Working with Empirical Formulas and Molecular Formulas loosely












- Slides: 20

Working with Empirical Formulas, and Molecular Formulas loosely based on Chap 4 Sec 2 & 3 of Jespersen 6 th ed Dr. C. Yau Spring 2014 1

Chemical Formula: Particulate vs. macroscopic levels When we see the formula H 2 S… at the particulate level we should be thinking of one molecule of H 2 S with 2 H atoms and 1 S atom, at the macroscopic level we should be thinking of one mol H 2 S with 2 mol H and 1 mol S. 2

Example 4. 5 p. 114 Calcium phosphate is widely found in nature in the form of natural minerals. It is also found in bones and some kidney stones. In many instances if we determine one element in a compound we can find out how much of the compound is present. In one case a sample is found to contain 0. 864 mole of phosphorus. How many moles of calcium phosphate will that represent. Hint: Do we need Avogadro's number? Do Practice Exercises 4. 5 & 4. 6 on p. 114 3

Example 4. 6 p. 115 Chlorophyll, the green pigment in leaves, has the formula C 55 H 72 Mg. N 4 O 5. If 0. 0011 g of Mg is available to a plant cell for chlorophyll synthesis, how many grams of carbon will be required to completely use up the magnesium? Do we need Avogadro’s number? Do Practice Exercises 7, 8, 9 on p. 116 4

Percent Composition LEARN THIS! "Percent composition" refers to the % of each element in a compound by mass. For example, the % composition of CO 2 is 27. 29 % C and 72. 71 % O. How do we come up with these numbers? (Unless specified otherwise, please always give answer to 4 sig. fig. ) 5

Example 4. 7 p. 116 A sample of a liquid with a mass of 8. 657 g was decomposed into its elements and gave 5. 217 g of carbon, 0. 9620 g of hydrogen and 2. 478 g of oxygen. What is the percentage composition of this compound? Remember: To find percent, always think. . Do Practice Exercises 10 & 11 p. 117 6

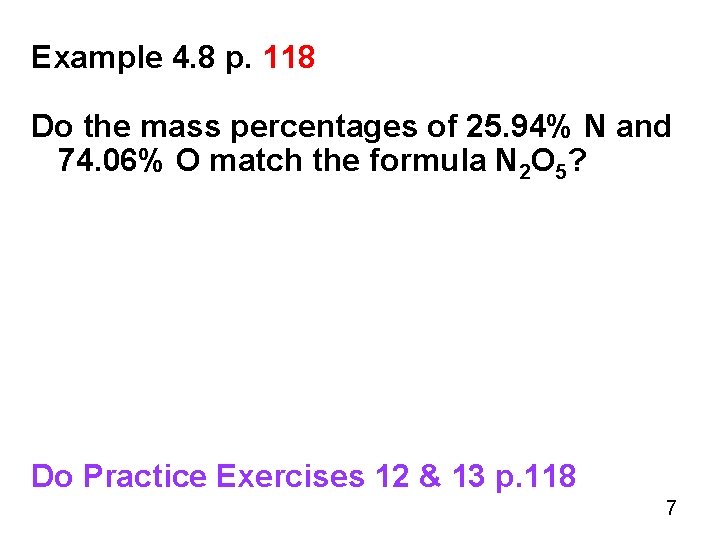
Example 4. 8 p. 118 Do the mass percentages of 25. 94% N and 74. 06% O match the formula N 2 O 5? Do Practice Exercises 12 & 13 p. 118 7

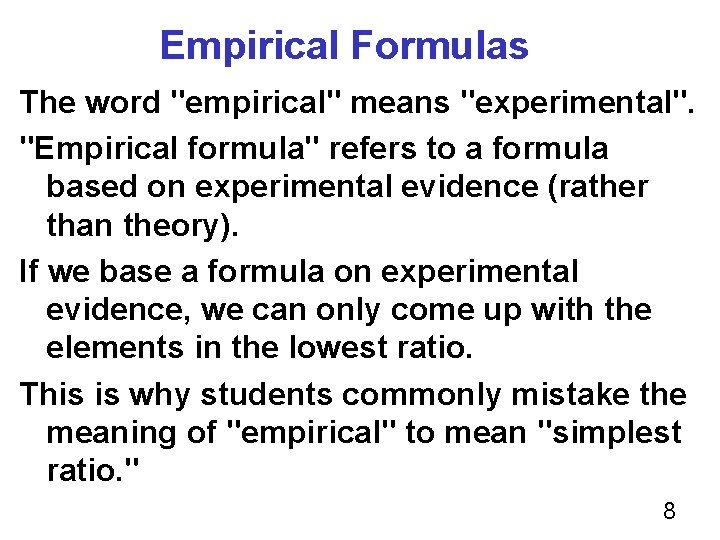
Empirical Formulas The word "empirical" means "experimental". "Empirical formula" refers to a formula based on experimental evidence (rather than theory). If we base a formula on experimental evidence, we can only come up with the elements in the lowest ratio. This is why students commonly mistake the meaning of "empirical" to mean "simplest ratio. " 8

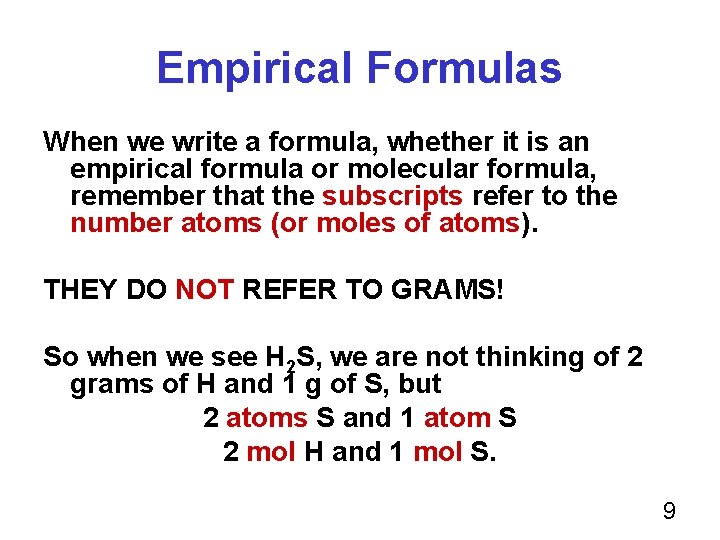
Empirical Formulas When we write a formula, whether it is an empirical formula or molecular formula, remember that the subscripts refer to the number atoms (or moles of atoms). THEY DO NOT REFER TO GRAMS! So when we see H 2 S, we are not thinking of 2 grams of H and 1 g of S, but 2 atoms S and 1 atom S 2 mol H and 1 mol S. 9

Empirical Formula Example 4. 9 p. 119 A 2. 57 g sample of a compound composed of only tin and chlorine was found to contain 1. 17 g of tin. what is the compound's empirical formula? Hint: We need a ratio of moles not grams! I STRONGLY recommend you set up the problem the way I am going to show you. You will be penalized if you have any "run-on" statements in your setup! My setup will help you avoid such "run-on" statements. Do practice exercises 4. 14 & 4. 15 p. 120. 10

Empirical Formula Example 4. 10 p. 121 One of the compounds of iron and oxygen, "black iron oxide, " occurs naturally in the mineral magnetite. When a 2. 448 g sample was analyzed it was found to have 1. 771 g of Fe. Calculate the empirical formula of this compound. Do Practice Exercises 16 & 17 p. 122 11

Empirical Formula Example 4. 11 p. 122 A white powder used in paints, enamels, and ceramics has the following percentage composition: Ba, 69. 6%; C, 6. 09%; and O, 24. 3%. What is its empirical formula? What is the name of this compound? Do practice exercises 18 & 19 p. 123. 12

Empirical Formula Example 4. 12 p. 125 A 0. 5438 g sample of a liquid consisting of only C, H, and O was burned in pure oxygen, and 1. 039 g of CO 2 and 0. 6369 g of H 2 O were obtained. What is the empirical formula of the compound. Do Practice Exercises 20 & 21 p. 127. 13

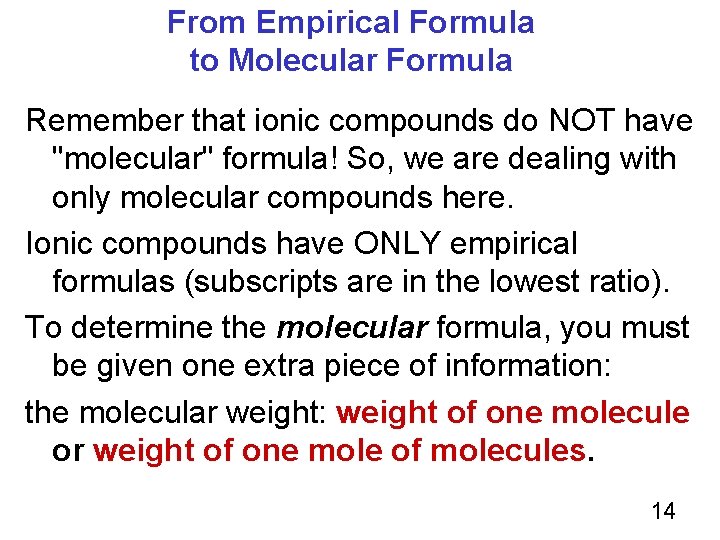
From Empirical Formula to Molecular Formula Remember that ionic compounds do NOT have "molecular" formula! So, we are dealing with only molecular compounds here. Ionic compounds have ONLY empirical formulas (subscripts are in the lowest ratio). To determine the molecular formula, you must be given one extra piece of information: the molecular weight: weight of one molecule or weight of one mole of molecules. 14

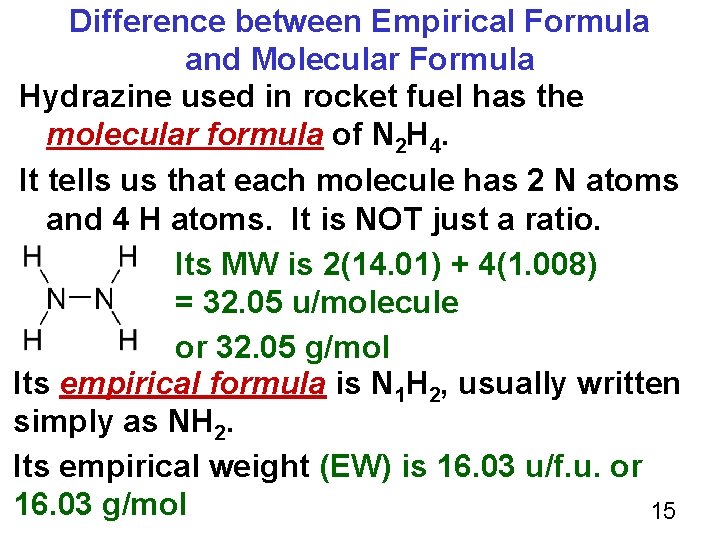
Difference between Empirical Formula and Molecular Formula Hydrazine used in rocket fuel has the molecular formula of N 2 H 4. It tells us that each molecule has 2 N atoms and 4 H atoms. It is NOT just a ratio. Its MW is 2(14. 01) + 4(1. 008) = 32. 05 u/molecule or 32. 05 g/mol Its empirical formula is N 1 H 2, usually written simply as NH 2. Its empirical weight (EW) is 16. 03 u/f. u. or 16. 03 g/mol 15

The Use of “f. u. ” • Previously you learned to use “f. u. ” in place of “molecule” when dealing with an ionic compound. • The smallest unit in an ionic compound is indeed its “f. u. ” • However, it does not mean “f. u. ” is limited to only ionic compounds. • For molecular compounds, if the molecular formula is reduced to its lowest ratio, it is also referred to as a “f. u. ” 16

The Use of “f. u. ” Thus, “f. u. ” is used for any substance when shown in the simplest ratio. IONIC COMPOUND: It is always referred to as a f. u. (e. g. Na 2 S) MOLECULAR COMPOUND: • If formula shows true # of atoms of each element (e. g. N 2 H 4), it is a molecule. • If formula shows only the lowest ratio of atoms of each element (e. g. NH 2) it is a f. u. N 2 H 4 is the molecular formula. 17 NH 2 is the empirical formula.

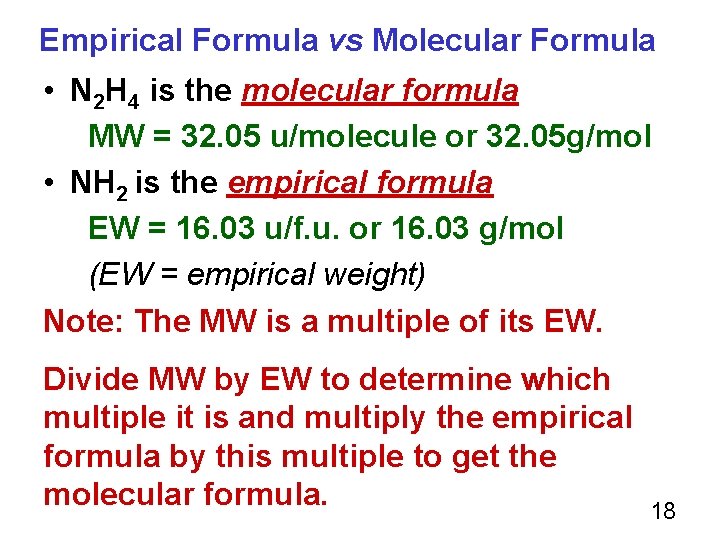
Empirical Formula vs Molecular Formula • N 2 H 4 is the molecular formula MW = 32. 05 u/molecule or 32. 05 g/mol • NH 2 is the empirical formula EW = 16. 03 u/f. u. or 16. 03 g/mol (EW = empirical weight) Note: The MW is a multiple of its EW. Divide MW by EW to determine which multiple it is and multiply the empirical formula by this multiple to get the molecular formula. 18

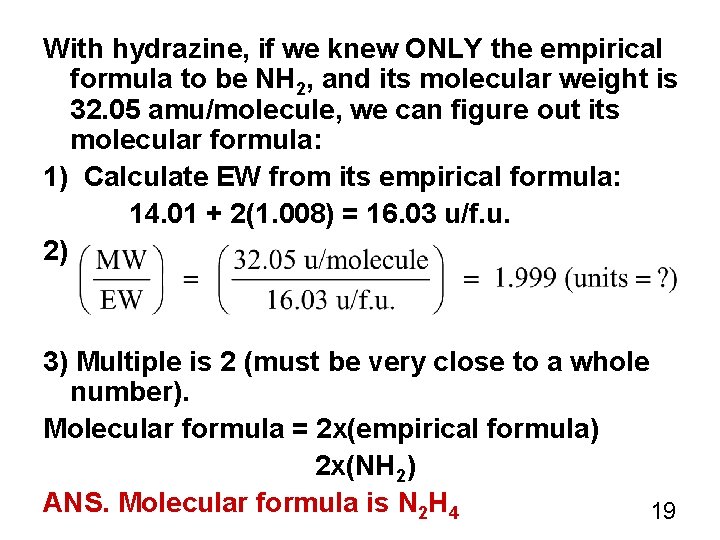
With hydrazine, if we knew ONLY the empirical formula to be NH 2, and its molecular weight is 32. 05 amu/molecule, we can figure out its molecular formula: 1) Calculate EW from its empirical formula: 14. 01 + 2(1. 008) = 16. 03 u/f. u. 2) 3) Multiple is 2 (must be very close to a whole number). Molecular formula = 2 x(empirical formula) 2 x(NH 2) ANS. Molecular formula is N 2 H 4 19

Molecular Formula Example 4. 13 p. 127 Styrene, the raw material for polystyrene foam plastics, has an empirical formula of CH. Its molecular mass is 104 g mol-1. What is its molecular formula. Do Practice Exercises 22 & 23 p. 128. 20