Chemical Formulas Percent Composition Empirical Formula Molecular Formula


- Slides: 14

Chemical Formulas Percent Composition Empirical Formula Molecular Formula I II III

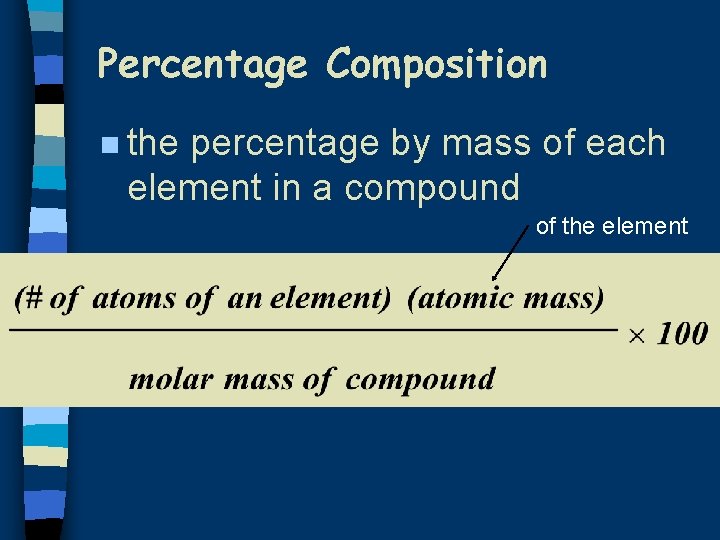
Percentage Composition n the percentage by mass of each element in a compound of the element

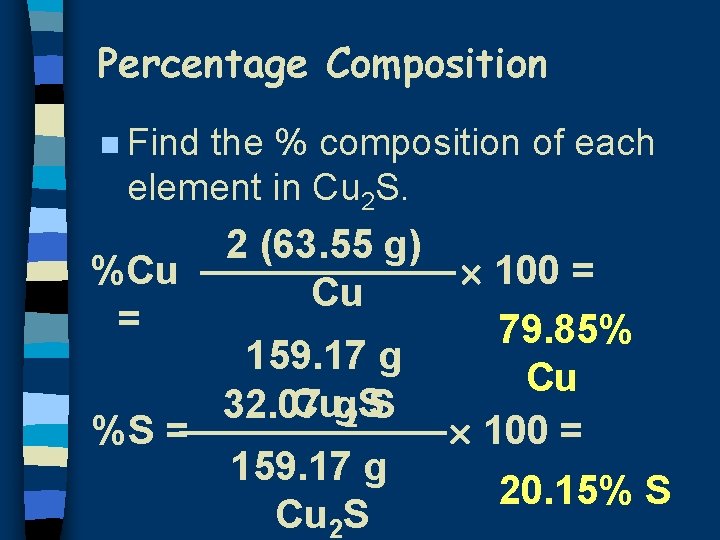
Percentage Composition n Find the % composition of each element in Cu 2 S. 2 (63. 55 g) 100 = %Cu Cu = 79. 85% 159. 17 g Cu Cug 2 SS 32. 07 %S = 100 = 159. 17 g 20. 15% S Cu 2 S

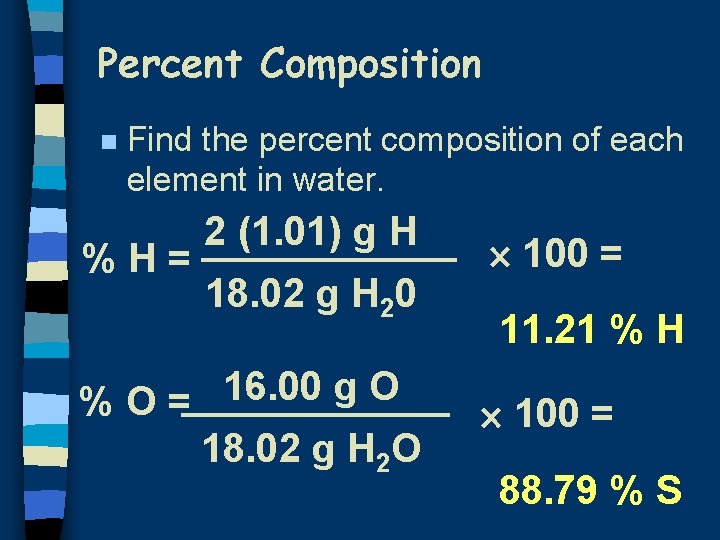
Percent Composition n Find the percent composition of each element in water. %H= 2 (1. 01) g H 18. 02 g H 20 16. 00 g O %O= 18. 02 g H 2 O 100 = 11. 21 % H 100 = 88. 79 % S

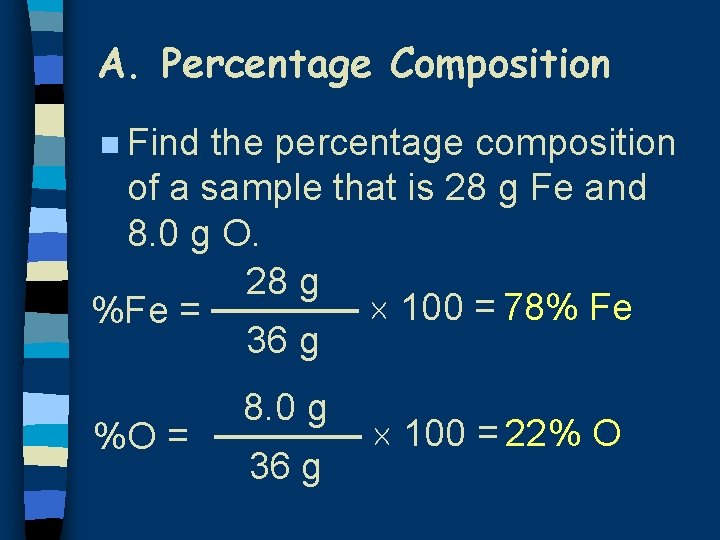
A. Percentage Composition n Find the percentage composition of a sample that is 28 g Fe and 8. 0 g O. 28 g 100 = 78% Fe %Fe = 36 g %O = 8. 0 g 36 g 100 = 22% O

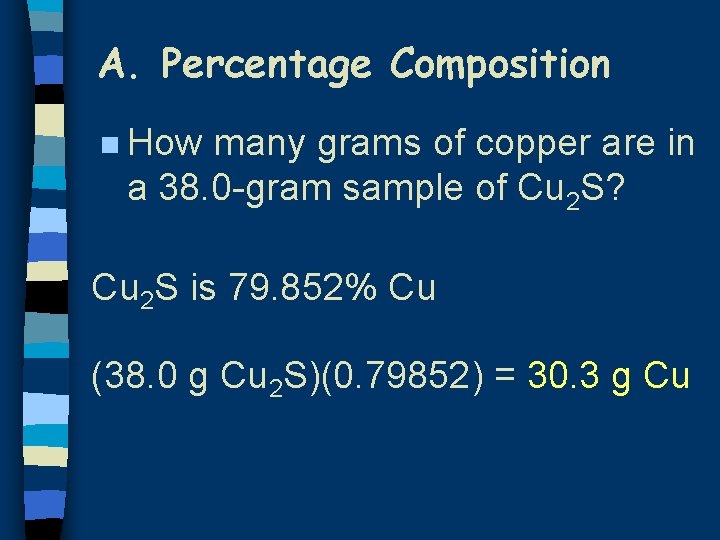
A. Percentage Composition n How many grams of copper are in a 38. 0 -gram sample of Cu 2 S? Cu 2 S is 79. 852% Cu (38. 0 g Cu 2 S)(0. 79852) = 30. 3 g Cu

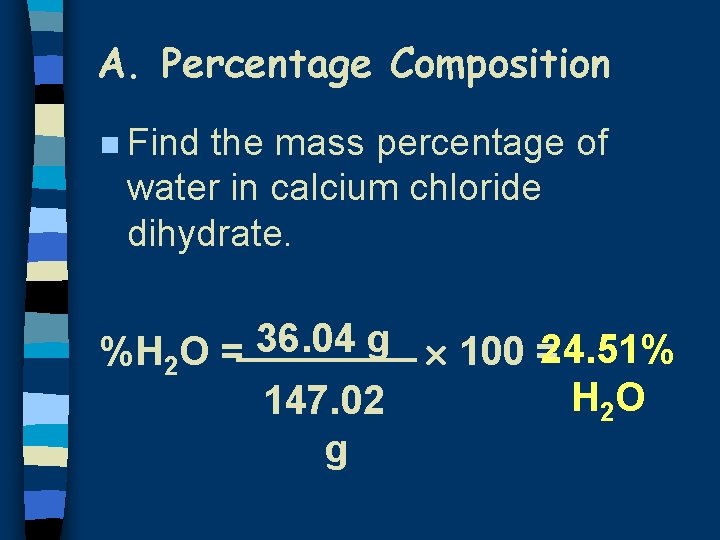
A. Percentage Composition n Find the mass percentage of water in calcium chloride dihydrate. 36. 04 g %H 2 O = 100 =24. 51% H 2 O 147. 02 g

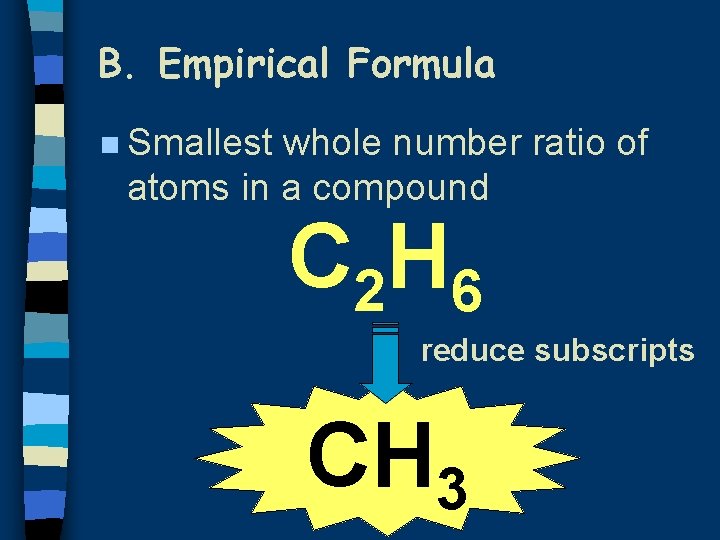
B. Empirical Formula n Smallest whole number ratio of atoms in a compound C 2 H 6 reduce subscripts CH 3

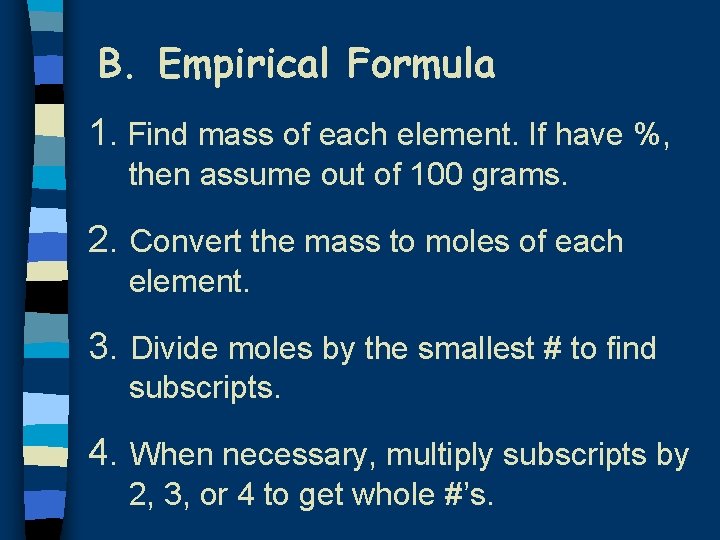
B. Empirical Formula 1. Find mass of each element. If have %, then assume out of 100 grams. 2. Convert the mass to moles of each element. 3. Divide moles by the smallest # to find subscripts. 4. When necessary, multiply subscripts by 2, 3, or 4 to get whole #’s.

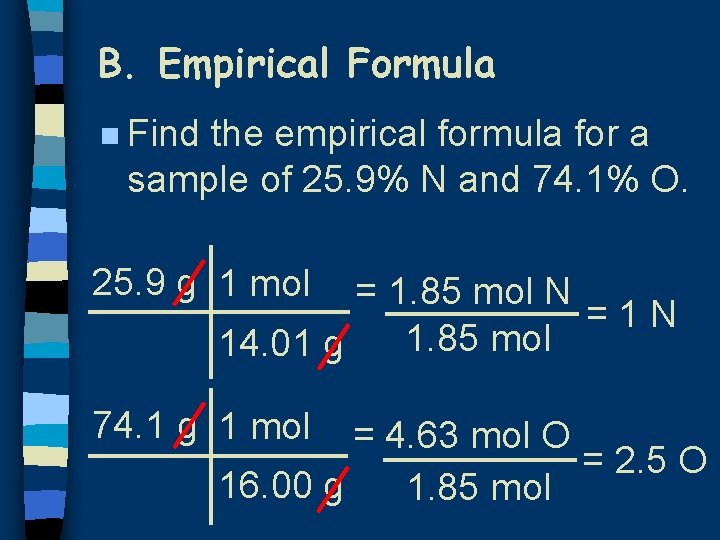
B. Empirical Formula n Find the empirical formula for a sample of 25. 9% N and 74. 1% O. 25. 9 g 1 mol = 1. 85 mol N =1 N 1. 85 mol 14. 01 g 74. 1 g 1 mol = 4. 63 mol O = 2. 5 O 16. 00 g 1. 85 mol

B. Empirical Formula N 1 O 2. 5 Need to make the subscripts whole numbers multiply by 2 N 2 O 5

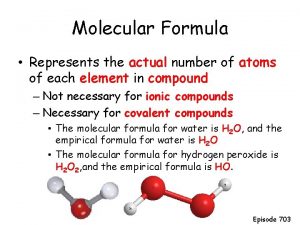
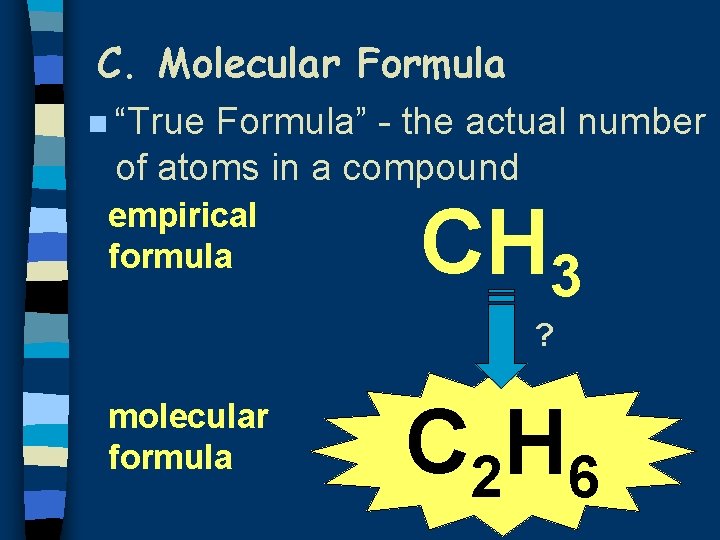
C. Molecular Formula n “True Formula” - the actual number of atoms in a compound empirical formula CH 3 ? molecular formula C 2 H 6

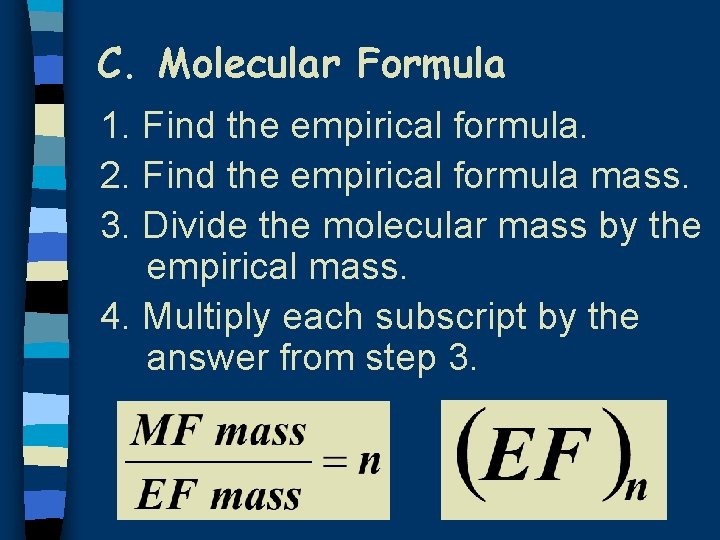
C. Molecular Formula 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the answer from step 3.

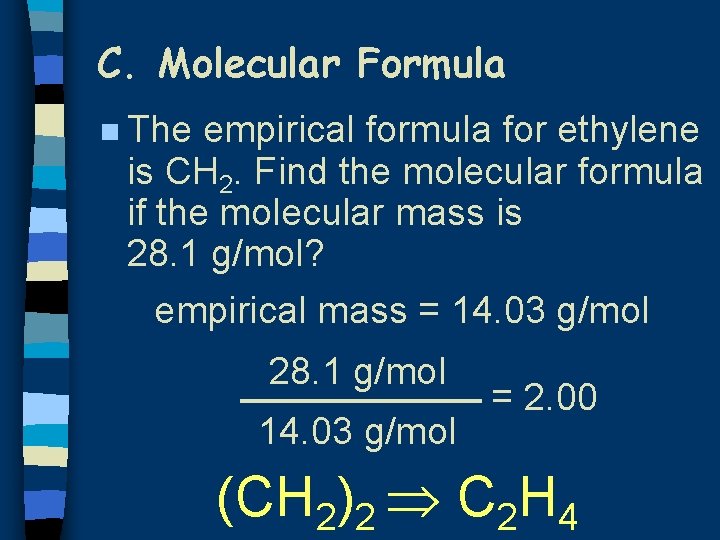
C. Molecular Formula n The empirical formula for ethylene is CH 2. Find the molecular formula if the molecular mass is 28. 1 g/mol? empirical mass = 14. 03 g/mol 28. 1 g/mol 14. 03 g/mol = 2. 00 (CH 2)2 C 2 H 4
 How to get empirical formula from percentages
How to get empirical formula from percentages Percentage composition of acetic acid
Percentage composition of acetic acid Atomic mass of kmno4
Atomic mass of kmno4 Percentage of composition
Percentage of composition Empirical formula from percent composition
Empirical formula from percent composition Empirical formula of adipic acid
Empirical formula of adipic acid Empirical formula to percent composition
Empirical formula to percent composition Percent by mass formula
Percent by mass formula Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Empirical formula of haemoglobin
Empirical formula of haemoglobin Define the molecular formula
Define the molecular formula Empirical formula vs
Empirical formula vs How to do empirical formula
How to do empirical formula Empirical formula
Empirical formula