EMPIRICAL AND MOLECULAR FORMULA DEFINITIONS Empirical Formula The
- Slides: 8

EMPIRICAL AND MOLECULAR FORMULA

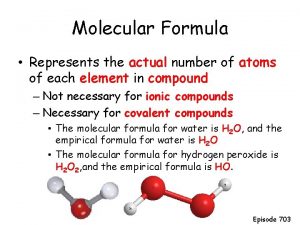
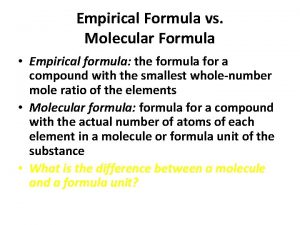
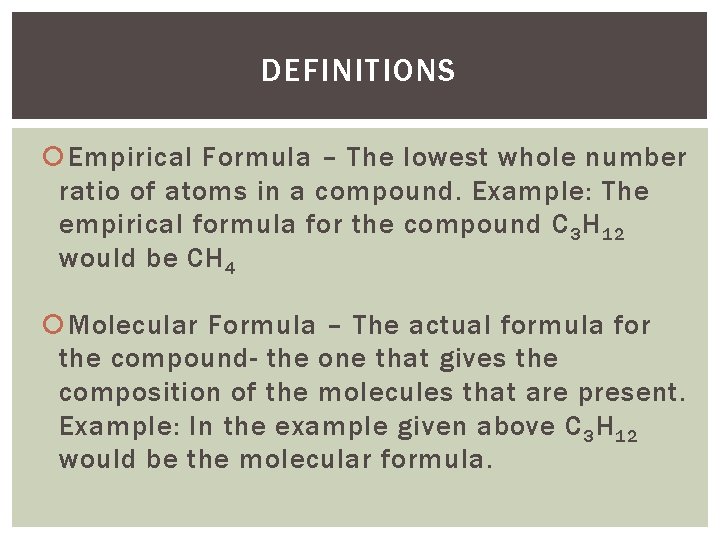
DEFINITIONS Empirical Formula – The lowest whole number ratio of atoms in a compound. Example: The empirical formula for the compound C 3 H 12 would be CH 4 Molecular Formula – The actual formula for the compound- the one that gives the composition of the molecules that are present. Example: In the example given above C 3 H 12 would be the molecular formula.

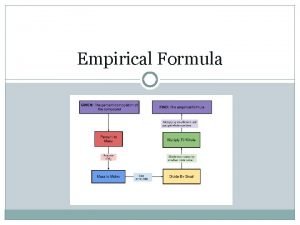
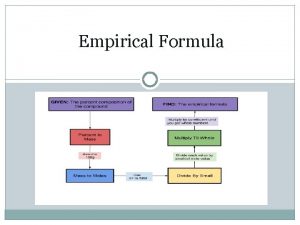
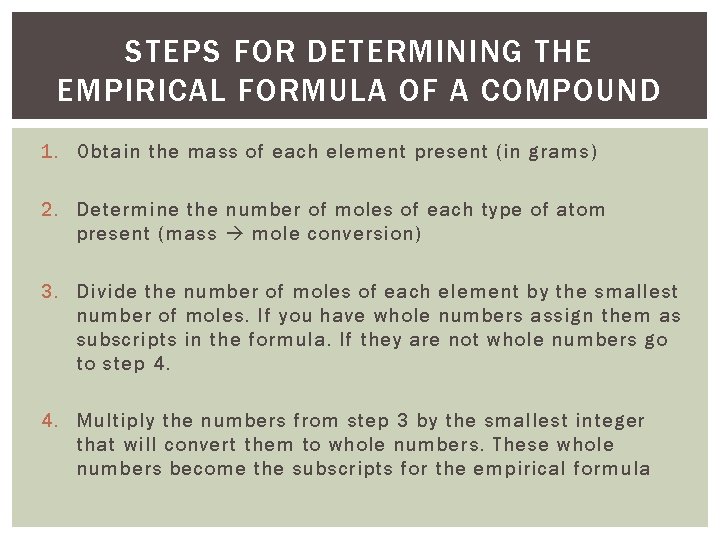
STEPS FOR DETERMINING THE EMPIRICAL FORMULA OF A COMPOUND 1. Obtain the mass of each element present (in grams) 2. Determine the number of moles of each type of atom present (mass mole conversion) 3. Divide the number of moles of each element by the smallest number of moles. If you have whole numbers assign them as subscripts in the formula. If they are not whole numbers go to step 4. 4. Multiply the numbers from step 3 by the smallest integer that will convert them to whole numbers. These whole numbers become the subscripts for the empirical formula

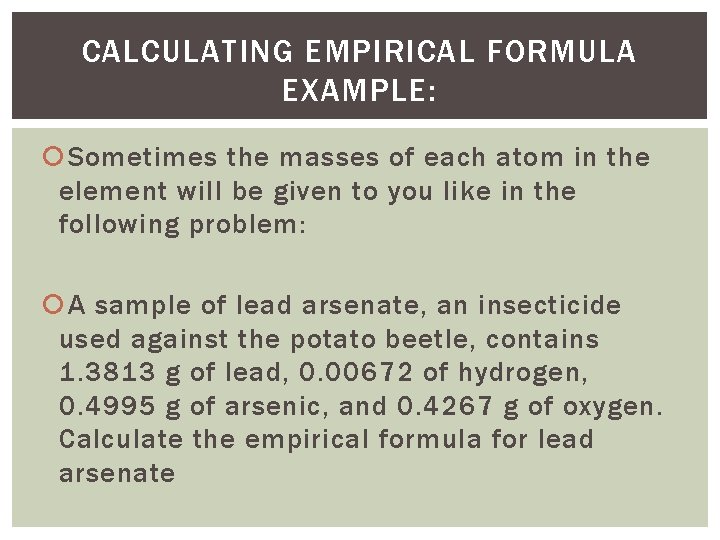
CALCULATING EMPIRICAL FORMULA EXAMPLE: Sometimes the masses of each atom in the element will be given to you like in the following problem: A sample of lead arsenate, an insecticide used against the potato beetle, contains 1. 3813 g of lead, 0. 00672 of hydrogen, 0. 4995 g of arsenic, and 0. 4267 g of oxygen. Calculate the empirical formula for lead arsenate

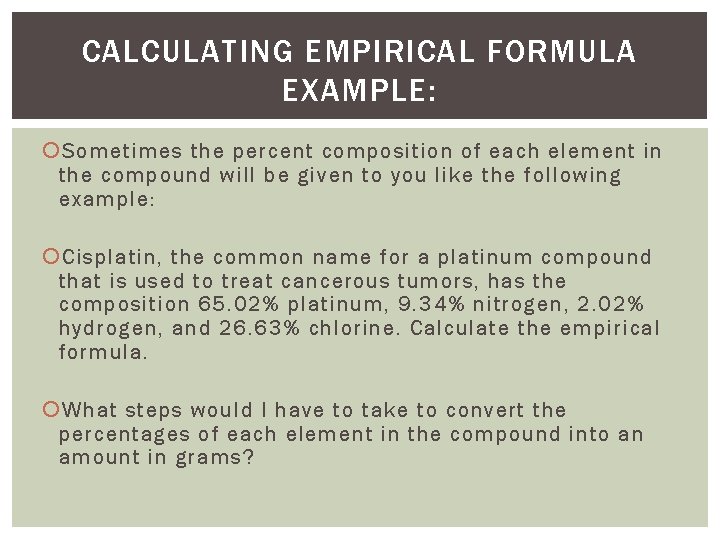
CALCULATING EMPIRICAL FORMULA EXAMPLE: Sometimes the percent composition of each element in the compound will be given to you like the following example: Cisplatin, the common name for a platinum compound that is used to treat cancerous tumors, has the composition 65. 02% platinum, 9. 34% nitrogen, 2. 02% hydrogen, and 26. 63% chlorine. Calculate the empirical formula. What steps would I have to take to convert the percentages of each element in the compound into an amount in grams?

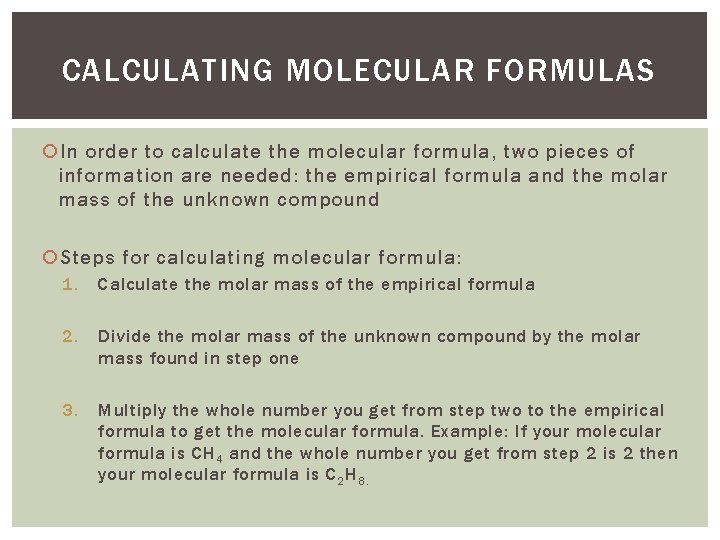
CALCULATING MOLECULAR FORMULAS In order to calculate the molecular formula, two pieces of information are needed: the empirical formula and the molar mass of the unknown compound Steps for calculating molecular formula: 1. Calculate the molar mass of the empirical formula 2. Divide the molar mass of the unknown compound by the molar mass found in step one 3. Multiply the whole number you get from step two to the empirical formula to get the molecular formula. Example: If your molecular formula is CH 4 and the whole number you get from step 2 is 2 then your molecular formula is C 2 H 8.

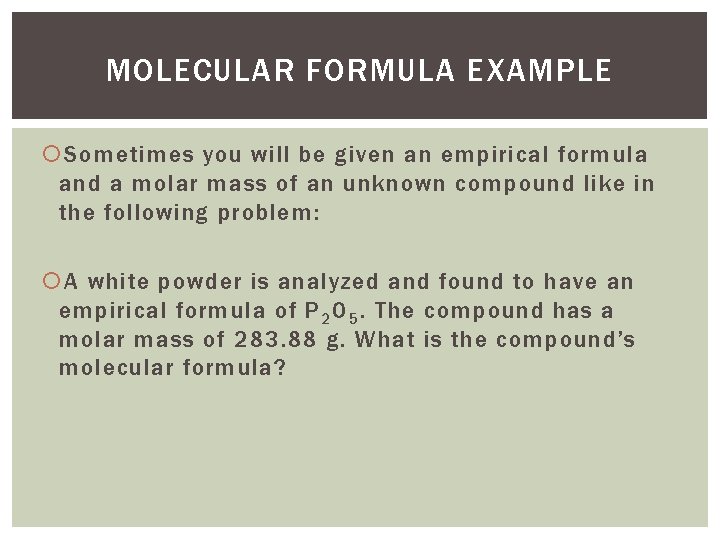
MOLECULAR FORMULA EXAMPLE Sometimes you will be given an empirical formula and a molar mass of an unknown compound like in the following problem: A white powder is analyzed and found to have an empirical formula of P 2 O 5. The compound has a molar mass of 283. 88 g. What is the compound’s molecular formula?

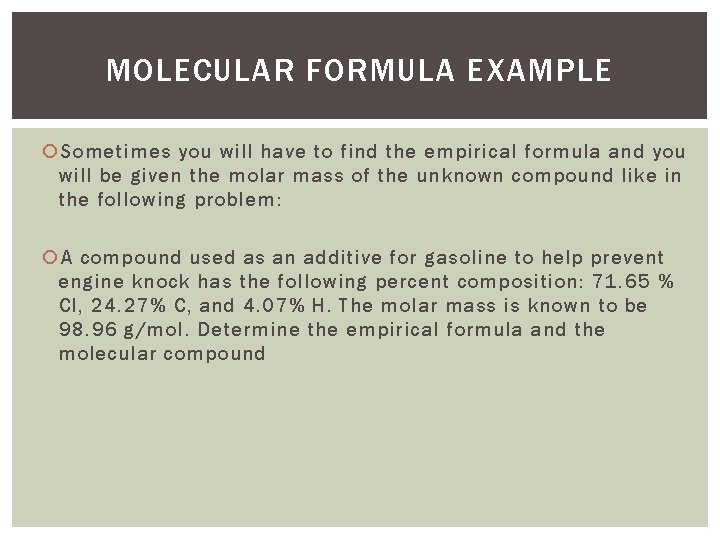
MOLECULAR FORMULA EXAMPLE Sometimes you will have to find the empirical formula and you will be given the molar mass of the unknown compound like in the following problem: A compound used as an additive for gasoline to help prevent engine knock has the following percent composition: 71. 65 % Cl, 24. 27% C, and 4. 07% H. The molar mass is known to be 98. 96 g/mol. Determine the empirical formula and the molecular compound