The Mole Unit 7 Formula Mass Formula mass






- Slides: 11

The Mole Unit 7

Formula Mass • Formula mass - (also called gram formula mass, molecular mass, formula weight, gram formula weight, molecular weight, gram molecular weight, molar mass, and molar weight): the mass of one mole of a compound, atom or ion.

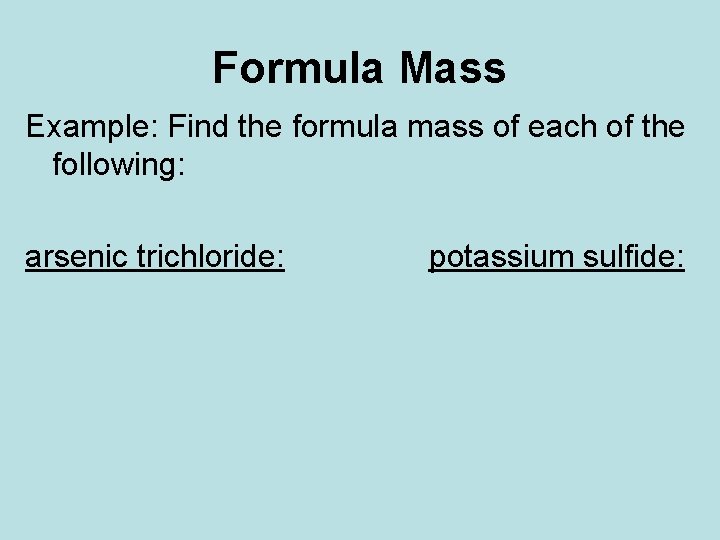
Formula Mass Example: Find the formula mass of each of the following: arsenic trichloride: potassium sulfide:

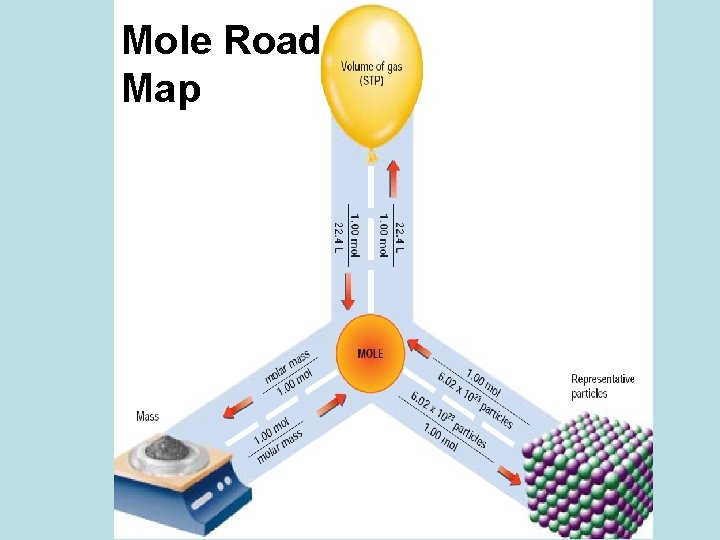
Measuring Matter There are three ways to measure matter: by counting representative particles (typically molecules or formula units), by mass (in grams), or by volume (in liters for gases). The method used is usually chosen by the ease of each method and the information needed. Once a measurement has been made, it is possible to convert between the units for the other methods.

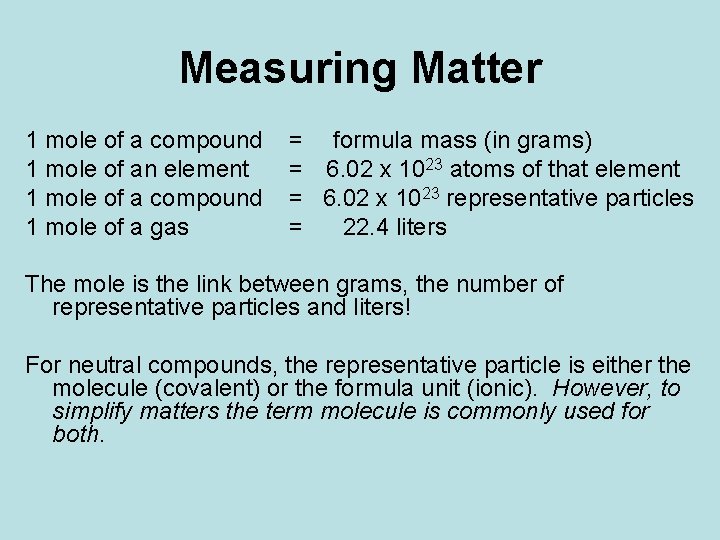
Measuring Matter 1 mole of a compound 1 mole of an element 1 mole of a compound 1 mole of a gas = formula mass (in grams) = 6. 02 x 1023 atoms of that element = 6. 02 x 1023 representative particles = 22. 4 liters The mole is the link between grams, the number of representative particles and liters! For neutral compounds, the representative particle is either the molecule (covalent) or the formula unit (ionic). However, to simplify matters the term molecule is commonly used for both.

Mole Road Map

Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. 1. How many moles are in 18. 0 grams of sugar (C 6 H 12 O 6)?

Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. 2. What is the weight in grams of 4. 50 moles of barium sulfide?

Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. 3. How many moles are in 3. 90 x 1028 molecules of methane (CH 4)?

Mole Practice: Multi Step Conversions: 7. How many molecules are in 198. 5 grams of sodium chloride?

Mole Practice: Multi Step Conversions: 9. How many grams are in 3. 21 x 1024 molecules of potassium hydroxide?