Empirical and Molecular Formulas Empirical Formula A formula












- Slides: 16

Empirical and Molecular Formulas

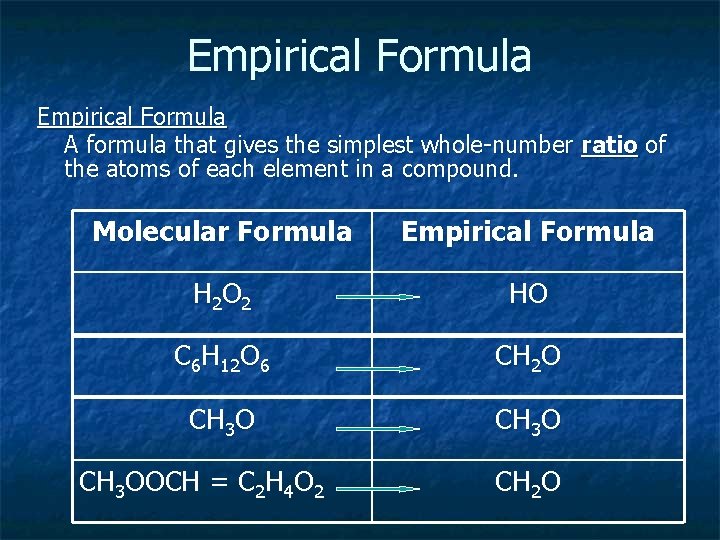
Empirical Formula A formula that gives the simplest whole-number ratio of the atoms of each element in a compound. Molecular Formula Empirical Formula H 2 O 2 HO C 6 H 12 O 6 CH 2 O CH 3 OOCH = C 2 H 4 O 2 CH 2 O

Determine the empirical formula for a compound containing 2. 128 g Cl and 1. 203 g Ca. Steps 1. Find mole amounts. 2. Divide each mole by the smallest mole.

1. Find mole amounts. 2. 128 g Cl x 1 mol Cl = 0. 0600 mol Cl 35. 45 g Cl 1. 203 g Ca x 1 mol Ca = 0. 0300 mol Ca 40. 08 g Ca

2. Divide each mole by the smallest mole. Cl = 0. 0600 mol Cl = 2. 00 mol Cl 0. 0300 Ca = 0. 0300 mol Ca = 1. 00 mol Ca 0. 0300 Ratio – 1 Ca: 2 Cl Empirical Formula = Ca. Cl 2

A compound weighing 298. 12 g consists of 72. 2% magnesium and 27. 8% nitrogen by mass. What is the empirical formula? Hint “Percent to mass Mass to mole Divide by small Multiply ‘til whole”

A compound weighing 298. 12 g consists of 72. 2% magnesium and 27. 8% nitrogen by mass. What is the empirical formula? Percent to mass: Mg – (72. 2%/100)*298. 12 g = 215. 24 g N – (27. 8%/100)*298. 12 g = 82. 88 g Mass to mole: Mg – 215. 24 g * ( 1 mole ) = 8. 86 mole 24. 3 g N – 82. 88 g * ( 1 mole ) = 5. 92 mole 14. 01 g Divide by small: Mg - 8. 86 mole/5. 92 mole = 1. 50 N - 5. 92 mole/5. 92 mole = 1. 00 mole Multiply ‘til whole: Mg – 1. 50 x 2 = 3. 00 N – 1. 00 x 2 = 2. 00 Mg 3 N 2

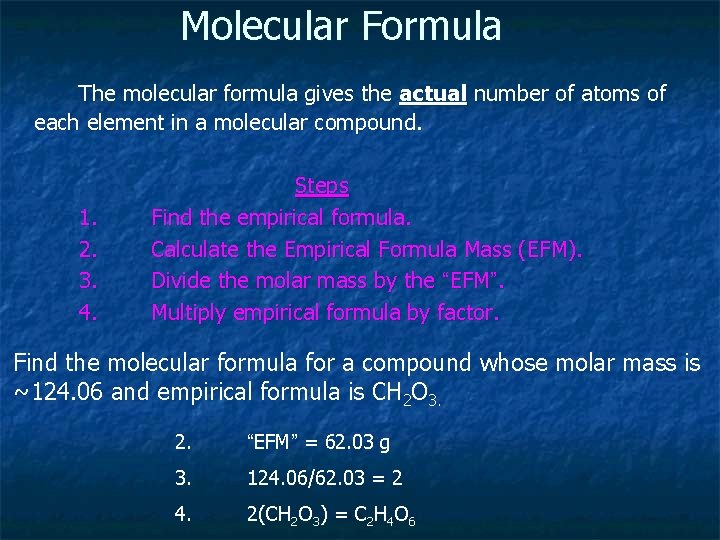
Molecular Formula The molecular formula gives the actual number of atoms of each element in a molecular compound. 1. 2. 3. 4. Steps Find the empirical formula. Calculate the Empirical Formula Mass (EFM). Divide the molar mass by the “EFM”. Multiply empirical formula by factor. Find the molecular formula for a compound whose molar mass is ~124. 06 and empirical formula is CH 2 O 3. 2. “EFM” = 62. 03 g 3. 124. 06/62. 03 = 2 4. 2(CH 2 O 3) = C 2 H 4 O 6

Find the molecular formula for a compound that contains 4. 90 g N and 11. 2 g O. The molar mass of the compound is 92. 0 g/mol. Steps 1. Find the empirical formula. 2. Calculate the Empirical Formula Mass. 3. Divide the molar mass by the “EFM”. 4. Multiply empirical formula by factor.

Empirical formula. A. Find mole amounts. 4. 90 g N x 1 mol N = 0. 350 mol N 14. 01 g N 11. 2 g O x 1 mol O = 0. 700 mol O 16. 00 g O

B. Divide each mole by the smallest mole. N = 0. 350 = 1. 00 mol N 0. 350 O = 0. 700 = 2. 00 mol O 0. 350 Empirical Formula = NO 2 Empirical Formula Mass = 46. 01 g/mol

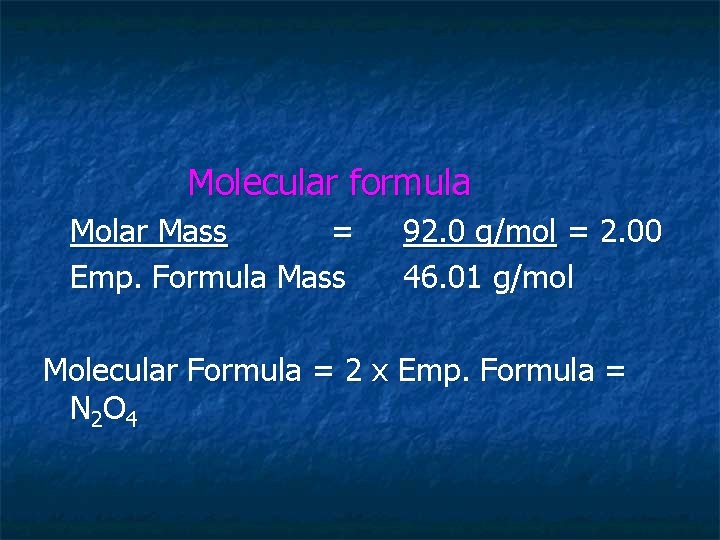
Molecular formula Molar Mass = Emp. Formula Mass 92. 0 g/mol = 2. 00 46. 01 g/mol Molecular Formula = 2 x Emp. Formula = N 2 O 4

A 528. 39 g compound containing only carbon, hydrogen, and oxygen is found to be 48. 38% carbon and 8. 12% hydrogen by mass. The molar mass of this compound is known to be ~222. 25 g/mol. What is its molecular formula?

A 528. 39 g compound containing only carbon, hydrogen, and oxygen is found to be 48. 38% carbon and 8. 12% hydrogen by mass. The molar mass of this compound is known to be ~222. 25 g/mol. What is its molecular formula? g C – (48. 38/100)*528. 39 g = 255. 64 g g H – (8. 12/100)*528. 39 g = 42. 91 g g O – (43. 5/100)*528. 39 g = 229. 85 g mole C - 255. 64 g * ( 1 mole ) = 21. 29 mol 12. 01 g mole H – 42. 91 g * ( 1 mole ) = 42. 49 mol 1. 01 g mole O – 229. 85 g * ( 1 mole ) = 14. 37 mol 16. 00 g

A 528. 39 g compound containing only carbon, hydrogen, and oxygen is found to be 48. 38% carbon and 8. 12% hydrogen by mass. The molar mass of this compound is known to be ~222. 25 g/mol. What is its molecular formula? From last slide: 21. 29 mol C, 42. 49 mol H, 14. 27 mol O C – 21. 29/14. 27 = 1. 49 H – 42. 49/14. 27 = 2. 98 (esentially 3) O – 14. 27/14. 27 = 1. 00 C – 1. 49 x 2 = 3 H– 3 x 2=6 O– 1 x 2=2 C 3 H 6 O 2

A 528. 39 g compound containing only carbon, hydrogen, and oxygen is found to be 48. 38% carbon and 8. 12% hydrogen by mass. The molar mass of this compound is known to be ~222. 25 g/mol. What is its molecular formula? From last slide: Empirical formula = C 3 H 6 O 2 “EFM” = 74. 09 Molar mass = 222. 24 = ~3 EFM 74. 09 3(C 3 H 6 O 2) = C 9 H 18 O 6