Empirical formula 1 Hydroquinone used as a photographic

- Slides: 17

Empirical formula: 1

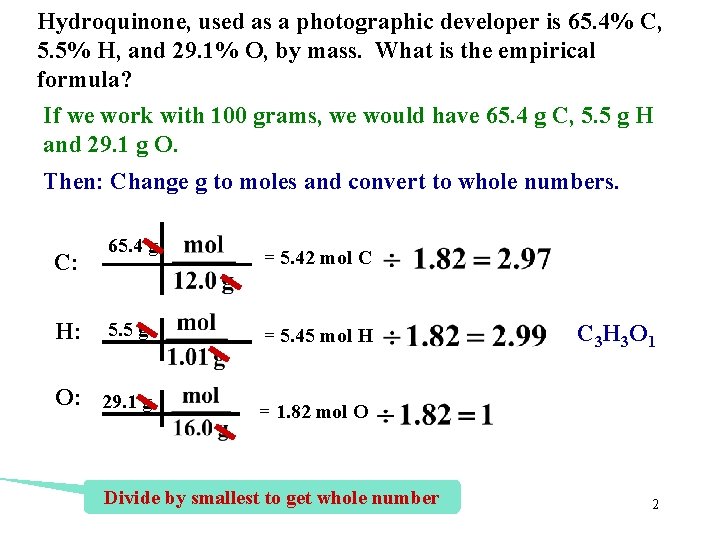
Hydroquinone, used as a photographic developer is 65. 4% C, 5. 5% H, and 29. 1% O, by mass. What is the empirical formula? If we work with 100 grams, we would have 65. 4 g C, 5. 5 g H and 29. 1 g O. Then: Change g to moles and convert to whole numbers. C: H: 65. 4 g = 5. 42 mol C 5. 5 g = 5. 45 mol H O: 29. 1 g = 1. 82 mol O Divide by smallest to get whole number C 3 H 3 O 1 2

A compound was sent out for analysis and was found to contain 6. 7% H, 39. 9% C, and 53. 4% O. It was also found to have a molecular mass of 60. 0 g/mol. Determine both the empircal and the molecular formulas. If we work with 100 grams, we could call the %’s grams! 3

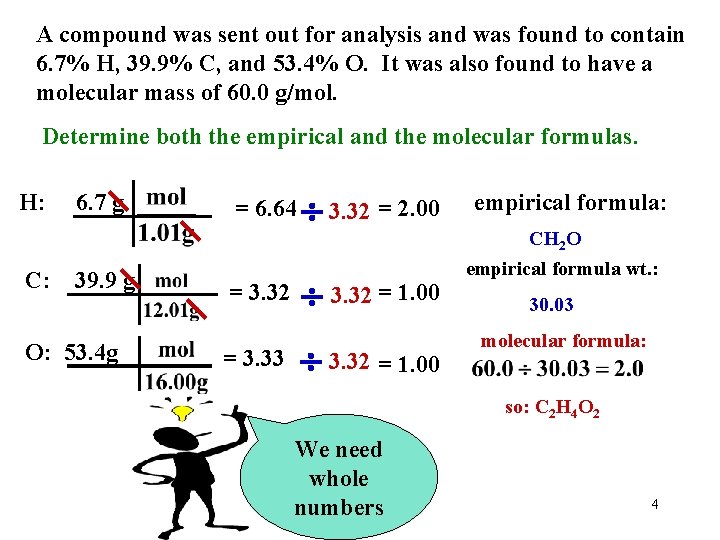
A compound was sent out for analysis and was found to contain 6. 7% H, 39. 9% C, and 53. 4% O. It was also found to have a molecular mass of 60. 0 g/mol. Determine both the empirical and the molecular formulas. H: C: 6. 7 g 39. 9 g O: 53. 4 g = 6. 64 = 3. 32 = 3. 33 3. 32 = 2. 00 3. 32 = 1. 00 empirical formula: CH 2 O empirical formula wt. : 30. 03 molecular formula: so: C 2 H 4 O 2 We need whole numbers 4

Stoichiometry: “working with ratios” 5

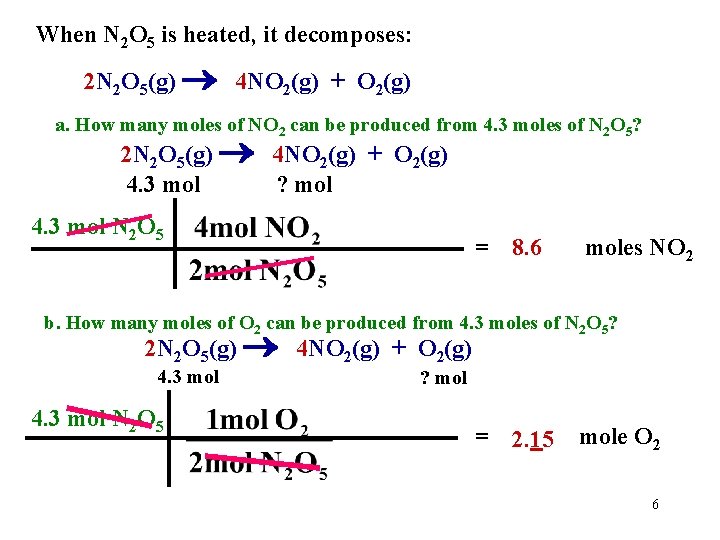
When N 2 O 5 is heated, it decomposes: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) a. How many moles of NO 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 4. 3 mol ? mol 4. 3 mol N 2 O 5 = 8. 6 moles NO 2 b. How many moles of O 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 4. 3 mol N 2 O 5 ? mol = 2. 15 mole O 2 6

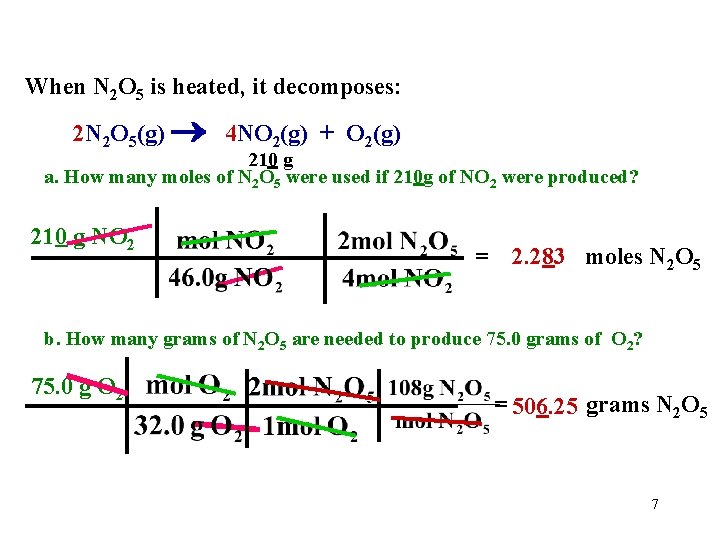
When N 2 O 5 is heated, it decomposes: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 210 g a. How many moles of N 2 O 5 were used if 210 g of NO 2 were produced? 210 g NO 2 = 2. 283 moles N 2 O 5 b. How many grams of N 2 O 5 are needed to produce 75. 0 grams of O 2? 75. 0 g O 2 = 506. 25 grams N 2 O 5 7

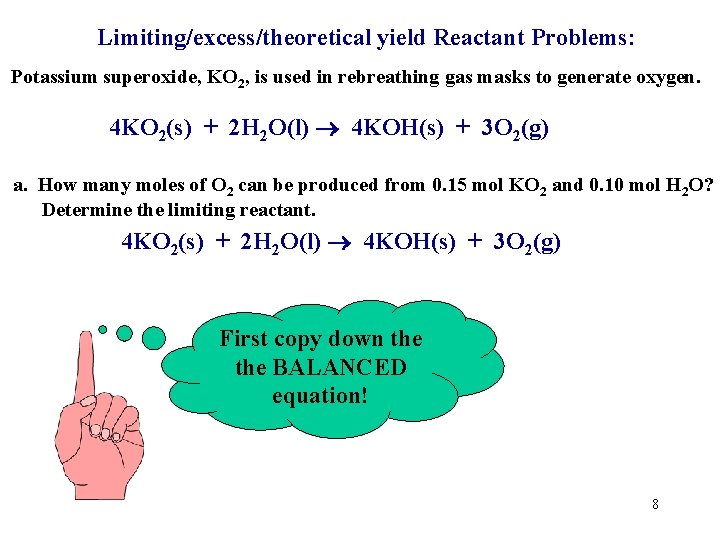
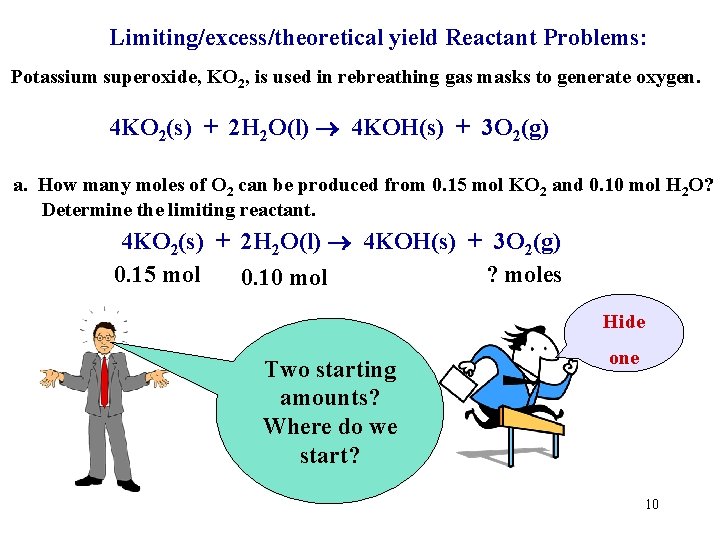
Limiting/excess/theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) First copy down the BALANCED equation! 8

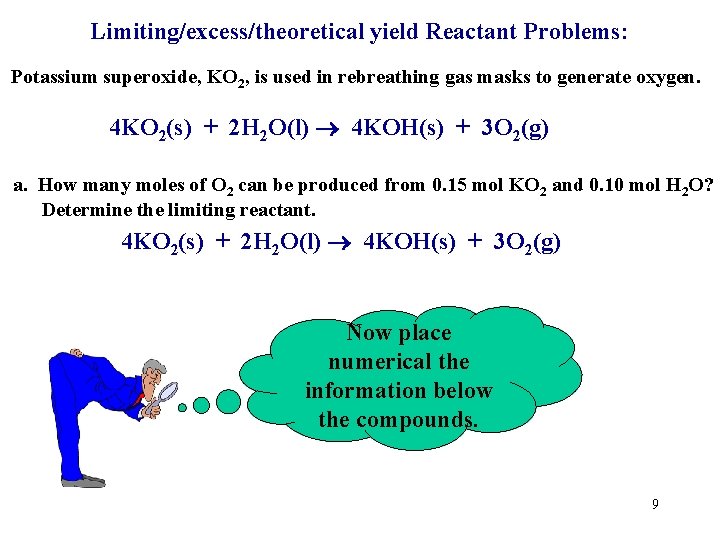
Limiting/excess/theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) Now place numerical the information below the compounds. 9

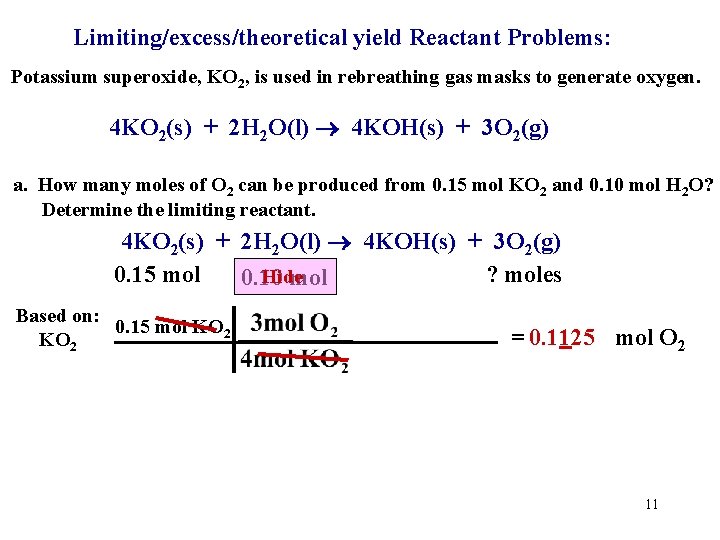
Limiting/excess/theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles 0. 10 mol Hide Two starting amounts? Where do we start? one 10

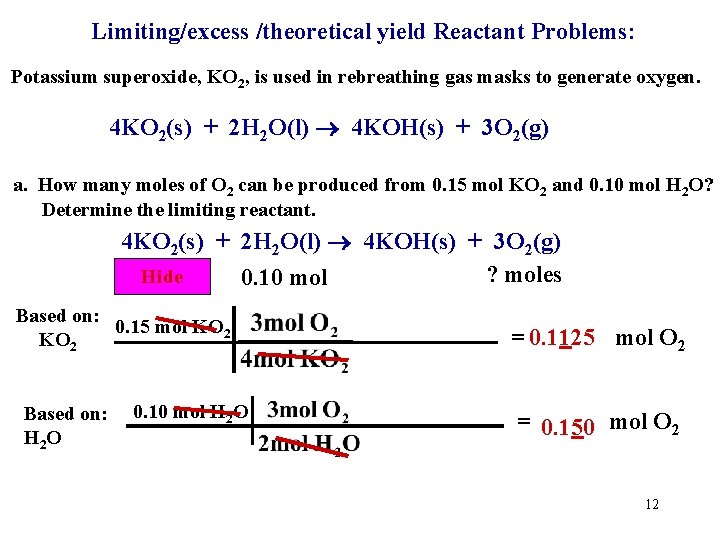
Limiting/excess/theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles Hide 0. 10 mol Based on: 0. 15 mol KO 2 = 0. 1125 mol O 2 11

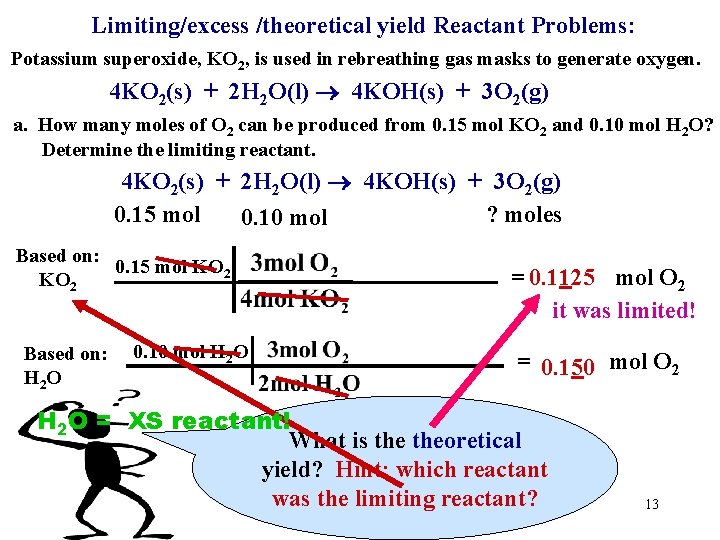
Limiting/excess /theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles Hide 0. 10 mol Based on: 0. 15 mol KO 2 Based on: H 2 O 0. 10 mol H 2 O = 0. 1125 mol O 2 = 0. 150 mol O 2 12

Limiting/excess /theoretical yield Reactant Problems: Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles 0. 10 mol Based on: 0. 15 mol KO 2 Based on: H 2 O 0. 10 mol H 2 O = 0. 1125 mol O 2 it was limited! = 0. 150 mol O 2 H 2 O = XS reactant! What is theoretical yield? Hint: which reactant was the limiting reactant? 13

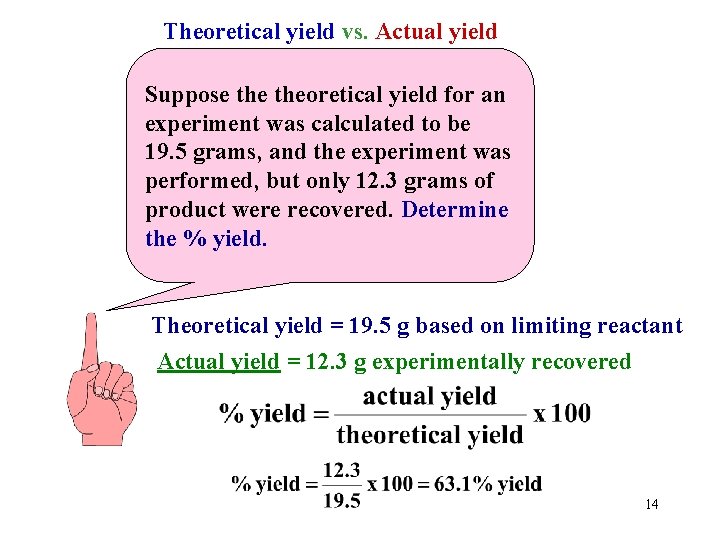
Theoretical yield vs. Actual yield Suppose theoretical yield for an experiment was calculated to be 19. 5 grams, and the experiment was performed, but only 12. 3 grams of product were recovered. Determine the % yield. Theoretical yield = 19. 5 g based on limiting reactant Actual yield = 12. 3 g experimentally recovered 14

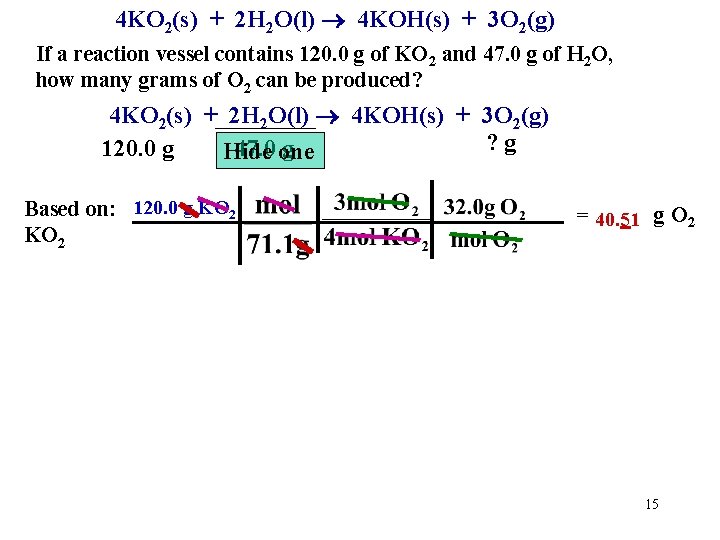
4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 one g Hide Based on: 120. 0 g KO 2 = 40. 51 g O 2 15

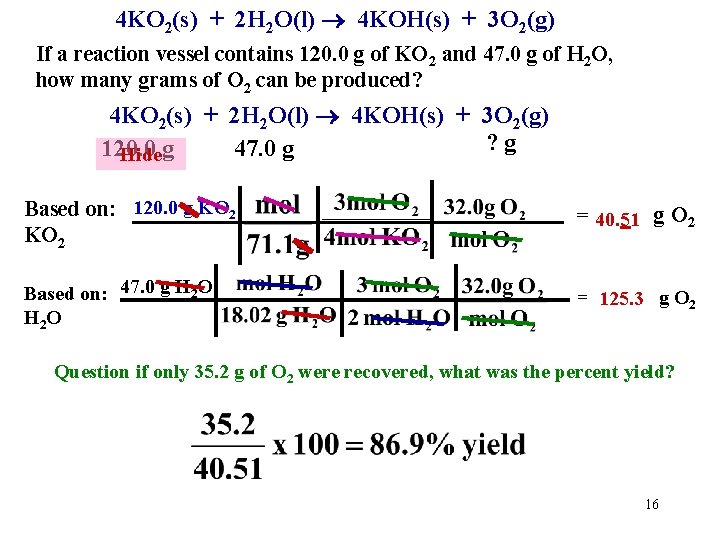
4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 47. 0 g Hideg Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O H 2 O = 125. 3 g O 2 Question if only 35. 2 g of O 2 were recovered, what was the percent yield? 16

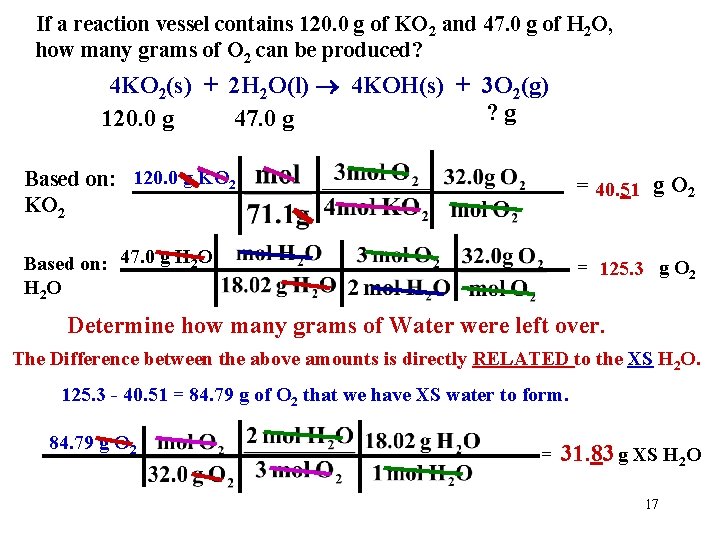
If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 g Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O H 2 O = 125. 3 g O 2 Determine how many grams of Water were left over. The Difference between the above amounts is directly RELATED to the XS H 2 O. 125. 3 - 40. 51 = 84. 79 g of O 2 that we have XS water to form. 84. 79 g O 2 = 31. 83 g XS H 2 O 17