Molecular Formulas Empirical Formula Empirical vs Molecular Formulas





- Slides: 12

Molecular Formulas

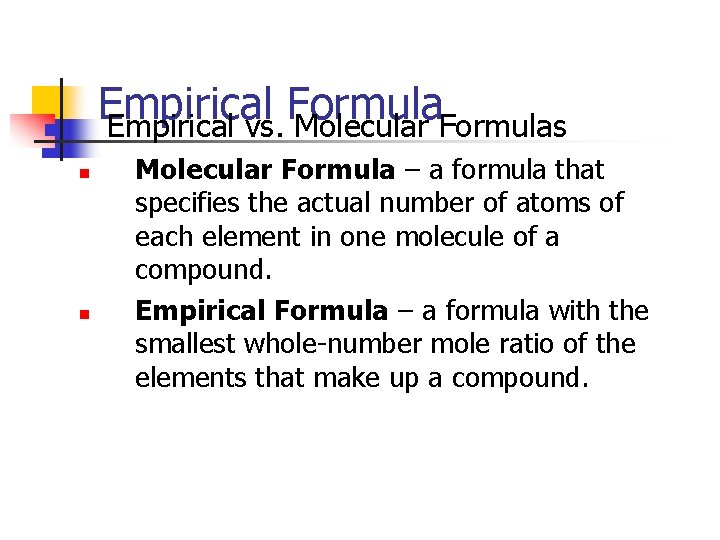
Empirical Formula Empirical vs. Molecular Formulas n n n Molecular Formula – a formula that specifies the actual number of atoms of each element in one molecule of a compound. Empirical Formula – a formula with the smallest whole-number mole ratio of the elements that make up a compound.

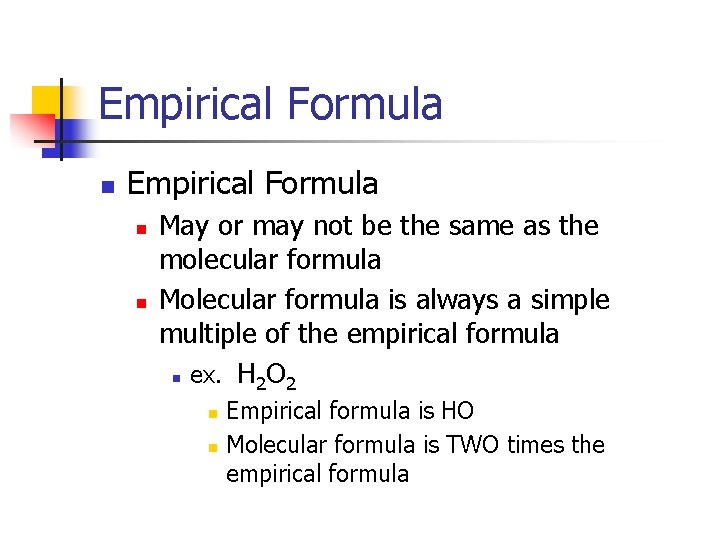
Empirical Formula n n May or may not be the same as the molecular formula Molecular formula is always a simple multiple of the empirical formula n ex. H O 2 2 n n Empirical formula is HO Molecular formula is TWO times the empirical formula

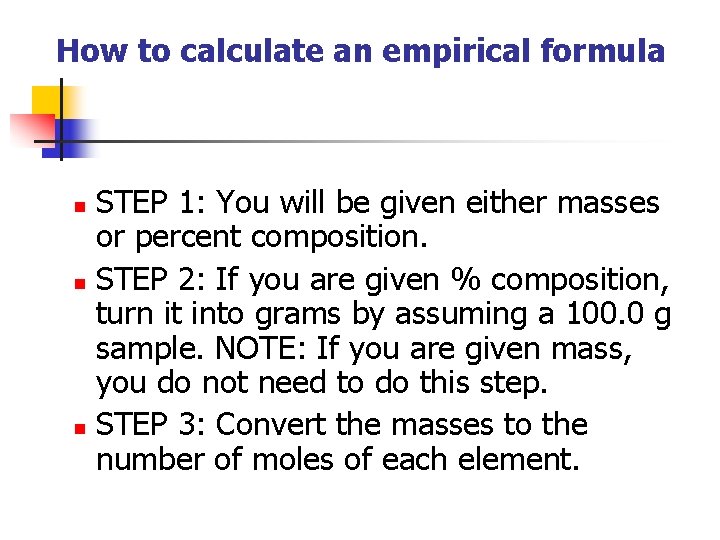
How to calculate an empirical formula STEP 1: You will be given either masses or percent composition. n STEP 2: If you are given % composition, turn it into grams by assuming a 100. 0 g sample. NOTE: If you are given mass, you do not need to do this step. n STEP 3: Convert the masses to the number of moles of each element. n

n n STEP 4: Figure out the proportion of moles of each element in the compound by dividing each by the smallest number of moles. STEP 5: If step 4 resulted in whole numbers, you are done! However, if there were decimals, you will need to multiply by small, whole numbers until you have whole numbers.

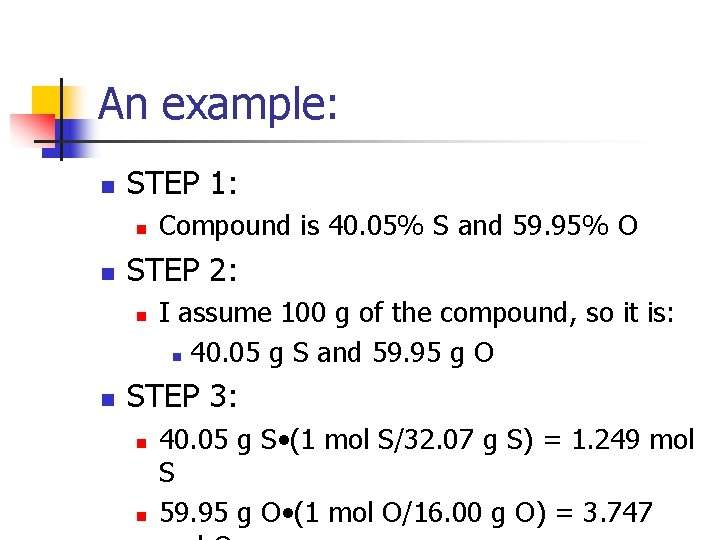
An example: n STEP 1: n n STEP 2: n n Compound is 40. 05% S and 59. 95% O I assume 100 g of the compound, so it is: n 40. 05 g S and 59. 95 g O STEP 3: n n 40. 05 g S • (1 mol S/32. 07 g S) = 1. 249 mol S 59. 95 g O • (1 mol O/16. 00 g O) = 3. 747

Continued… n STEP 4: n n STEP 5: n n 1. 249 mol S : 3. 747 mol O Divide each by 1. 249 (smallest number in ratio) 1 mol S : 3 mol O SO 3 You are done! The compound is sulfur trioxide.

A way to remember those steps: n n n A Poem by Joel Thompson: Percent to mass Mass to mole Divide by small Multiply ‘til whole

Molecular Formula n n Molecular Formula – this tells us how many atoms of each type there really are in the compound. Can two substances have the same empirical formula but be different? n n YES! Benzene vs. acetylene: C 6 H 6 vs. C 2 H 2 What is their empirical formula? How is this different from ionic compounds?

Calculating Molecular Formula n STEP 1: n n STEP 2: n n Calculate the empirical mass (mass of the empirical formula). STEP 3: n n You will be given the molar mass of the compound and the empirical formula. Divide the given molar mass by the empirical mass. You should get a small whole number. STEP 4: n Multiply the subscripts of the empirical formula with the number obtained.

Molecular Formula Example n STEP 1: n n STEP 2: n n The empirical mass is 12. 01 g + 2*1. 01 g + 16. 00 g = 30. 03 g STEP 3: n n The empirical formula is CH 2 O and the molar mass is 180. 18 g/ 30. 03 g = 6 STEP 4: n CH 2 O becomes C 6 H 12 O 6

n n Closure: Acetylene is a gas that is used as a fuel for welding. Benzene is a liquid solvent. n n How are they similar? How are they different? Why is one a gas at room temperature and one a liquid?