Empirical and Molecular Formulas Empirical vs Molecular Formula










- Slides: 36

Empirical and Molecular Formulas

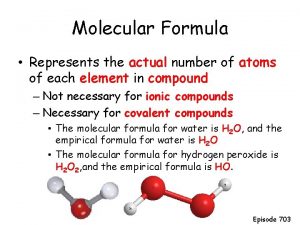
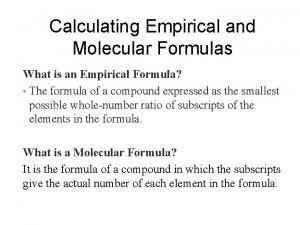
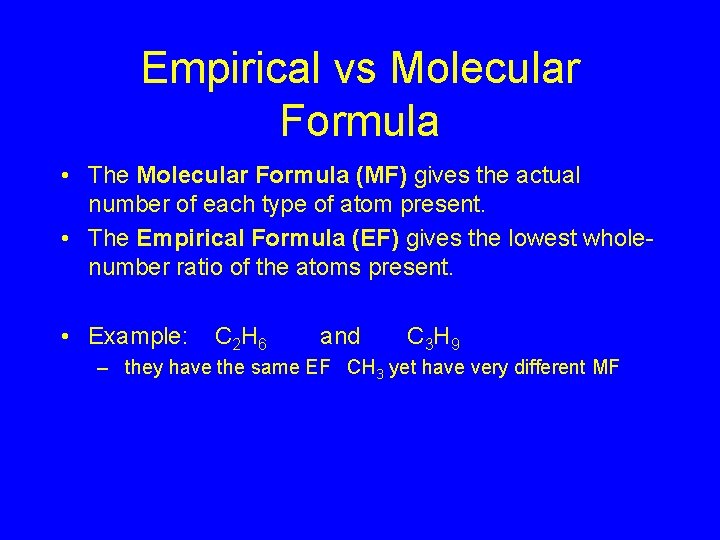
Empirical vs Molecular Formula • The Molecular Formula (MF) gives the actual number of each type of atom present. • The Empirical Formula (EF) gives the lowest wholenumber ratio of the atoms present. • Example: C 2 H 6 and C 3 H 9 – they have the same EF CH 3 yet have very different MF

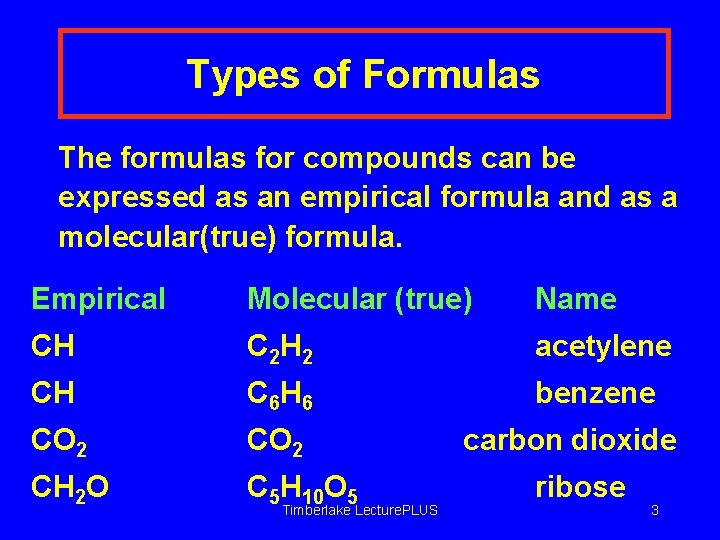
Types of Formulas The formulas for compounds can be expressed as an empirical formula and as a molecular(true) formula. Empirical Molecular (true) Name CH C 2 H 2 acetylene CH C 6 H 6 benzene CO 2 CH 2 O C 5 H 10 O 5 Timberlake Lecture. PLUS carbon dioxide ribose 3

• An empirical formula represents the simplest whole number ratio of the atoms in a compound. • The molecular formula is the true or actual ratio of the atoms in a compound. Timberlake Lecture. PLUS 4

EF vs. MF When you have an ionic compound: the EF = MF For some molecular compounds: the EF = MF, but not always

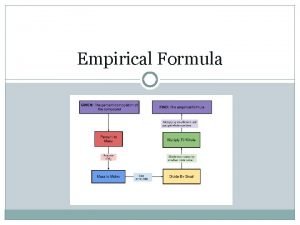
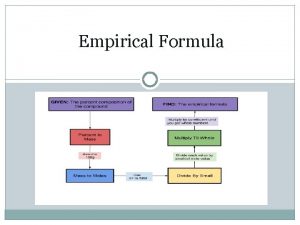
Determining the EF Remember: The EF is the lowest whole-number ratio of the moles of each atom present. Example: CH 4 has 1 mol C atoms 4 mol H atoms

Determining the EF If a compound consists of: 62. 1% C 13. 8% H 24. 1% N The percentages are based on MASS not MOLES We can compare moles, not masses

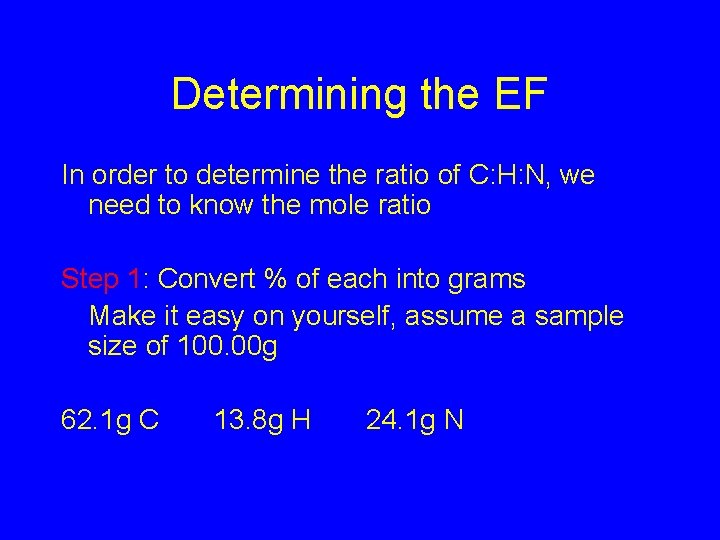
Determining the EF In order to determine the ratio of C: H: N, we need to know the mole ratio Step 1: Convert % of each into grams Make it easy on yourself, assume a sample size of 100. 00 g 62. 1 g C 13. 8 g H 24. 1 g N

Determining the EF Now that you know how many grams of each atom you have: Step 2: Convert grams to moles using the molar mass of each 62. 1 g C 13. 8 g H 24. 1 g N

Determining the EF Now that you have the mol ratio, you need to make them Whole-Numbers. Step 3: Divide each mol by the smallest mol value from step #2 5. 17 mol C 13. 7 mol H 1. 72 mol N

Determining the EF This results in 3 mol C, 8 mol H and 1 mol N therefore the EF = C 3 H 8 N

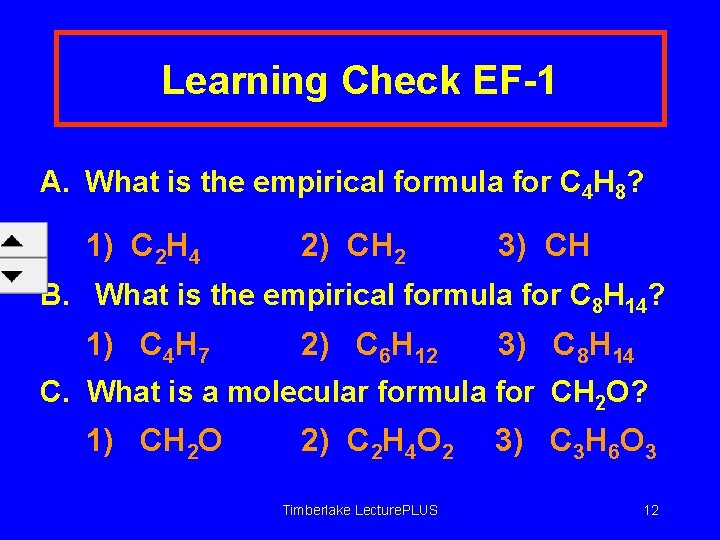
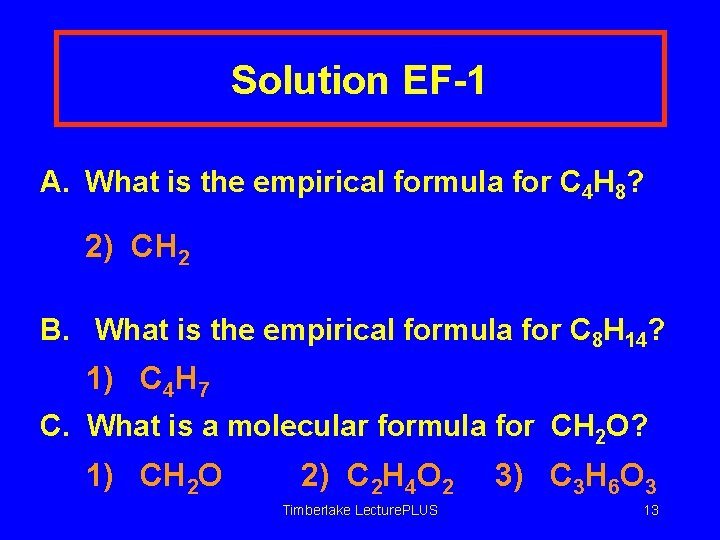
Learning Check EF-1 A. What is the empirical formula for C 4 H 8? 1) C 2 H 4 2) CH 2 3) CH B. What is the empirical formula for C 8 H 14? 1) C 4 H 7 2) C 6 H 12 3) C 8 H 14 C. What is a molecular formula for CH 2 O? 1) CH 2 O 2) C 2 H 4 O 2 Timberlake Lecture. PLUS 3) C 3 H 6 O 3 12

Solution EF-1 A. What is the empirical formula for C 4 H 8? 2) CH 2 B. What is the empirical formula for C 8 H 14? 1) C 4 H 7 C. What is a molecular formula for CH 2 O? 1) CH 2 O 2) C 2 H 4 O 2 Timberlake Lecture. PLUS 3) C 3 H 6 O 3 13

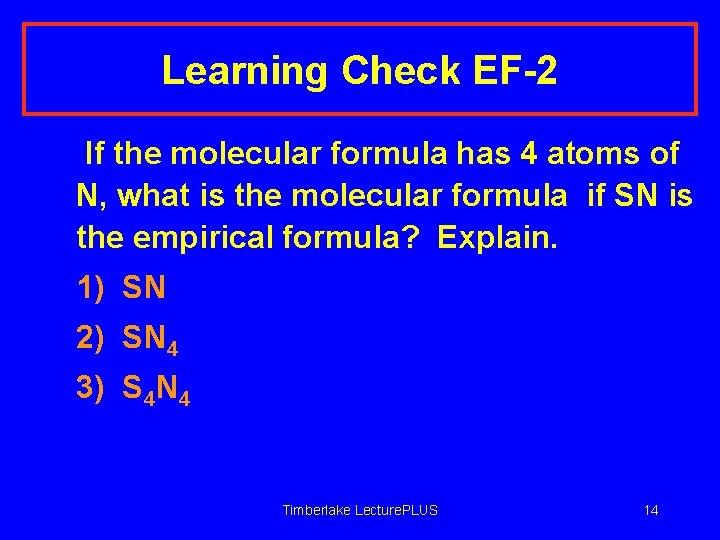
Learning Check EF-2 If the molecular formula has 4 atoms of N, what is the molecular formula if SN is the empirical formula? Explain. 1) SN 2) SN 4 3) S 4 N 4 Timberlake Lecture. PLUS 14

Solution EF-2 If the molecular formula has 4 atoms of N, what is the molecular formula if SN is the empirical formula? Explain. 3) S 4 N 4 If the actual formula has 4 atoms of N, and S is related 1: 1, then there must also be 4 atoms of S. Timberlake Lecture. PLUS 15

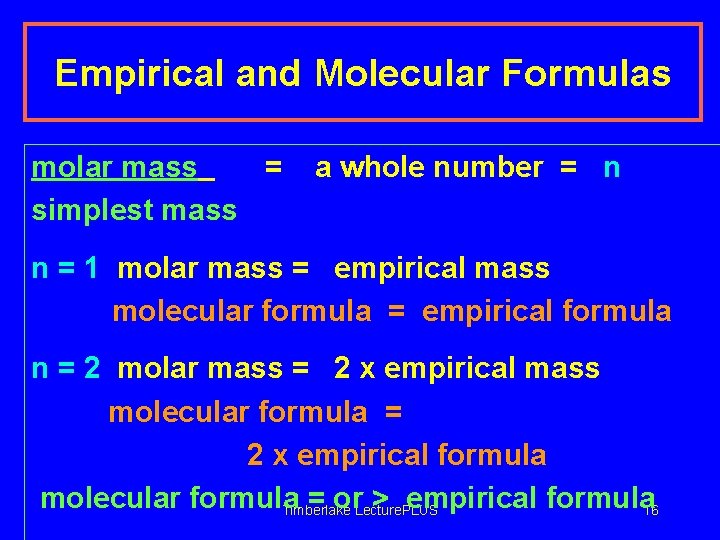
Empirical and Molecular Formulas molar mass = simplest mass a whole number = n n = 1 molar mass = empirical mass molecular formula = empirical formula n = 2 molar mass = 2 x empirical mass molecular formula = 2 x empirical formula molecular formula = or > empirical formula 16 Timberlake Lecture. PLUS

EF to MF To go from the EF to the MF, you need two additional pieces of information: 1 – calculate the mass from your EF 2 – You must be given the mass of the MF (X) EFmass = MFmass X = MFmass / EFmass

EF to MF Example: You found the EF to be HO, and the MFmass = 34. 02 g 1. 2. 3. 4. Calculate the EFmass = 17. 01 g Calculate X = 34. 02 g / 17. 01 g X=2 EF = HO MF = H 2 O 2

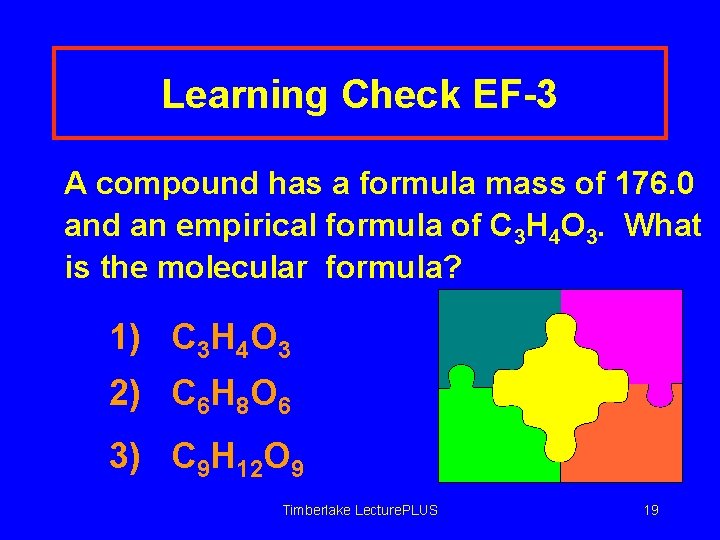
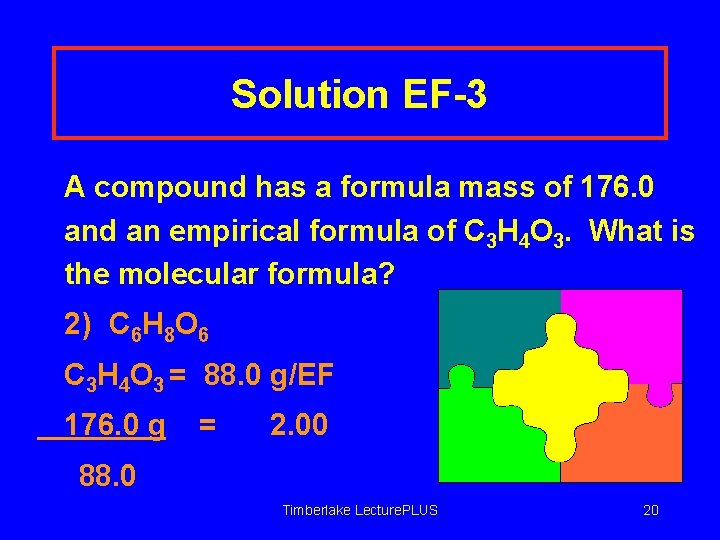
Learning Check EF-3 A compound has a formula mass of 176. 0 and an empirical formula of C 3 H 4 O 3. What is the molecular formula? 1) C 3 H 4 O 3 2) C 6 H 8 O 6 3) C 9 H 12 O 9 Timberlake Lecture. PLUS 19

Solution EF-3 A compound has a formula mass of 176. 0 and an empirical formula of C 3 H 4 O 3. What is the molecular formula? 2) C 6 H 8 O 6 C 3 H 4 O 3 = 88. 0 g/EF 176. 0 g = 2. 00 88. 0 Timberlake Lecture. PLUS 20

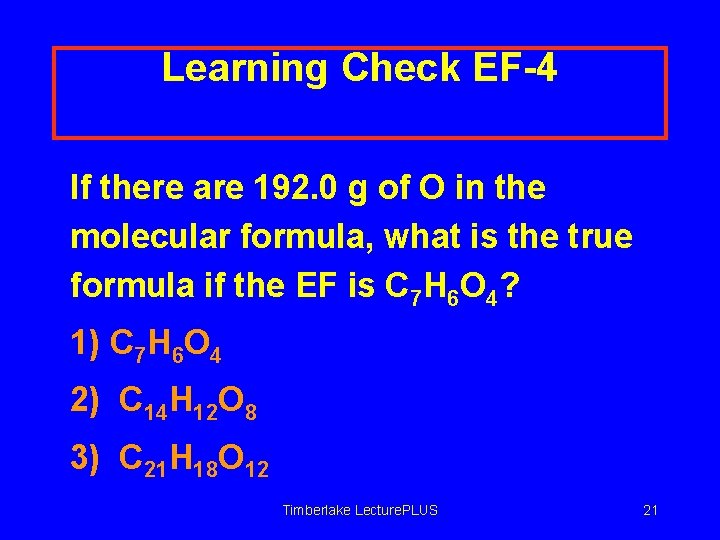
Learning Check EF-4 If there are 192. 0 g of O in the molecular formula, what is the true formula if the EF is C 7 H 6 O 4? 1) C 7 H 6 O 4 2) C 14 H 12 O 8 3) C 21 H 18 O 12 Timberlake Lecture. PLUS 21

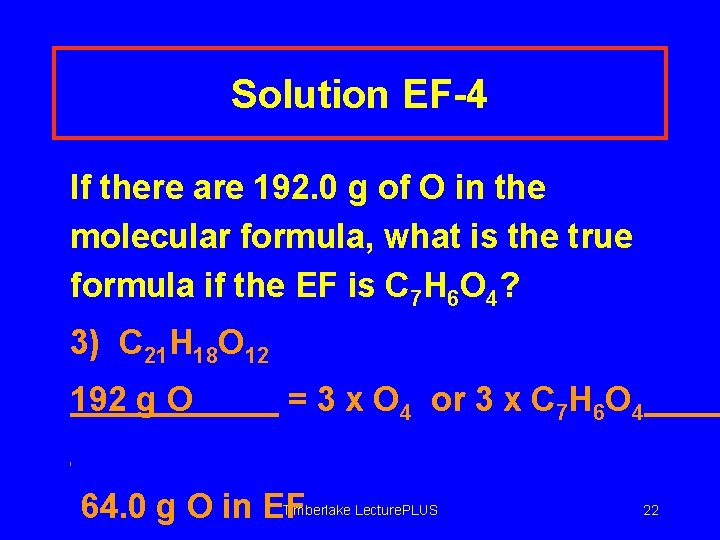
Solution EF-4 If there are 192. 0 g of O in the molecular formula, what is the true formula if the EF is C 7 H 6 O 4? 3) C 21 H 18 O 12 192 g O = 3 x O 4 or 3 x C 7 H 6 O 4 Timberlake Lecture. PLUS 64. 0 g O in EF 22

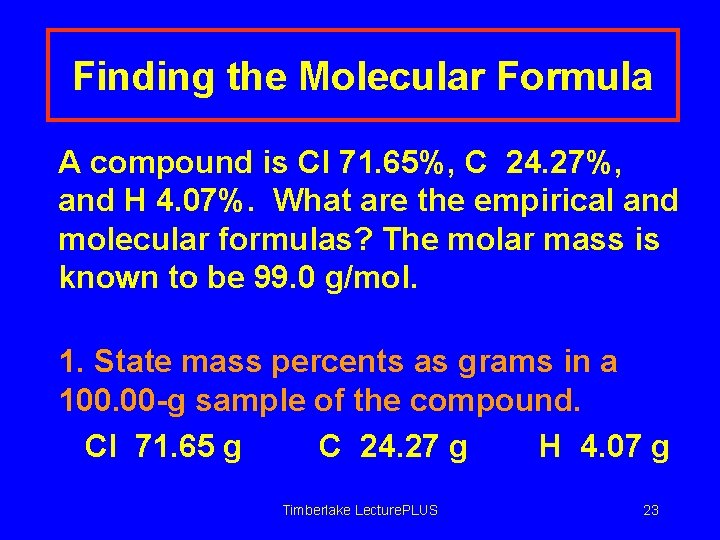
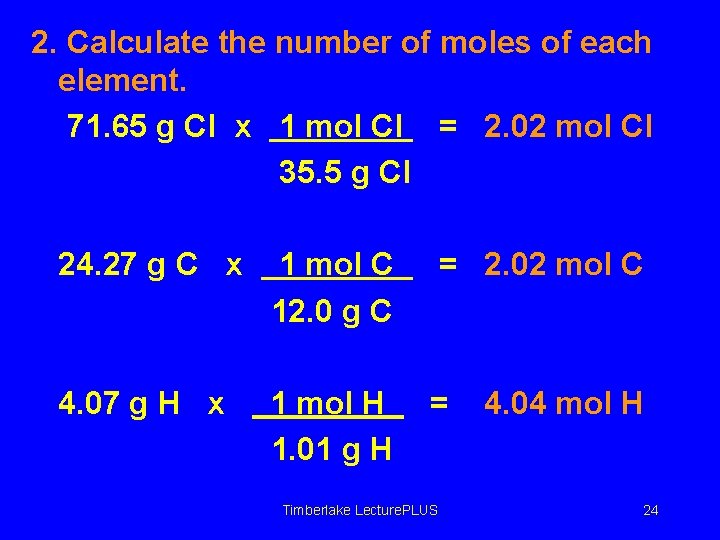
Finding the Molecular Formula A compound is Cl 71. 65%, C 24. 27%, and H 4. 07%. What are the empirical and molecular formulas? The molar mass is known to be 99. 0 g/mol. 1. State mass percents as grams in a 100. 00 -g sample of the compound. Cl 71. 65 g C 24. 27 g H 4. 07 g Timberlake Lecture. PLUS 23

2. Calculate the number of moles of each element. 71. 65 g Cl x 1 mol Cl = 2. 02 mol Cl 35. 5 g Cl 24. 27 g C x 1 mol C 12. 0 g C = 2. 02 mol C 4. 07 g H x 1 mol H 1. 01 g H = Timberlake Lecture. PLUS 4. 04 mol H 24

Why moles? Why do you need the number of moles of each element in the compound? Timberlake Lecture. PLUS 25

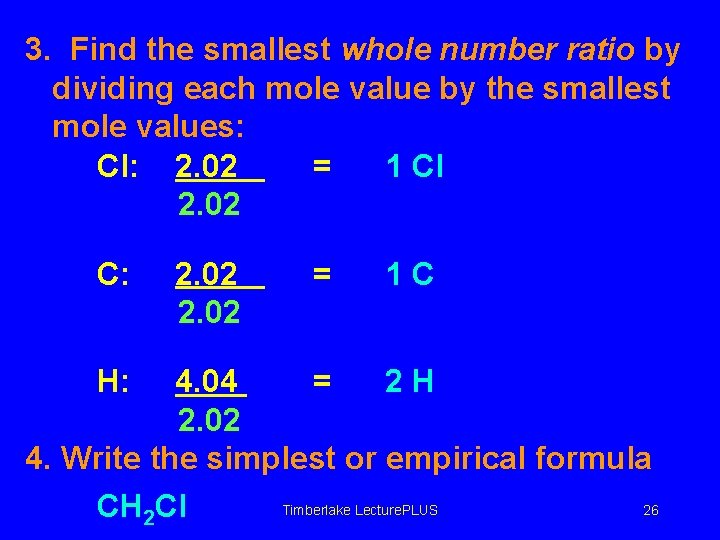
3. Find the smallest whole number ratio by dividing each mole value by the smallest mole values: Cl: 2. 02 = 1 Cl 2. 02 C: H: 2. 02 = 1 C 4. 04 = 2 H 2. 02 4. Write the simplest or empirical formula Timberlake Lecture. PLUS 26 CH 2 Cl

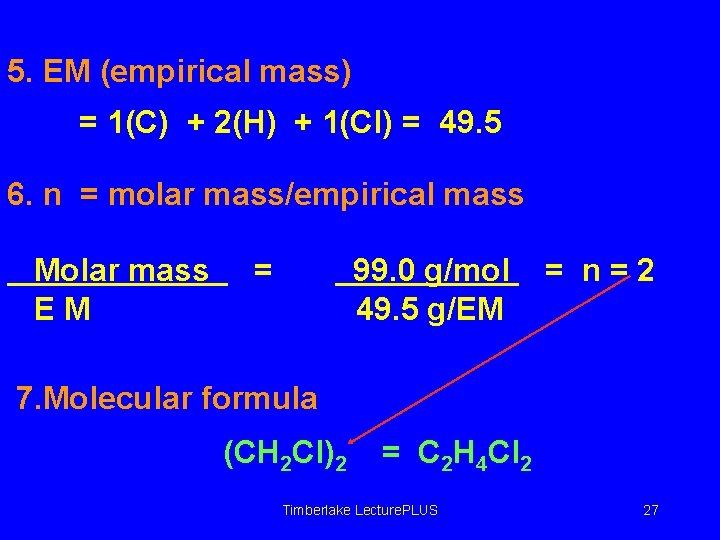
5. EM (empirical mass) = 1(C) + 2(H) + 1(Cl) = 49. 5 6. n = molar mass/empirical mass Molar mass EM = 99. 0 g/mol 49. 5 g/EM = n=2 7. Molecular formula (CH 2 Cl)2 = C 2 H 4 Cl 2 Timberlake Lecture. PLUS 27

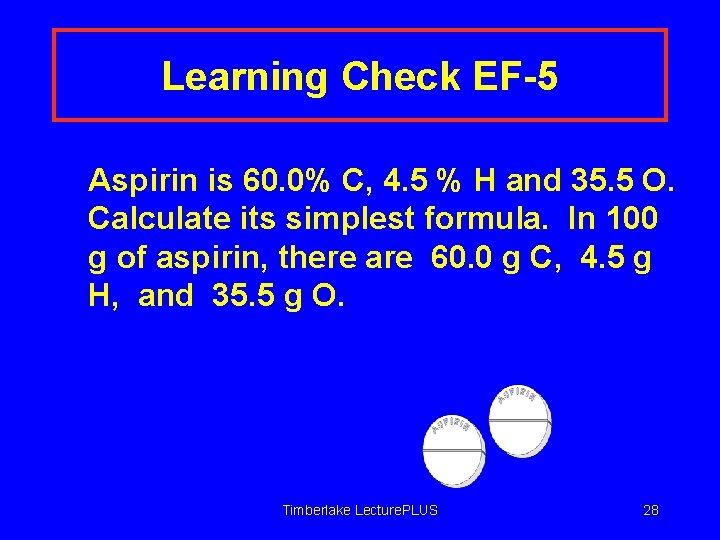
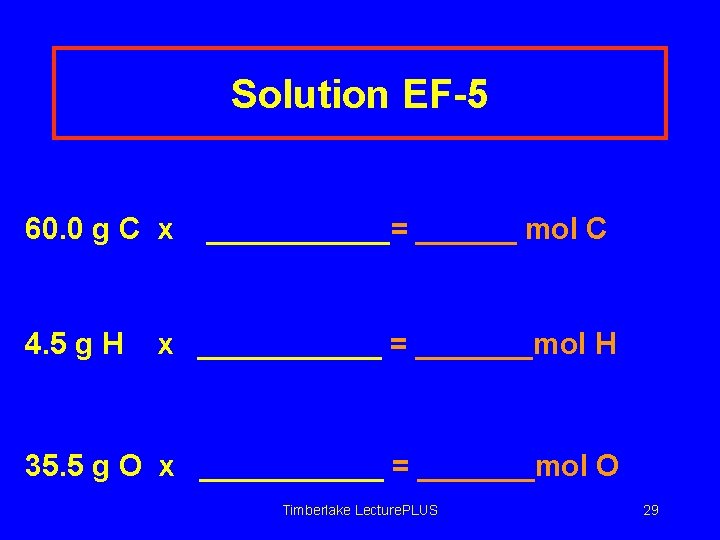
Learning Check EF-5 Aspirin is 60. 0% C, 4. 5 % H and 35. 5 O. Calculate its simplest formula. In 100 g of aspirin, there are 60. 0 g C, 4. 5 g H, and 35. 5 g O. Timberlake Lecture. PLUS 28

Solution EF-5 60. 0 g C x 4. 5 g H ______= ______ mol C x ______ = _______mol H 35. 5 g O x ______ = _______mol O Timberlake Lecture. PLUS 29

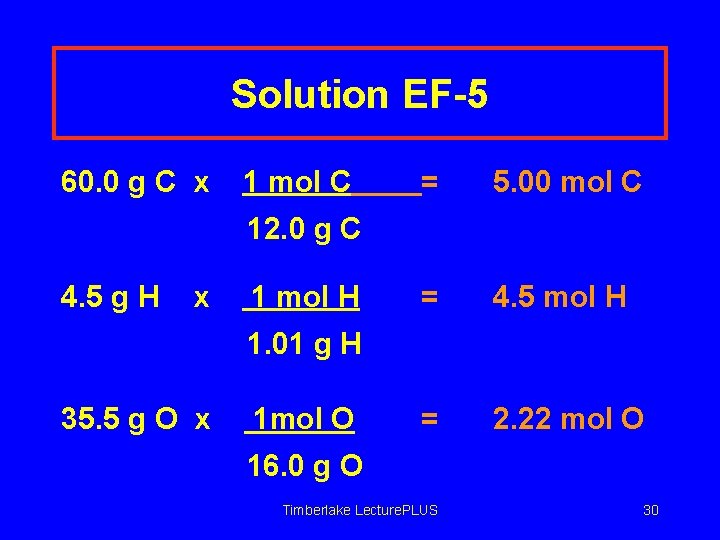
Solution EF-5 60. 0 g C x 1 mol C = 5. 00 mol C = 4. 5 mol H = 2. 22 mol O 12. 0 g C 4. 5 g H x 1 mol H 1. 01 g H 35. 5 g O x 1 mol O 16. 0 g O Timberlake Lecture. PLUS 30

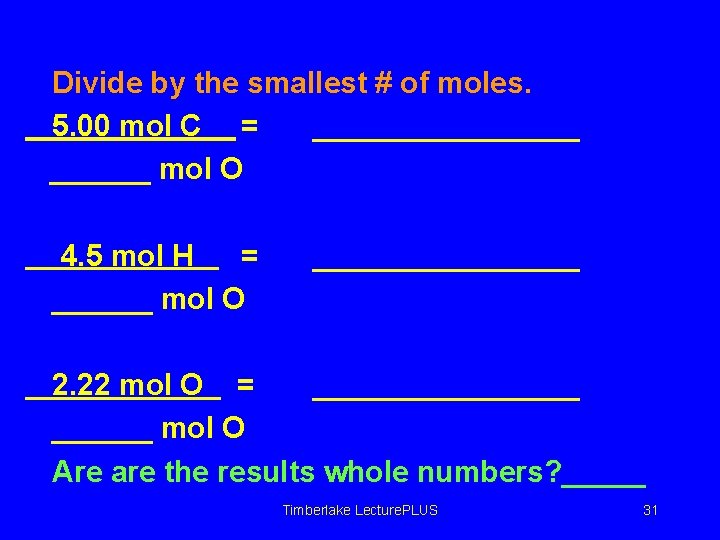
Divide by the smallest # of moles. 5. 00 mol C = ________ mol O 4. 5 mol H = ______ mol O ________ 2. 22 mol O = ________ mol O Are are the results whole numbers? _____ Timberlake Lecture. PLUS 31

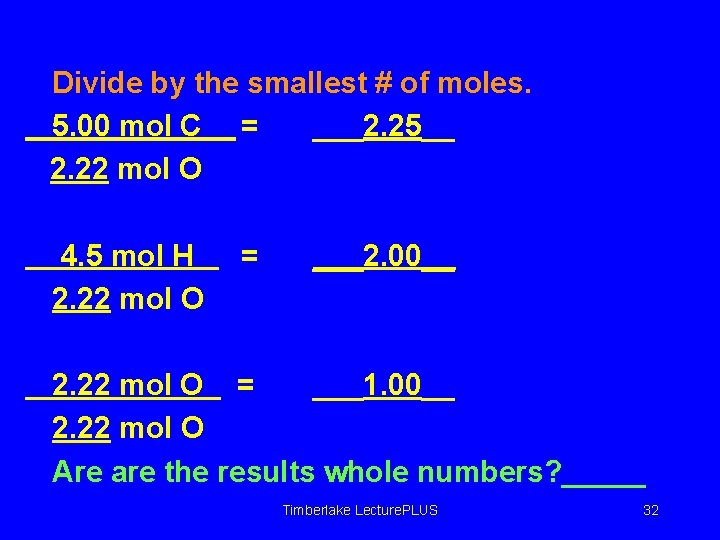
Divide by the smallest # of moles. 5. 00 mol C = ___2. 25__ 2. 22 mol O 4. 5 mol H 2. 22 mol O = ___2. 00__ 2. 22 mol O = ___1. 00__ 2. 22 mol O Are are the results whole numbers? _____ Timberlake Lecture. PLUS 32

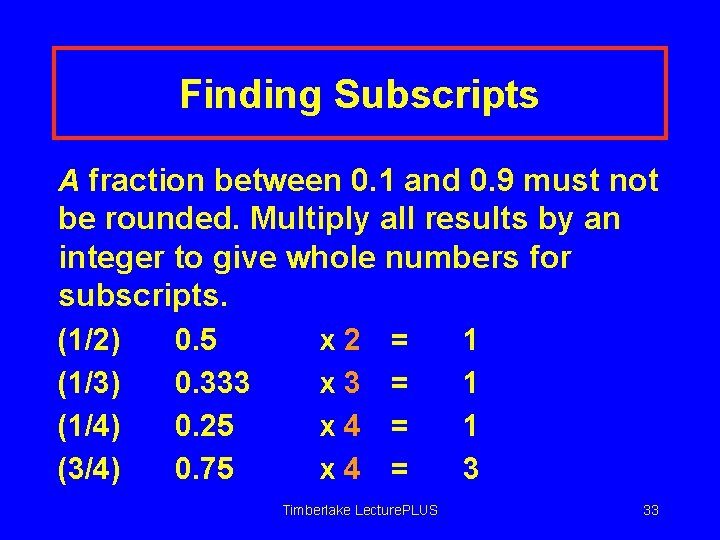
Finding Subscripts A fraction between 0. 1 and 0. 9 must not be rounded. Multiply all results by an integer to give whole numbers for subscripts. (1/2) (1/3) (1/4) (3/4) 0. 5 0. 333 0. 25 0. 75 x 2 x 3 x 4 = = Timberlake Lecture. PLUS 1 1 1 3 33

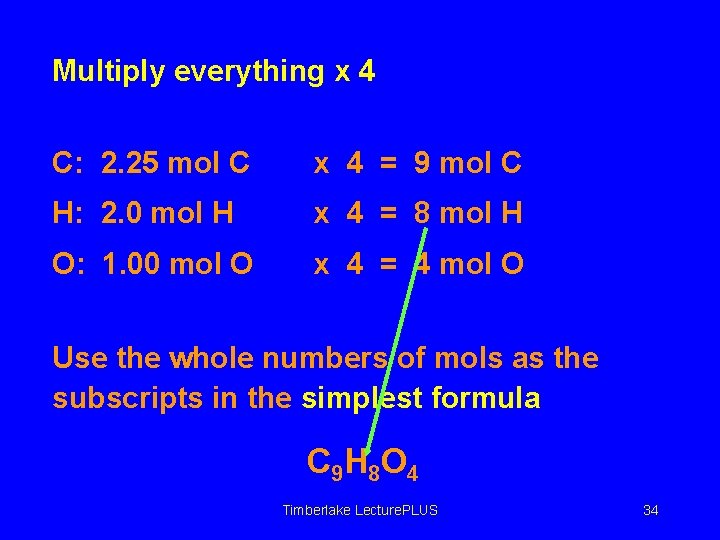
Multiply everything x 4 C: 2. 25 mol C x 4 = 9 mol C H: 2. 0 mol H x 4 = 8 mol H O: 1. 00 mol O x 4 = 4 mol O Use the whole numbers of mols as the subscripts in the simplest formula C 9 H 8 O 4 Timberlake Lecture. PLUS 34

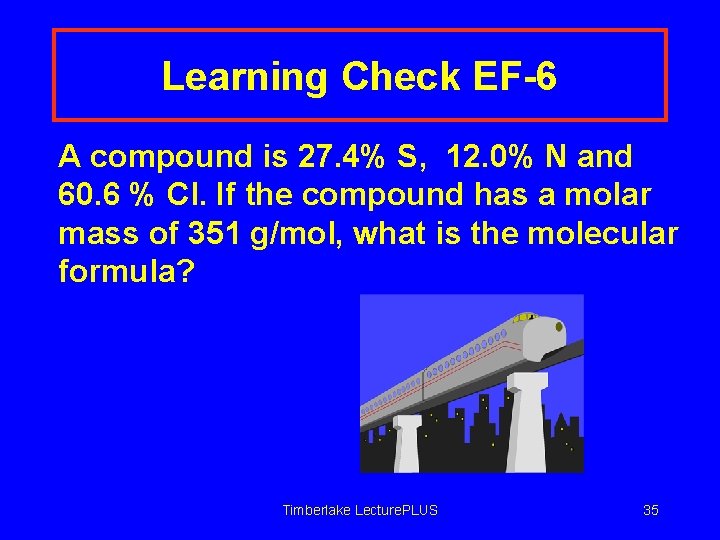
Learning Check EF-6 A compound is 27. 4% S, 12. 0% N and 60. 6 % Cl. If the compound has a molar mass of 351 g/mol, what is the molecular formula? Timberlake Lecture. PLUS 35

Solution EF 6 0. 853 mol S /0. 853 = 1 S 0. 857 mol N /0. 853 = 1 N 1. 71 mol Cl /0. 853 = 2 Cl Empirical formula = SNCl 2 = 117. 1 g/EF Mol. Mass/ Empirical mass 351/117. 1 = 3 Molecular formula = S 3 N 3 Cl 6 Timberlake Lecture. PLUS 36
 Empirical and molecular formula worksheet
Empirical and molecular formula worksheet Ibuprofen percent composition
Ibuprofen percent composition Molecular formula and empirical formula
Molecular formula and empirical formula What is meant by empirical formula
What is meant by empirical formula Emprical formula
Emprical formula Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Empirical formula vs
Empirical formula vs Empirical formula and molecular formula
Empirical formula and molecular formula Empirical formula of haemoglobin
Empirical formula of haemoglobin Percentage to empirical formula
Percentage to empirical formula Definition of molecular formula
Definition of molecular formula Molecular formular
Molecular formular Water percentage composition
Water percentage composition Empirical and molecular formula quiz
Empirical and molecular formula quiz Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical formula poem
Empirical formula poem Naming compounds and writing formulas
Naming compounds and writing formulas What is the empirical formula of caffeine
What is the empirical formula of caffeine How to do empirical formula
How to do empirical formula Lesson 28: sniffing around molecular formulas answer key
Lesson 28: sniffing around molecular formulas answer key Chemical formula vs molecular formula
Chemical formula vs molecular formula Chemical formula covalent compounds
Chemical formula covalent compounds How to find molecular formula
How to find molecular formula Physical state of covalent compounds
Physical state of covalent compounds Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Empirical formula poem
Empirical formula poem Hydrate formula
Hydrate formula Empirical probability formula
Empirical probability formula Standard deviation of discrete random variable
Standard deviation of discrete random variable Empirical formula from percentages
Empirical formula from percentages Empirical formula for adrenaline
Empirical formula for adrenaline Empirical formula from percent composition
Empirical formula from percent composition Empirical formula with percentages
Empirical formula with percentages How to calculate empirical formula with percentages
How to calculate empirical formula with percentages Empirical formula rhyme
Empirical formula rhyme