Empirical and molecular formulas The empirical formula is

- Slides: 9

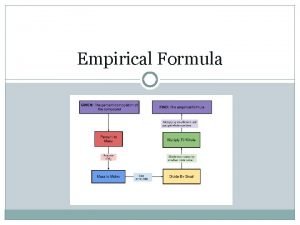
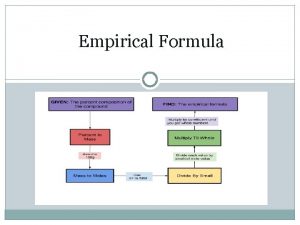
Empirical and molecular formulas Ø Ø Ø The empirical formula is the simplest ratio of atoms within a chemical compound. Example: Find the empirical formula of the compound which has the following percentage by mass composition: Carbon 12. 12%; Oxygen 16. 16%; Chlorine 71. 17%. Suppose 3. 2 g of sulfur reacts with oxygen to produce 6. 4 g of sulfur oxide. What is the formula of the oxide? Use the fact that the Ar of sulfur is 32 and the Ar of oxygen is 16.

The molecular formula is the representation of the actual whole number ratio between the elements of the compound. Ø Ø Ø A molecule has the empirical formula CH 2 O and a relative molecular mass of 60. What is its molecular formula? A molecule has the following composition by mass: N: 25. 9%, O: 74. 1%. What is its empirical formula? An unknown sugar is found to have a formula mass of 180. 18 g/mol. The sugar contains: 40. 0 % C, 6. 7 % H and 53. 3 % O. Find the empirical and molecular formula of this sugar.

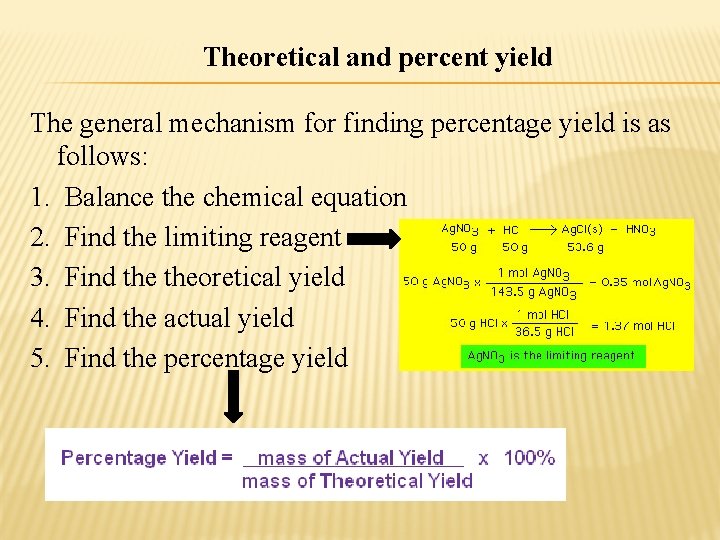
Theoretical and percent yield The general mechanism for finding percentage yield is as follows: 1. Balance the chemical equation 2. Find the limiting reagent 3. Find theoretical yield 4. Find the actual yield 5. Find the percentage yield


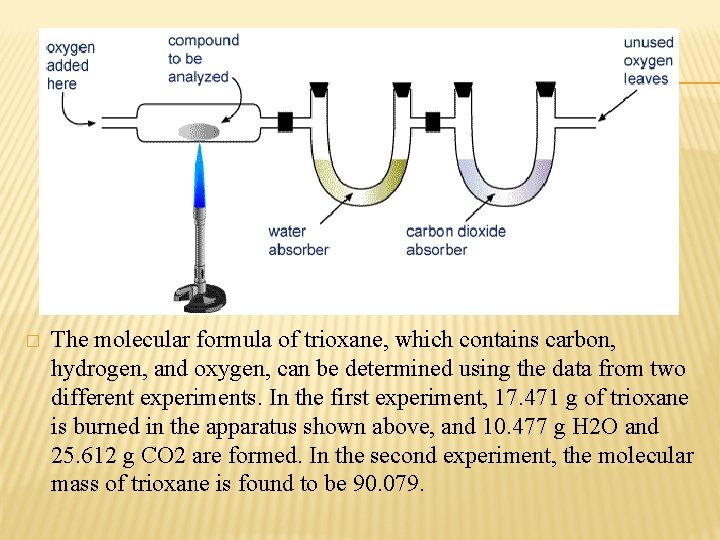
� The molecular formula of trioxane, which contains carbon, hydrogen, and oxygen, can be determined using the data from two different experiments. In the first experiment, 17. 471 g of trioxane is burned in the apparatus shown above, and 10. 477 g H 2 O and 25. 612 g CO 2 are formed. In the second experiment, the molecular mass of trioxane is found to be 90. 079.

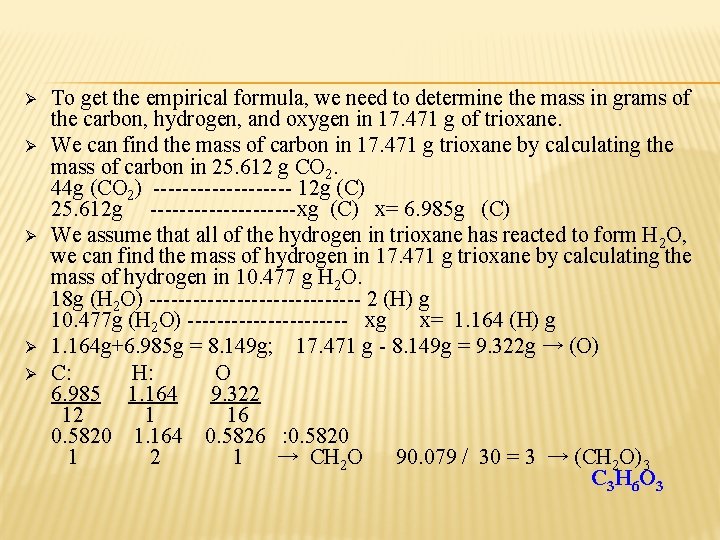
Ø Ø Ø To get the empirical formula, we need to determine the mass in grams of the carbon, hydrogen, and oxygen in 17. 471 g of trioxane. We can find the mass of carbon in 17. 471 g trioxane by calculating the mass of carbon in 25. 612 g CO 2. 44 g (CO 2) ---------- 12 g (C) 25. 612 g ----------xg (C) x= 6. 985 g (C) We assume that all of the hydrogen in trioxane has reacted to form H 2 O, we can find the mass of hydrogen in 17. 471 g trioxane by calculating the mass of hydrogen in 10. 477 g H 2 O. 18 g (H 2 O) --------------- 2 (H) g 10. 477 g (H 2 O) ----------- xg x= 1. 164 (H) g 1. 164 g+6. 985 g = 8. 149 g; 17. 471 g - 8. 149 g = 9. 322 g → (O) C: H: O 6. 985 1. 164 9. 322 12 1 16 0. 5820 1. 164 0. 5826 : 0. 5820 1 2 1 → CH 2 O 90. 079 / 30 = 3 → (CH 2 O)3 C 3 H 6 O 3

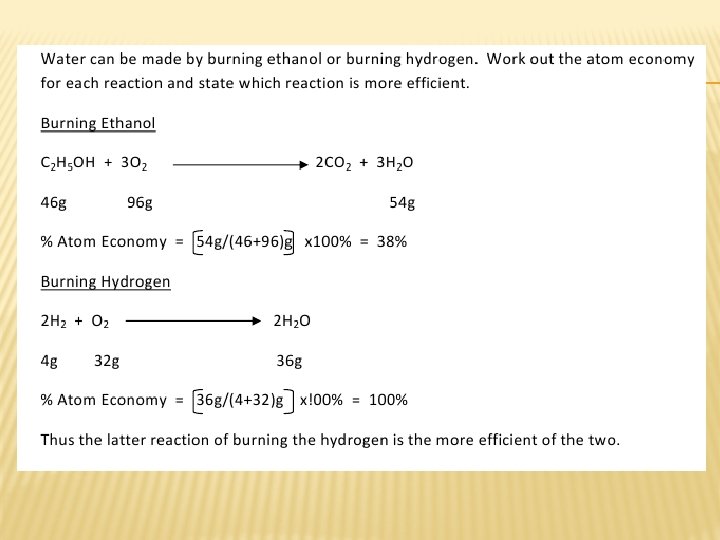
Atom economy The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. For the general chemical reaction: reactants ↔ desired product + waste products The atom economy can be calculated in either of two ways: Equation (I) is identical to equation (II) because by the Law of Mass Conservation: total mass of all reactants = mass of desired product + mass of waste products

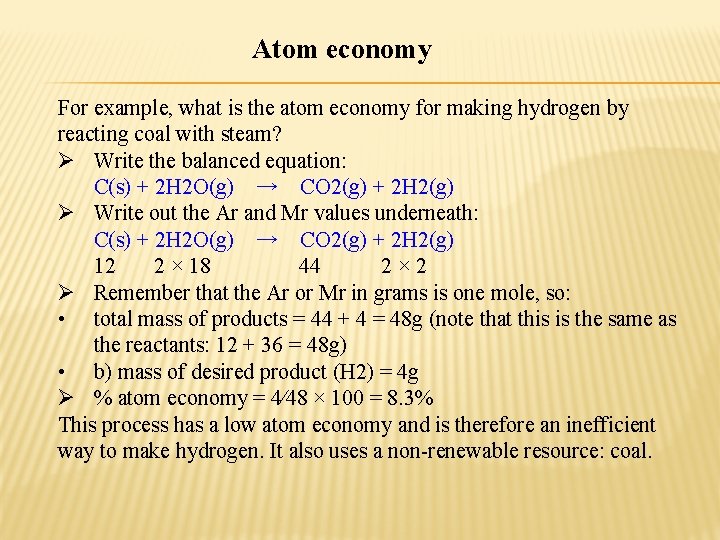
Atom economy For example, what is the atom economy for making hydrogen by reacting coal with steam? Ø Write the balanced equation: C(s) + 2 H 2 O(g) → CO 2(g) + 2 H 2(g) Ø Write out the Ar and Mr values underneath: C(s) + 2 H 2 O(g) → CO 2(g) + 2 H 2(g) 12 2 × 18 44 2× 2 Ø Remember that the Ar or Mr in grams is one mole, so: • total mass of products = 44 + 4 = 48 g (note that this is the same as the reactants: 12 + 36 = 48 g) • b) mass of desired product (H 2) = 4 g Ø % atom economy = 4⁄48 × 100 = 8. 3% This process has a low atom economy and is therefore an inefficient way to make hydrogen. It also uses a non-renewable resource: coal.

 Empirical and molecular formula worksheet
Empirical and molecular formula worksheet Find the empirical/simplest formula fe
Find the empirical/simplest formula fe Molecular formula
Molecular formula Molecular formula
Molecular formula Empirical formula chemistry
Empirical formula chemistry Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil How to find molecular formula
How to find molecular formula Compound ratio
Compound ratio Empirical formula vs molecular formula
Empirical formula vs molecular formula Empirical formula with percentages
Empirical formula with percentages