EMPIRICAL VS MOLECULAR FORMULA Define molecular formula and






- Slides: 14

EMPIRICAL VS. MOLECULAR FORMULA Define molecular formula and empirical formula. • Find the empirical formula given the molecular formula. • Find the molecular formula given the empirical formula & the molecular mass. •

Aim: How can we determine the molecular formula from the empirical formula? Key Words: ions, molecules, simplest whole number ratio, molecular mass, empirical formula, molecular formula, percent composition Essential Questions: What is the difference between empirical and molecular formulas? 2. In order to find the molecular formula of an unknown compound, what two units of information do you need? 3. How do you calculate the empirical formula from the molecular formula and vice versa? Use examples to explain your answer. 1.

Do Now What is the molecular formula for hydrogen peroxide? (Show the steps of the crisscross method) What is the formula for magnesium oxide? (Show the steps of the crisscross method)

How Do We Find the Atomic Ratios of Molecular Compounds?

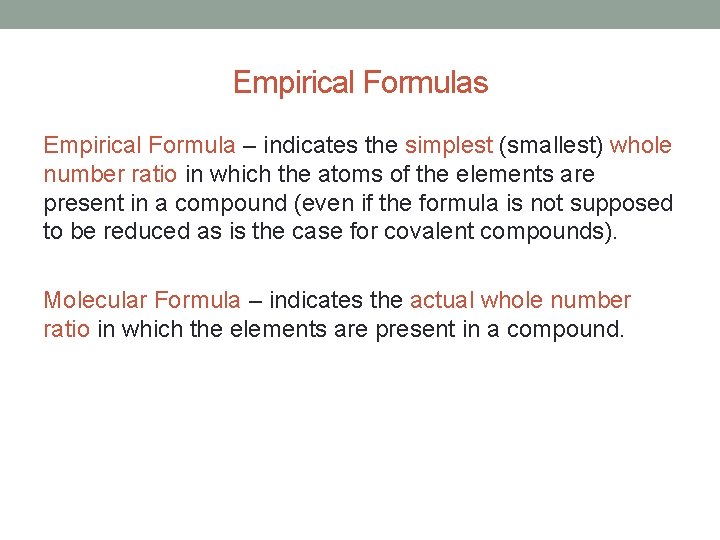
Empirical Formulas Empirical Formula – indicates the simplest (smallest) whole number ratio in which the atoms of the elements are present in a compound (even if the formula is not supposed to be reduced as is the case for covalent compounds). Molecular Formula – indicates the actual whole number ratio in which the elements are present in a compound.

All formulas for ionic compounds are empirical formulas!

Find the Empirical Formula for Glucose from you knowledge of its molecular formula

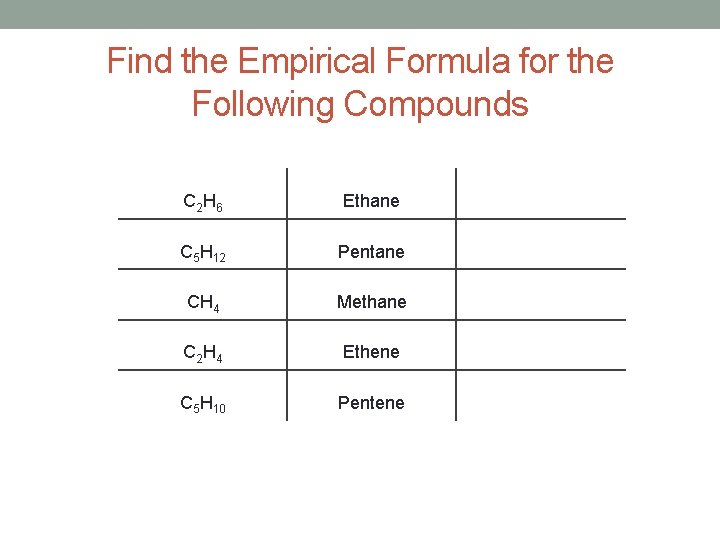
Find the Empirical Formula for the Following Compounds C 2 H 6 Ethane C 5 H 12 Pentane CH 4 Methane C 2 H 4 Ethene C 5 H 10 Pentene

Now that we know how to write convert a molecular formula empirical formula, can we do the reverse: empirical molecular? If so, what additional information do you think we would need to know?

4. A compound has an empirical formula of CH 3 and a molecular mass of 30 amu. What is its molecular formula?

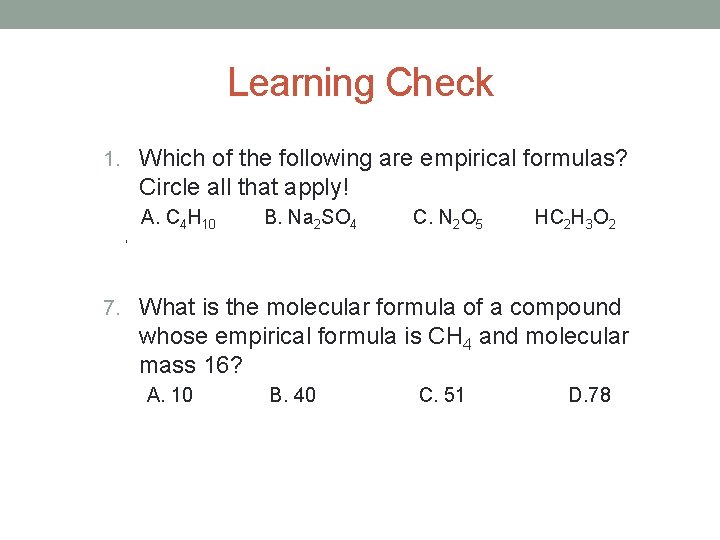
Learning Check 1. Which of the following are empirical formulas? Circle all that apply! A. C 4 H 10 B. Na 2 SO 4 C. N 2 O 5 HC 2 H 3 O 2 ‘ 7. What is the molecular formula of a compound whose empirical formula is CH 4 and molecular mass 16? A. 10 B. 40 C. 51 D. 78

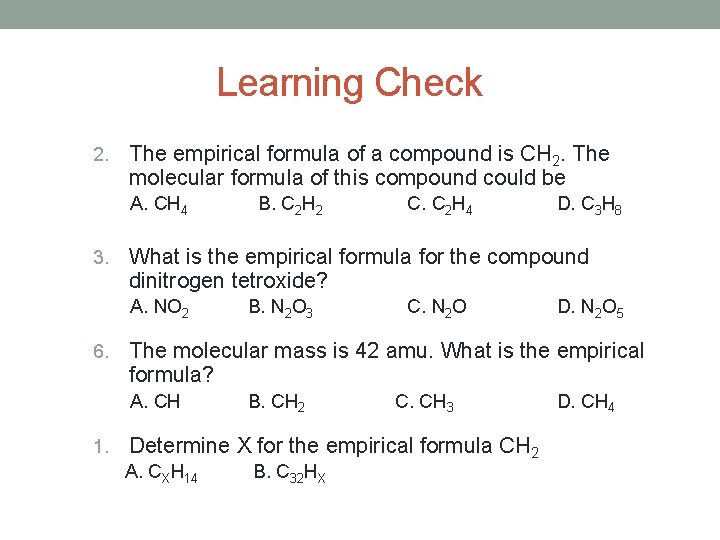
Learning Check 2. The empirical formula of a compound is CH 2. The molecular formula of this compound could be A. CH 4 3. D. C 3 H 8 B. N 2 O 3 C. N 2 O D. N 2 O 5 The molecular mass is 42 amu. What is the empirical formula? A. CH 1. C. C 2 H 4 What is the empirical formula for the compound dinitrogen tetroxide? A. NO 2 6. B. C 2 H 2 B. CH 2 C. CH 3 Determine X for the empirical formula CH 2 A. CXH 14 B. C 32 HX D. CH 4

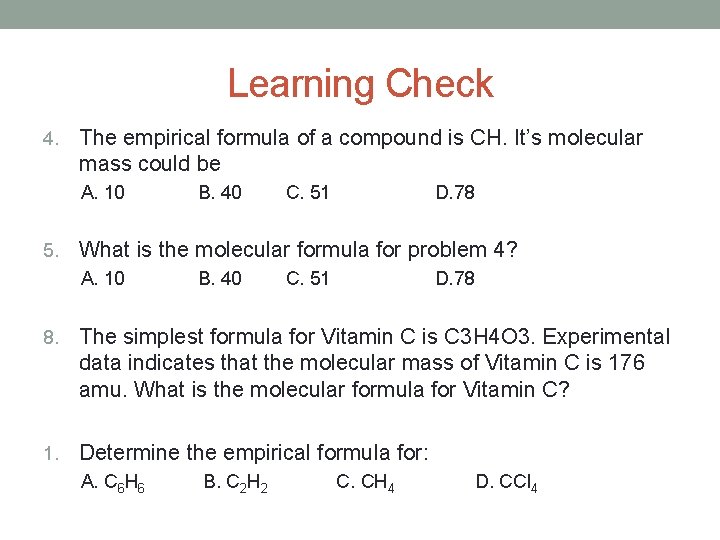
Learning Check 4. The empirical formula of a compound is CH. It’s molecular mass could be A. 10 5. B. 40 C. 51 D. 78 What is the molecular formula for problem 4? A. 10 B. 40 C. 51 D. 78 8. The simplest formula for Vitamin C is C 3 H 4 O 3. Experimental data indicates that the molecular mass of Vitamin C is 176 amu. What is the molecular formula for Vitamin C? 1. Determine the empirical formula for: A. C 6 H 6 B. C 2 H 2 C. CH 4 D. CCl 4

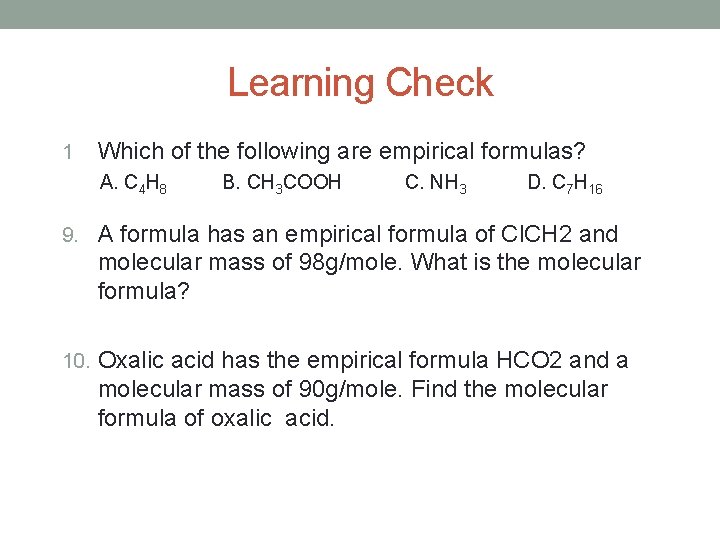
Learning Check 1 Which of the following are empirical formulas? A. C 4 H 8 B. CH 3 COOH C. NH 3 D. C 7 H 16 9. A formula has an empirical formula of Cl. CH 2 and molecular mass of 98 g/mole. What is the molecular formula? 10. Oxalic acid has the empirical formula HCO 2 and a molecular mass of 90 g/mole. Find the molecular formula of oxalic acid.