Empirical and Molecular Formulas Empirical Formula What are







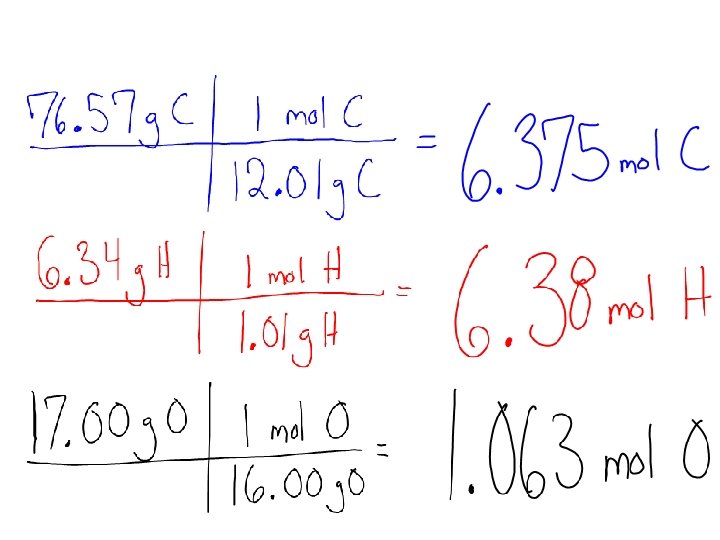










- Slides: 23

Empirical and Molecular Formulas

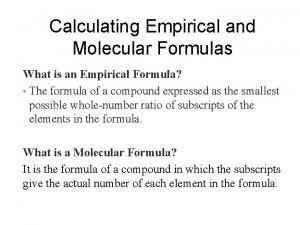
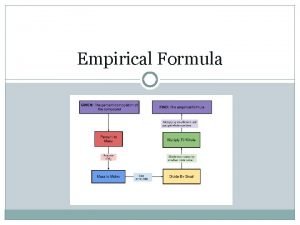
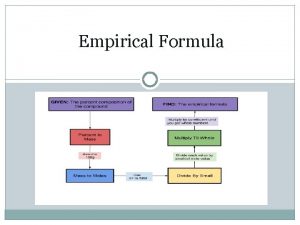
Empirical Formula • What are we talking about? ? ? • Empirical Formula represents the smallest ratio of atoms in a formula. • In other words it represents the simplest chemical formula for a particular compound.

Steps to Solve! • You need to follow these basic steps to determine your answer. • 1) Make sure you are starting in grams • 2) Convert all grams to moles (Molar Mass) • 3) Divide each by the smallest number of moles. • 4) Create whole number ratio of subscripts • 5) Multiply if necessary

Example 1 • In an unknown molecule, there are 4. 15 grams of Carbon and 1. 38 grams of Hydrogen. Determine the empirical formula for the substance! • Step 1) Make sure you are starting in grams • Step 2) Convert each mass in grams to moles.


Now What? • Step 3) Divide each by the smallest number of moles. Set up the Ratio of elements

Solution! • The 1 and the 4 represent the whole number ratio of the elements in the chemical formula. • The 1 represents that there is only one Carbon atom • The 4 represents that there are four Hydrogen atoms • The Solution: The empirical formula is CH 4

• Phenol, a general disinfectant, is 76. 57 % Carbon, 6. 43 % Hydrogen, and 17. 00 % Oxygen. Determine it’s empirical formula. • Step 1) Make sure you are starting in grams……. • How can we go from percent to grams? • Easy! Assume we have a 100 gram sample so there would be how much of each element?
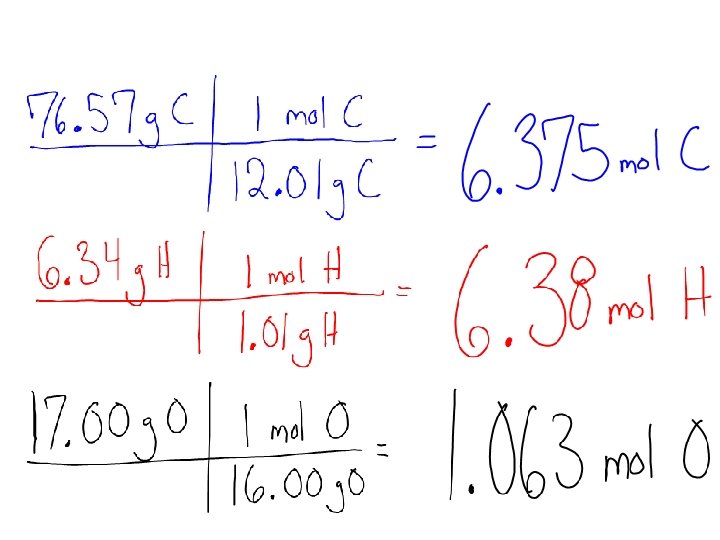
• Step 2) Convert each mass in grams to moles.


Try this! • Element “A” is 78. 1% abundant and has a molar mass of 10. 81 g/mol. Element “B” is 21. 9% abundant and has a molar mass of 1. 01 g/mol. Determine the empirical formula. • • 78. 1 g A X 1 mol/10. 81 g = 7. 22 mol B • 21. 9 g B X 1 mol/1. 01 g = 21. 7 mol H • 7. 22 mol ÷ 7. 22 mol =1 • 21. 7 mol ÷ 7. 22 mol = 3. 01 • Ratio of 1: 3 • AB 3

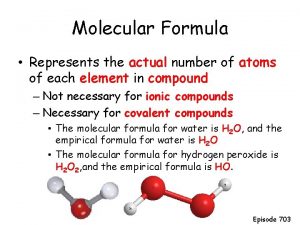
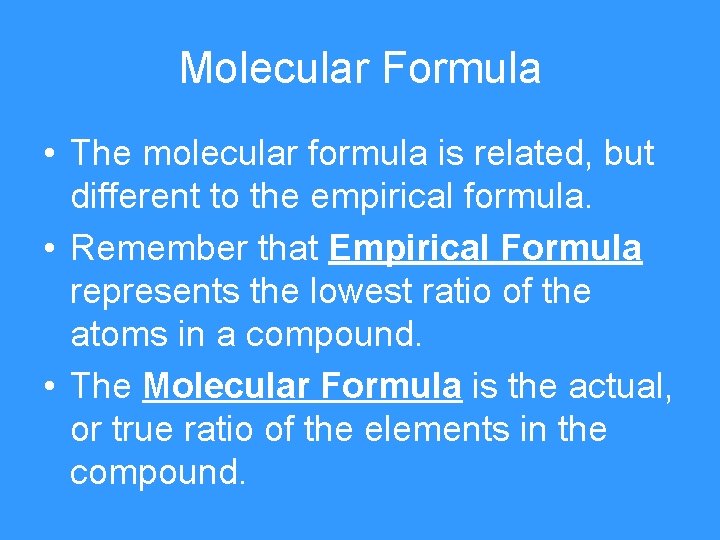
Molecular Formula • The molecular formula is related, but different to the empirical formula. • Remember that Empirical Formula represents the lowest ratio of the atoms in a compound. • The Molecular Formula is the actual, or true ratio of the elements in the compound.

Needs and Steps! • 1. In order to solve you need the molar mass of the molecular formula for a compound in g/mol. • 2. Determine the empirical formula. • 3. Calculate the molar mass of the empirical formula • 4. Divide the molar mass of the molecular formula by the molar mass of the empirical formula. Apply ratio to all subscripts.

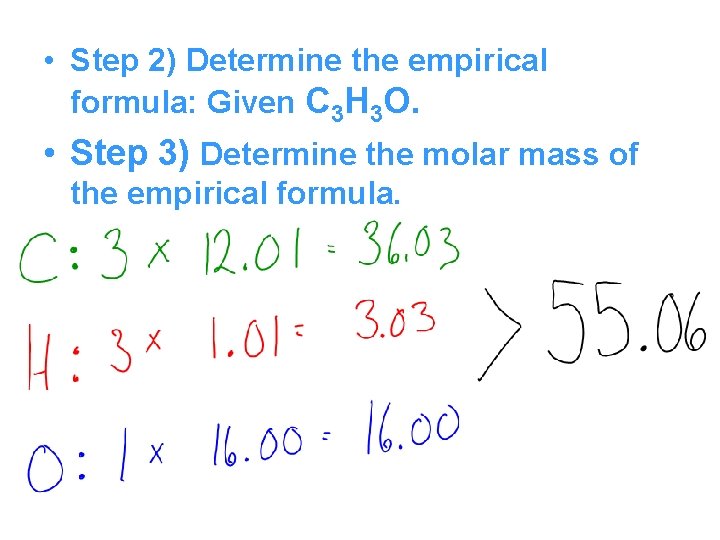
Example • The empirical formula for hydroquinone, a chemical used in photography, is C 3 H 3 O. The molecular weight of the compound Is 110 g/mol. Determine the molecular formula. • Step 1) Determine the molecular weight of the compound: Given at 110 g/mol.

• Step 2) Determine the empirical formula: Given C 3 H 3 O. • Step 3) Determine the molar mass of the empirical formula.

Think…I Know it’s hard… • Step 4) Think about the molar mass of the molecular formula compared to the molar mass of the empirical formula. • Mol. Formula Emp. Formula • 110 g/mol 55. 06 g/mol • Divide and get the ratio • 110 g/mol / 55. 06 g/mol = 2

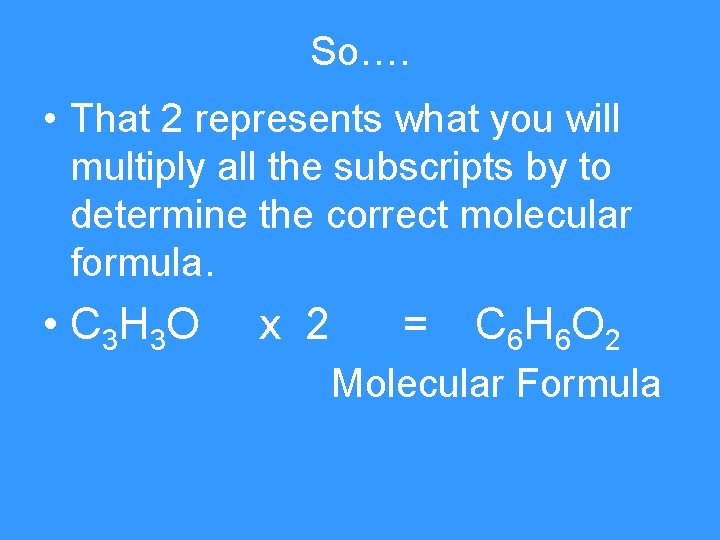
So…. • That 2 represents what you will multiply all the subscripts by to determine the correct molecular formula. • C 3 H 3 O x 2 = C 6 H 6 O 2 Molecular Formula

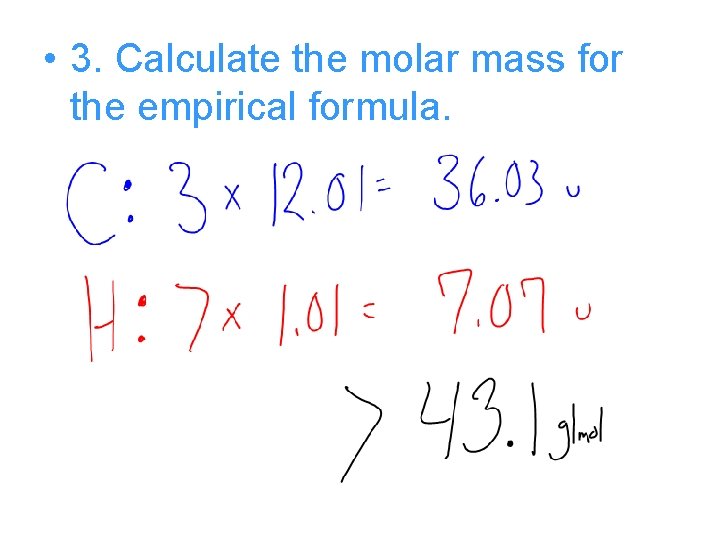
Try this • The empirical formula for a compound is C 3 H 7. If the molecular weight is 86 g/mol, then what is the molecular formula? • 1. The molecular weight is given: – 86 g/mol • 2. The empirical formula is given: - C 3 H 7

• 3. Calculate the molar mass for the empirical formula.

Compare the two…. • The Molecular weight was 86 g/mol • The Empirical weight was 43. 1 g/mol • Divide Molecular by Empirical to determine the ratio! • 86 g/mol / 43. 1 g/mol = 2

• So 2 is the ratio, multiply all the subscripts by 2 • C 3 H 7 x 2 = C 6 H 14 is the Mol. Formula

Practice! A) Empirical Formula is S Molecular weight is 256 grams per mol B) Empirical Formula is NO 2 Molecular Weight is 46 g/mol

 Mikael ferm
Mikael ferm Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Molar mass of ibuprofen
Molar mass of ibuprofen Empirical formula poem
Empirical formula poem Cho empirical formula
Cho empirical formula Molecular formula = n × empirical formula
Molecular formula = n × empirical formula Empirical formula pogil
Empirical formula pogil Empirical formula of ethane
Empirical formula of ethane Empirical formula rhyme
Empirical formula rhyme Empirical formula vs molecular formula
Empirical formula vs molecular formula How to find molecular formula
How to find molecular formula Molecular formula
Molecular formula Empirical formula to molecular formula
Empirical formula to molecular formula How to find empirical formula from percentages
How to find empirical formula from percentages Empirical and molecular formula quiz
Empirical and molecular formula quiz Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical formula poem
Empirical formula poem Naming compounds and writing formulas
Naming compounds and writing formulas Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Lesson 28: sniffing around molecular formulas answer key
Lesson 28: sniffing around molecular formulas answer key Determine the empirical formula
Determine the empirical formula