Empirical and Molecular Formulas Review n We learned






- Slides: 16

Empirical and Molecular Formulas

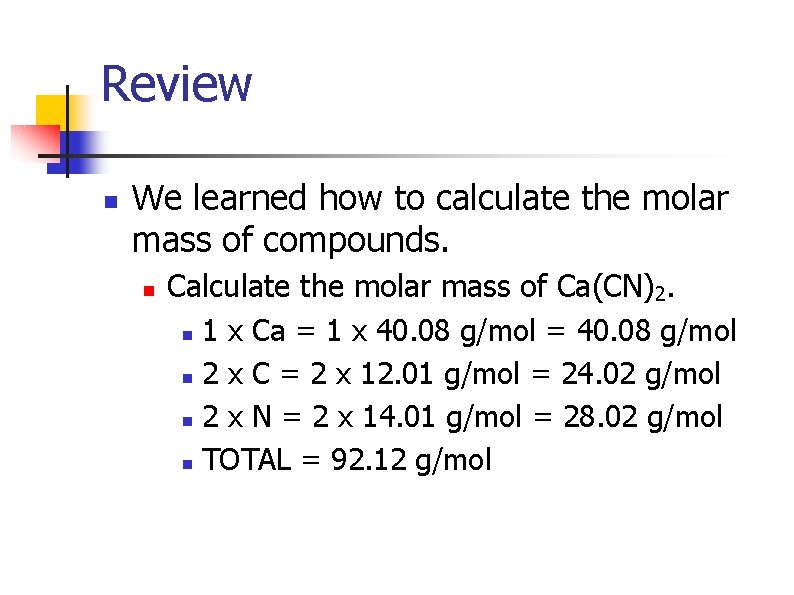
Review n We learned how to calculate the molar mass of compounds. n Calculate the molar mass of Ca(CN)2. n 1 x Ca = 1 x 40. 08 g/mol = 40. 08 g/mol n 2 x C = 2 x 12. 01 g/mol = 24. 02 g/mol n 2 x N = 2 x 14. 01 g/mol = 28. 02 g/mol n TOTAL = 92. 12 g/mol

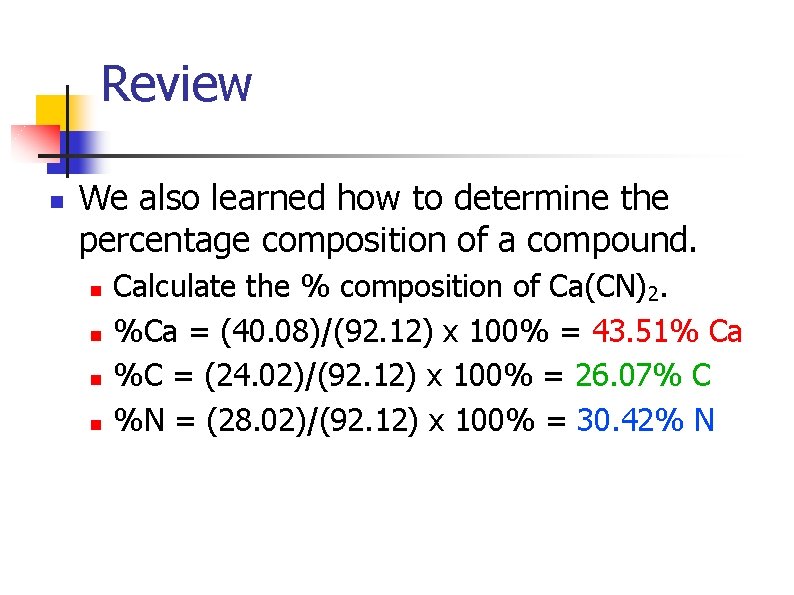
Review n We also learned how to determine the percentage composition of a compound. n n Calculate the % composition of Ca(CN)2. %Ca = (40. 08)/(92. 12) x 100% = 43. 51% Ca %C = (24. 02)/(92. 12) x 100% = 26. 07% C %N = (28. 02)/(92. 12) x 100% = 30. 42% N

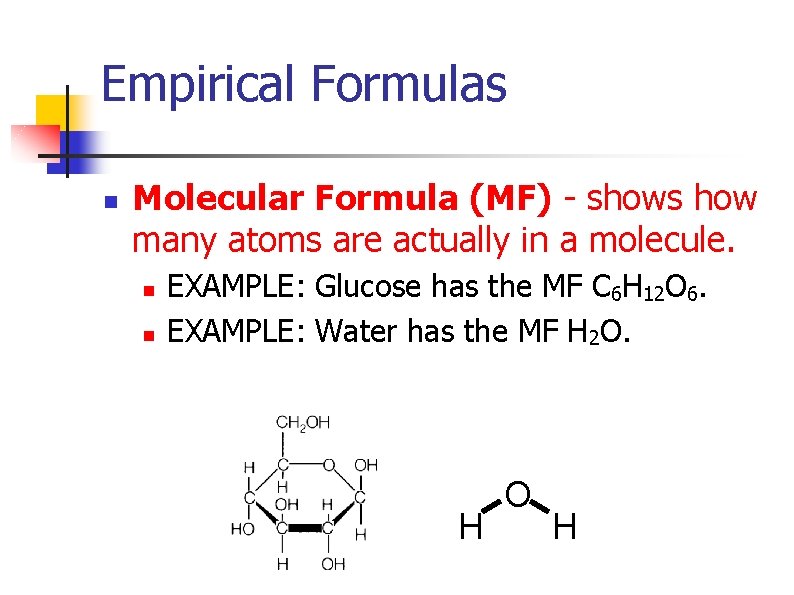
Empirical Formulas n Molecular Formula (MF) - shows how many atoms are actually in a molecule. n n EXAMPLE: Glucose has the MF C 6 H 12 O 6. EXAMPLE: Water has the MF H 2 O. H O H

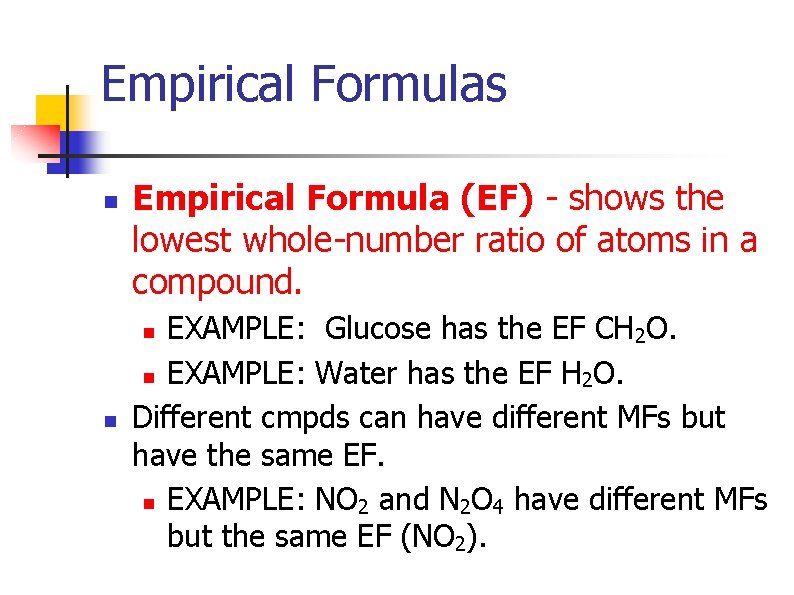
Empirical Formulas n Empirical Formula (EF) - shows the lowest whole-number ratio of atoms in a compound. EXAMPLE: Glucose has the EF CH 2 O. n EXAMPLE: Water has the EF H 2 O. Different cmpds can have different MFs but have the same EF. n EXAMPLE: NO 2 and N 2 O 4 have different MFs but the same EF (NO 2). n n

Empirical Formulas n You can discover the empirical formula of a compound if you know the % composition. n Will NOT tell you which molecular formula is correct!

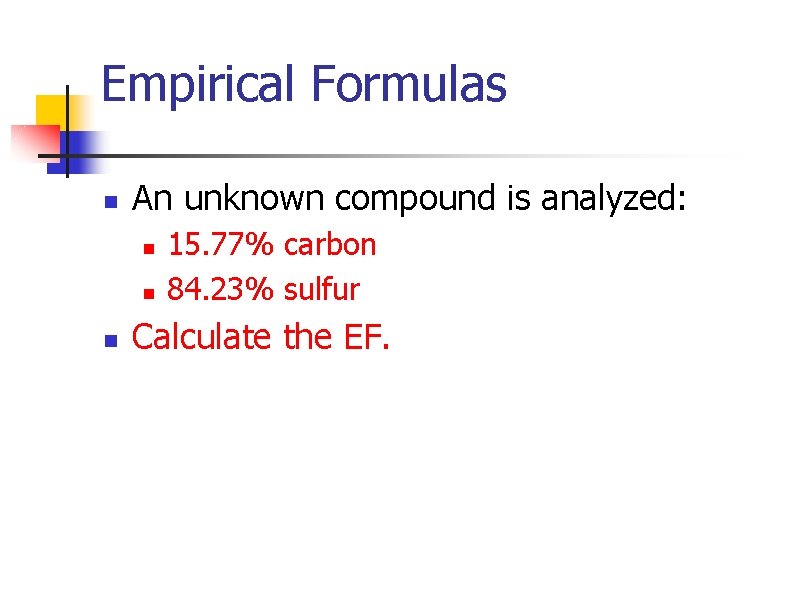
Empirical Formulas n An unknown compound is analyzed: n n n 15. 77% carbon 84. 23% sulfur Calculate the EF.

Empirical Formulas n First, assume you have exactly 100 grams of the sample. n n n Why 100 grams? Because percents become grams. In 100 grams of this compound you would have: n n 15. 77 g C 84. 23 g S

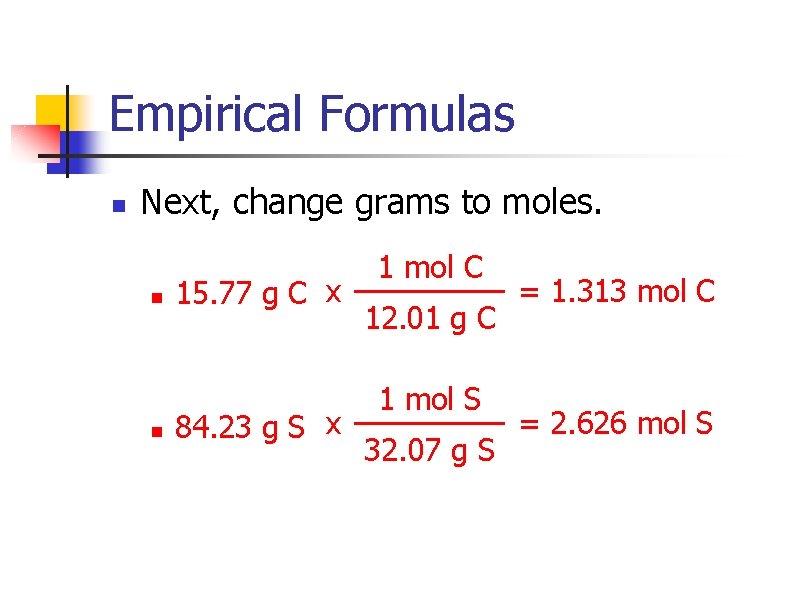
Empirical Formulas n Next, change grams to moles. n n 15. 77 g C x 84. 23 g S x 1 mol C 12. 01 g C 1 mol S 32. 07 g S = 1. 313 mol C = 2. 626 mol S

Empirical Formulas n The formula so far: n n Divide all subscripts by the lowest one. n n C 1. 313 S 2. 626 CS 2 This is the empirical formula of our mystery compound. n n We don’t know if it’s the correct molecular formula. Could be CS 2, C 2 S 4, C 3 S 6, C 4 S 8, etc. . .

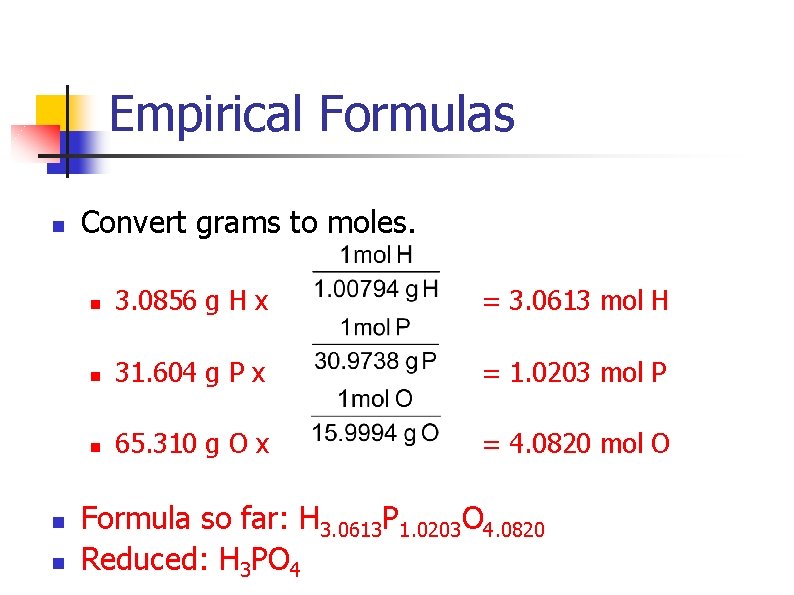
Empirical Formulas n A mystery compound has the following composition: n n 3. 0856% hydrogen 31. 604% phosphorus 65. 310% oxygen What is this compound’s empirical formula?

Empirical Formulas n n n Convert grams to moles. n 3. 0856 g H x = 3. 0613 mol H n 31. 604 g P x = 1. 0203 mol P n 65. 310 g O x = 4. 0820 mol O Formula so far: H 3. 0613 P 1. 0203 O 4. 0820 Reduced: H 3 PO 4

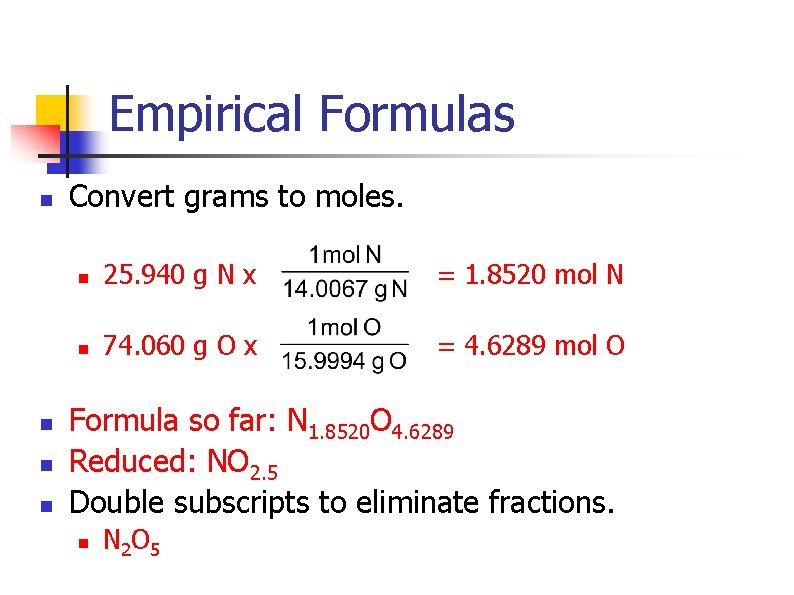
Empirical Formulas n A mystery compound has the following composition: n n n 25. 940% nitrogen 74. 060% oxygen What is this compound’s empirical formula?

Empirical Formulas n n Convert grams to moles. n 25. 940 g N x = 1. 8520 mol N n 74. 060 g O x = 4. 6289 mol O Formula so far: N 1. 8520 O 4. 6289 Reduced: NO 2. 5 Double subscripts to eliminate fractions. n N 2 O 5

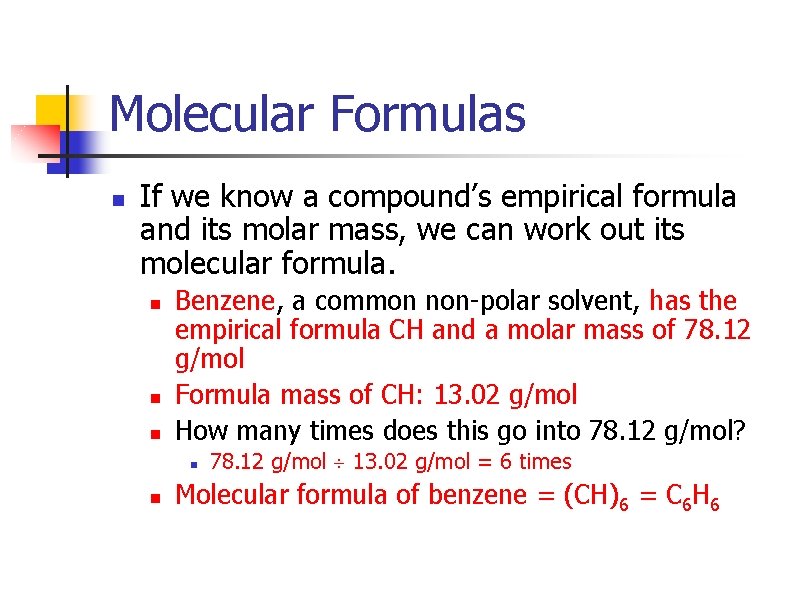
Molecular Formulas n If we know a compound’s empirical formula and its molar mass, we can work out its molecular formula. n n n Benzene, a common non-polar solvent, has the empirical formula CH and a molar mass of 78. 12 g/mol Formula mass of CH: 13. 02 g/mol How many times does this go into 78. 12 g/mol? n n 78. 12 g/mol 13. 02 g/mol = 6 times Molecular formula of benzene = (CH)6 = C 6 H 6

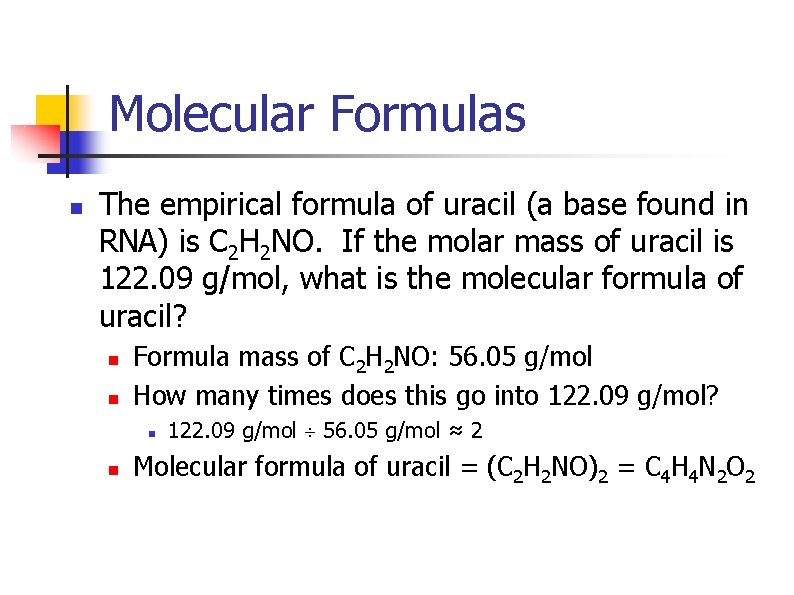
Molecular Formulas n The empirical formula of uracil (a base found in RNA) is C 2 H 2 NO. If the molar mass of uracil is 122. 09 g/mol, what is the molecular formula of uracil? n n Formula mass of C 2 H 2 NO: 56. 05 g/mol How many times does this go into 122. 09 g/mol? n n 122. 09 g/mol 56. 05 g/mol ≈ 2 Molecular formula of uracil = (C 2 H 2 NO)2 = C 4 H 4 N 2 O 2