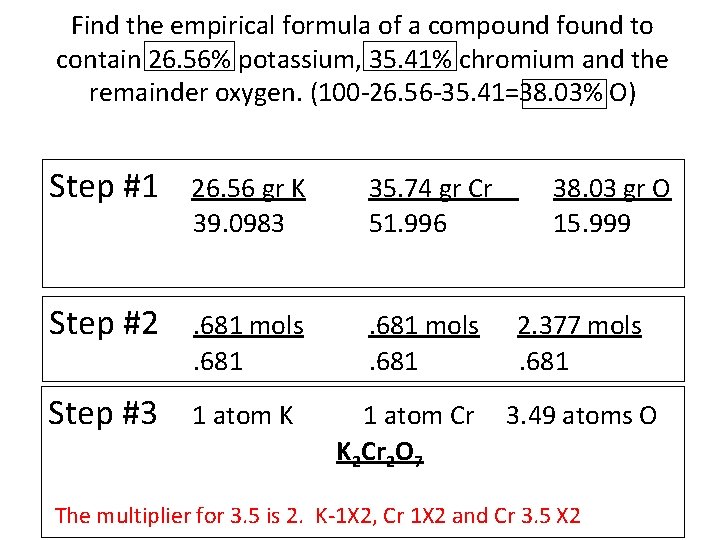
Find the empirical formula of a compound found to contain 26. 56% potassium, 35. 41% chromium and the remainder oxygen. (100 -26. 56 -35. 41=38. 03% O) Step #1 26. 56 gr K 39. 0983 35. 74 gr Cr 51. 996 Step #2 . 681 mols. 681 Step #3 1 atom K 1 atom Cr K 2 Cr 2 O 7 38. 03 gr O 15. 999 2. 377 mols. 681 3. 49 atoms O The multiplier for 3. 5 is 2. K-1 X 2, Cr 1 X 2 and Cr 3. 5 X 2

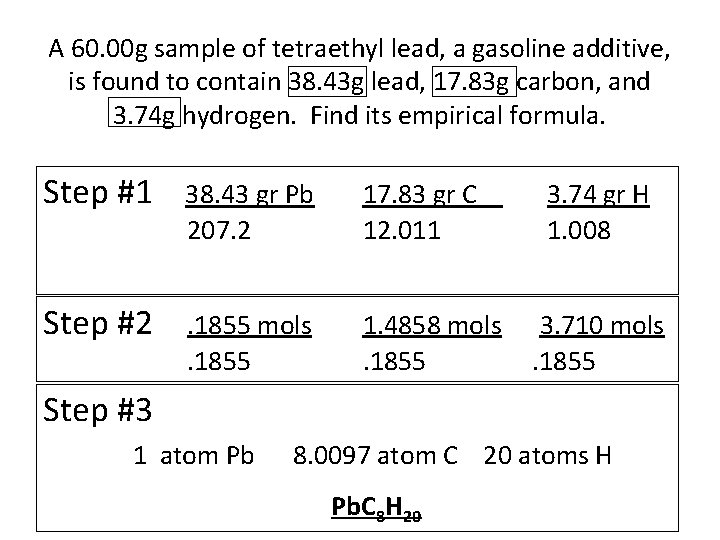
A 60. 00 g sample of tetraethyl lead, a gasoline additive, is found to contain 38. 43 g lead, 17. 83 g carbon, and 3. 74 g hydrogen. Find its empirical formula. Step #1 38. 43 gr Pb 207. 2 17. 83 gr C 12. 011 Step #2 . 1855 mols. 1855 1. 4858 mols. 1855 3. 74 gr H 1. 008 3. 710 mols. 1855 Step #3 1 atom Pb 8. 0097 atom C 20 atoms H Pb. C 8 H 20

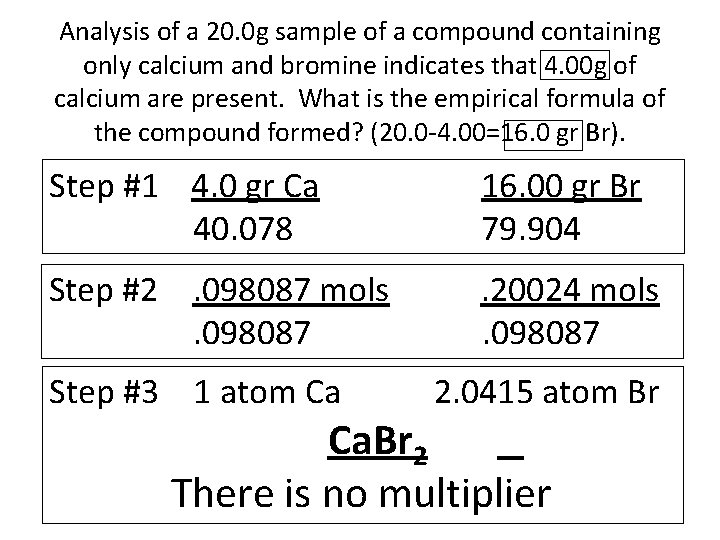
Analysis of a 20. 0 g sample of a compound containing only calcium and bromine indicates that 4. 00 g of calcium are present. What is the empirical formula of the compound formed? (20. 0 -4. 00=16. 0 gr Br). Step #1 4. 0 gr Ca 40. 078 16. 00 gr Br 79. 904 Step #2. 098087 mols. 098087 . 20024 mols. 098087 Step #3 1 atom Ca 2. 0415 atom Br Ca. Br 2 There is no multiplier

Interactive Website http: //www. softschools. com/quizzes/chemistry /stoichiometry_empirical_molecular_formulas/ quiz 1129. html