Calculating Empirical and Molecular Formulas What is an
- Slides: 7

Calculating Empirical and Molecular Formulas What is an Empirical Formula? • The formula of a compound expressed as the smallest possible whole-number ratio of subscripts of the elements in the formula. What is a Molecular Formula? It is the formula of a compound in which the subscripts give the actual number of each element in the formula.

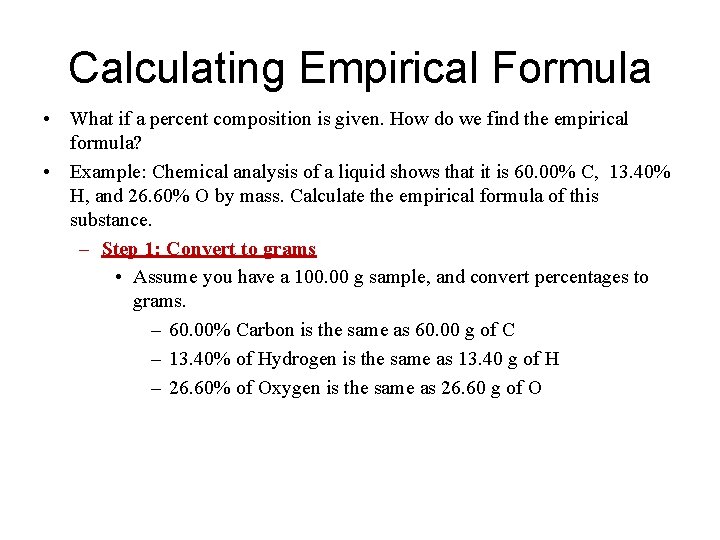
Calculating Empirical Formula • What if a percent composition is given. How do we find the empirical formula? • Example: Chemical analysis of a liquid shows that it is 60. 00% C, 13. 40% H, and 26. 60% O by mass. Calculate the empirical formula of this substance. – Step 1: Convert to grams • Assume you have a 100. 00 g sample, and convert percentages to grams. – 60. 00% Carbon is the same as 60. 00 g of C – 13. 40% of Hydrogen is the same as 13. 40 g of H – 26. 60% of Oxygen is the same as 26. 60 g of O

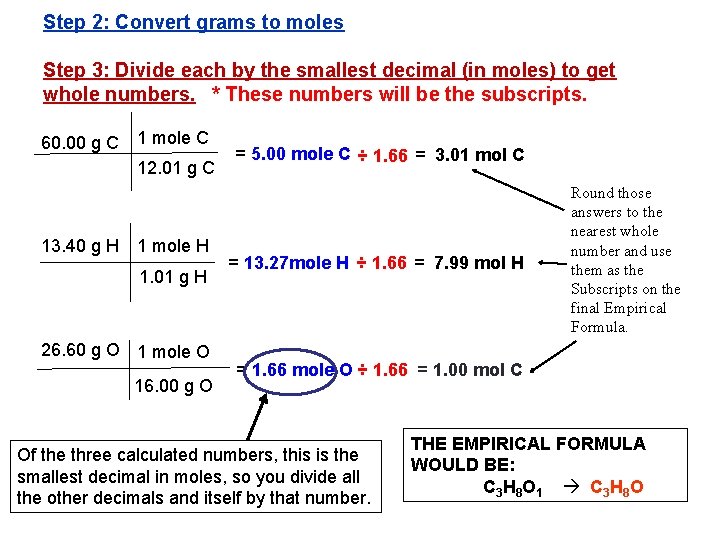
Step 2: Convert grams to moles Step 3: Divide each by the smallest decimal (in moles) to get whole numbers. * These numbers will be the subscripts. 60. 00 g C 1 mole C 12. 01 g C 13. 40 g H 1 mole H 1. 01 g H 26. 60 g O 1 mole O 16. 00 g O = 5. 00 mole C ÷ 1. 66 = 3. 01 mol C = 13. 27 mole H ÷ 1. 66 = 7. 99 mol H Round those answers to the nearest whole number and use them as the Subscripts on the final Empirical Formula. = 1. 66 mole O ÷ 1. 66 = 1. 00 mol C Of the three calculated numbers, this is the smallest decimal in moles, so you divide all the other decimals and itself by that number. THE EMPIRICAL FORMULA WOULD BE: C 3 H 8 O 1 C 3 H 8 O

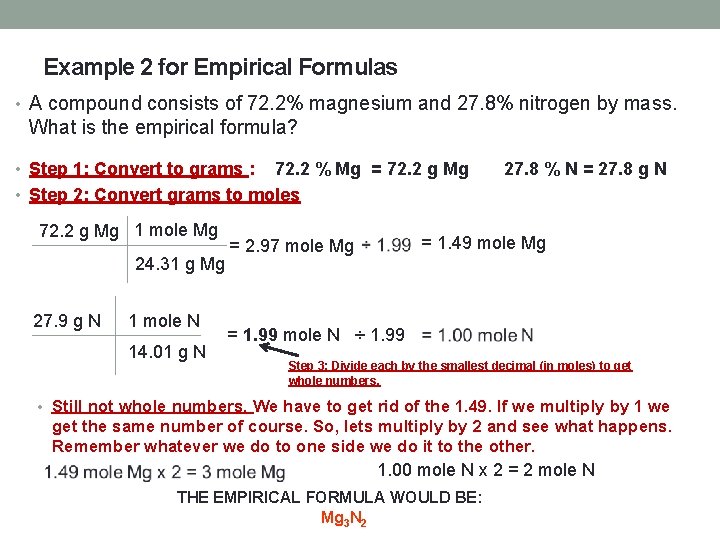
Example 2 for Empirical Formulas • A compound consists of 72. 2% magnesium and 27. 8% nitrogen by mass. What is the empirical formula? • Step 1: Convert to grams : 72. 2 % Mg = 72. 2 g Mg • Step 2: Convert grams to moles 72. 2 g Mg 1 mole Mg 24. 31 g Mg 27. 9 g N 1 mole N 14. 01 g N 27. 8 % N = 27. 8 g N = 1. 49 mole Mg = 2. 97 mole Mg = 1. 99 mole N ÷ 1. 99 Step 3: Divide each by the smallest decimal (in moles) to get whole numbers. • Still not whole numbers. We have to get rid of the 1. 49. If we multiply by 1 we get the same number of course. So, lets multiply by 2 and see what happens. Remember whatever we do to one side we do it to the other. 1. 00 mole N x 2 = 2 mole N THE EMPIRICAL FORMULA WOULD BE: Mg 3 N 2

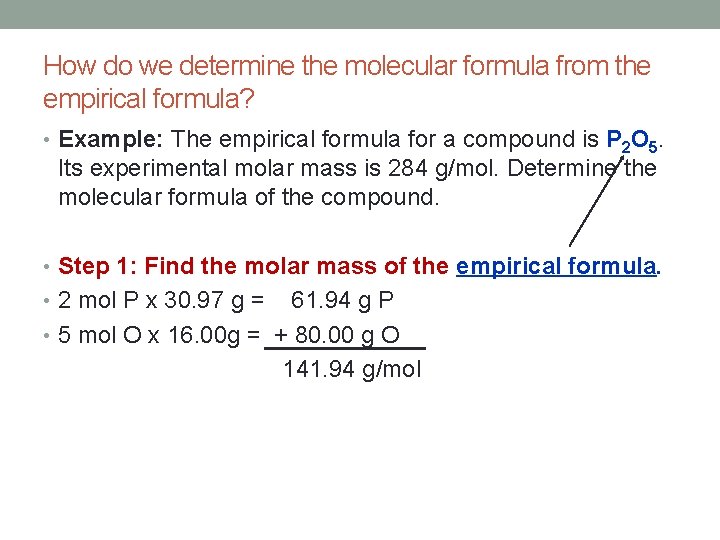
How do we determine the molecular formula from the empirical formula? • Example: The empirical formula for a compound is P 2 O 5. Its experimental molar mass is 284 g/mol. Determine the molecular formula of the compound. • Step 1: Find the molar mass of the empirical formula. • 2 mol P x 30. 97 g = 61. 94 g P • 5 mol O x 16. 00 g = + 80. 00 g O 141. 94 g/mol

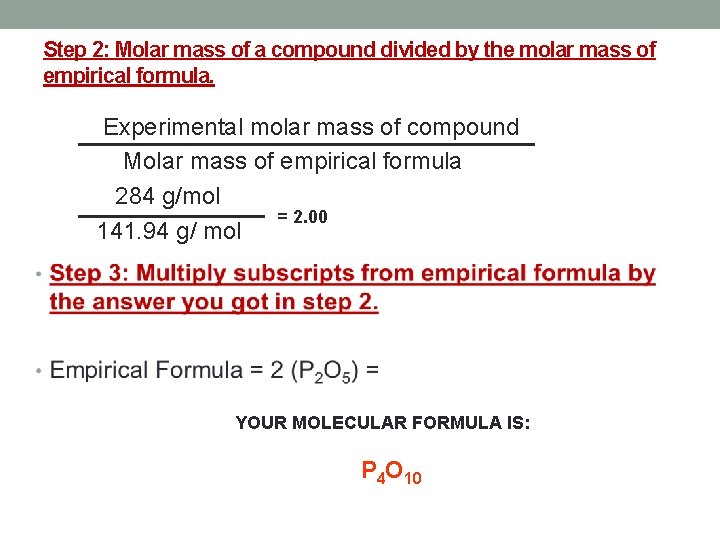
Step 2: Molar mass of a compound divided by the molar mass of empirical formula. Experimental molar mass of compound Molar mass of empirical formula 284 g/mol = 2. 00 141. 94 g/ mol YOUR MOLECULAR FORMULA IS: P 4 O 10

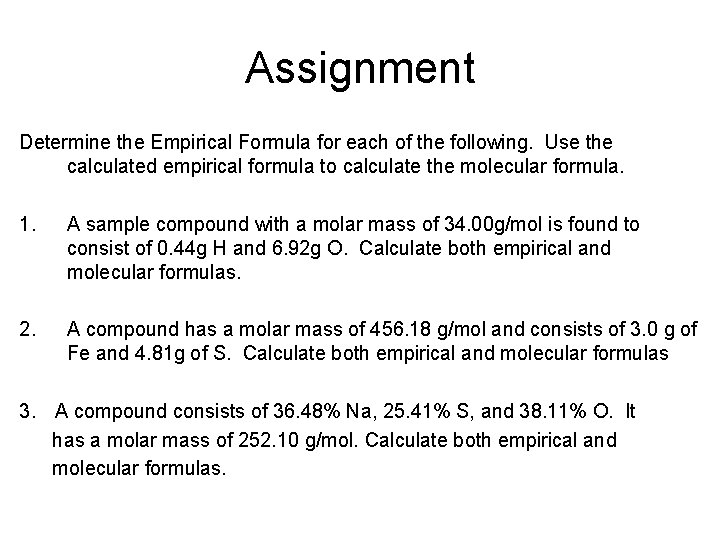
Assignment Determine the Empirical Formula for each of the following. Use the calculated empirical formula to calculate the molecular formula. 1. A sample compound with a molar mass of 34. 00 g/mol is found to consist of 0. 44 g H and 6. 92 g O. Calculate both empirical and molecular formulas. 2. A compound has a molar mass of 456. 18 g/mol and consists of 3. 0 g of Fe and 4. 81 g of S. Calculate both empirical and molecular formulas 3. A compound consists of 36. 48% Na, 25. 41% S, and 38. 11% O. It has a molar mass of 252. 10 g/mol. Calculate both empirical and molecular formulas.