Empirical and Molecular Formulas Empirical vs Molecular Empirical










- Slides: 15

Empirical and Molecular Formulas

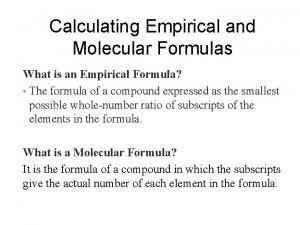
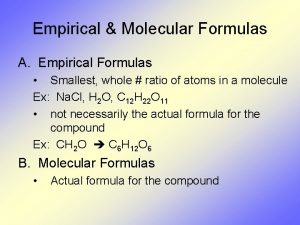
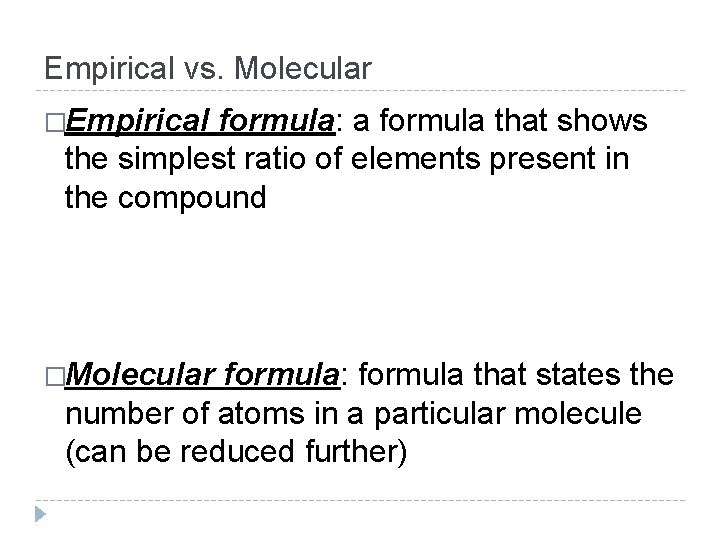
Empirical vs. Molecular �Empirical formula: a formula that shows the simplest ratio of elements present in the compound �Molecular formula: formula that states the number of atoms in a particular molecule (can be reduced further)

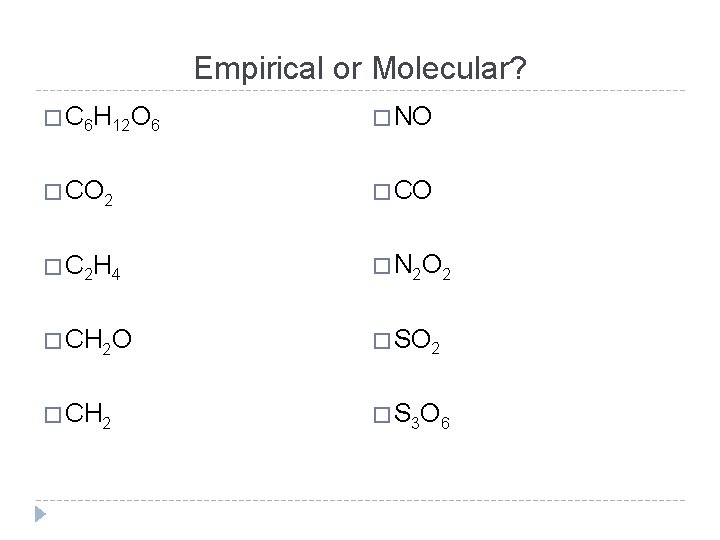
Empirical or Molecular? � C 6 H 12 O 6 � NO � CO 2 � CO � C 2 H 4 � N 2 O 2 � CH 2 O � SO 2 � CH 2 � S 3 O 6

Question 1 � Analysis of a sample of a covalent compound showed that it contained 85. 6% carbon and 14. 4% hydrogen. What is the empirical formula for this compound?

Question 2 � What is the empirical formula for a compound containing 68. 3% lead, 10. 6% sulfur and the remainder oxygen?

Question 3 �A compound contains sulfur, oxygen, and chlorine. Analysis shows that it contains 26. 95% sulfur, and 59. 61% chlorine. What is the simplest formula for this compound?

Question 4 �A compound contains carbon, oxygen, and hydrogen. Analysis of a sample showed that it contained by mass 68. 83% carbon and 4. 96% hydrogen. What is the simplest formula for this compound?

A compound with an empirical formula of CO 2 and a molar mass of 132 grams per mole. �What is the molecular formula of this compound?

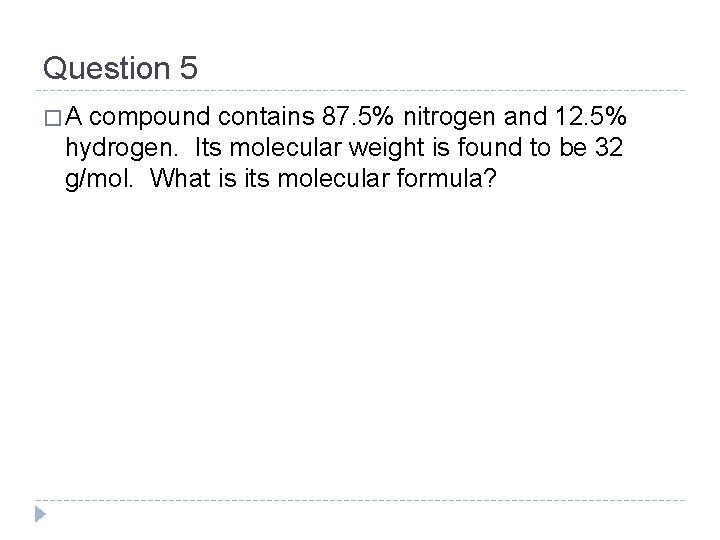
Question 5 �A compound contains 87. 5% nitrogen and 12. 5% hydrogen. Its molecular weight is found to be 32 g/mol. What is its molecular formula?

Question 6 �A compound contains only carbon, hydrogen, and oxygen. Analysis of a sample showed that it contained 54. 52% C and 9. 15% H. Its molecular weight was determined to be approximately 88 g/mol. What is its molecular formula?

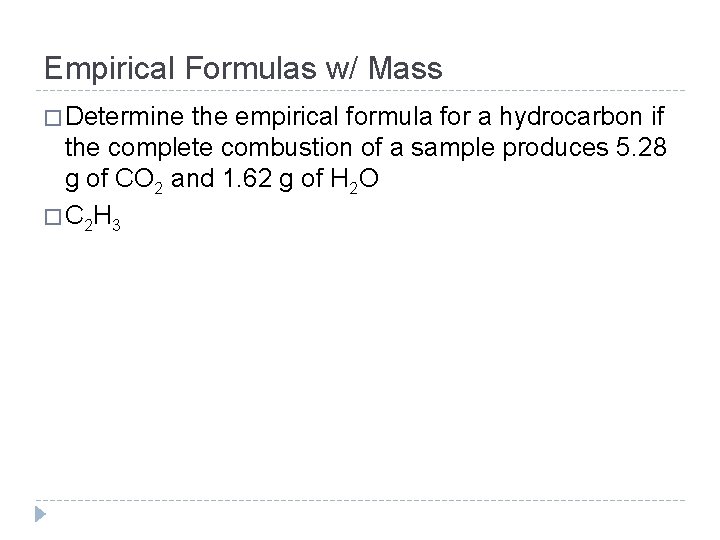
Empirical Formulas w/ Mass � Determine the empirical formula for a hydrocarbon if the complete combustion of a sample produces 5. 28 g of CO 2 and 1. 62 g of H 2 O � C 2 H 3

Practice 1 � Determine the simplest formula for a hydrocarbon if the complete combustion of a sample produces 3. 96 g of CO 2 and 2. 16 g of H 2 O. � C 3 H 8

Practice 2 �A compound is known to contain only carbon, hydrogen, and oxygen. If the complete combustion of a 0. 150 g sample of this compound produces 0. 225 g of CO 2 and 0. 0614 g of H 2 O, what is the empirical formula of this compound? � C 3 H 4 O 3

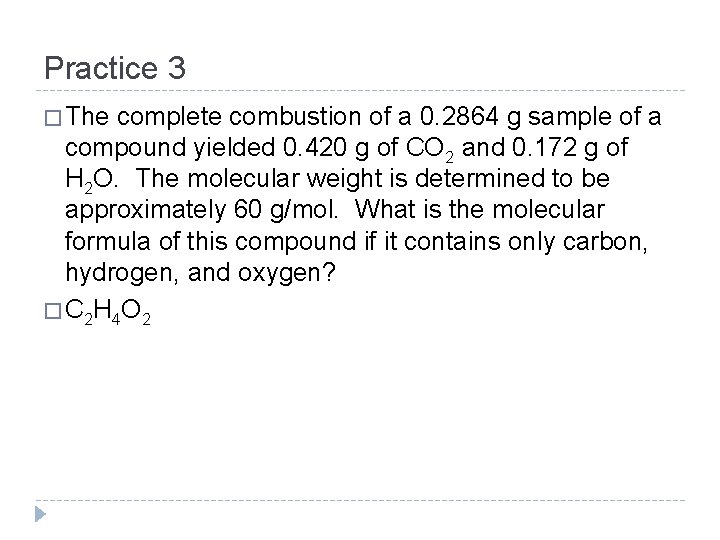
Practice 3 � The complete combustion of a 0. 2864 g sample of a compound yielded 0. 420 g of CO 2 and 0. 172 g of H 2 O. The molecular weight is determined to be approximately 60 g/mol. What is the molecular formula of this compound if it contains only carbon, hydrogen, and oxygen? � C 2 H 4 O 2

Practice 4 � 41 g of CO 2, 25 g of H 2 O, 29. 90 g of Total Compound containing C, H, and O � CH 3 O
 Empirical and molecular formula worksheet
Empirical and molecular formula worksheet Find the empirical/simplest formula fe
Find the empirical/simplest formula fe Naming compounds and writing formulas
Naming compounds and writing formulas Water percentage composition
Water percentage composition Empirical formula vs
Empirical formula vs What is meant by empirical formula
What is meant by empirical formula Empirical and molecular formula
Empirical and molecular formula Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chemical formulas quiz
Chemical formulas quiz Empirical formula of ethane
Empirical formula of ethane Mass practice problems
Mass practice problems Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc What are the empirical formulas
What are the empirical formulas Empirical formulas
Empirical formulas Lesson 28: sniffing around molecular formulas answer key
Lesson 28: sniffing around molecular formulas answer key