Foundation year Lecture 2 Molecular Mass and Formula











- Slides: 37

Foundation year Lecture 2 Molecular Mass and Formula mass 101 CHEM Done by: L. Amal Abu-Mostafa

Session Objectives

Session Objectives • • • - Atoms - Atomic Symbols and Models - Symbols of some common elements - Using the Periodic Table - Atomic mass - Molecule of elements - Molecule of compounds - Ions - Molecular mass / Formula unit mass

Atom: • The fundamental building block of matter is the atom. • An atom is the smallest particle of an element that may or may not exist independently and retains all its chemical properties. • Atoms are very small in size and smaller than anything we can imagine or compare with.

• Atomic radius is measured in nanometers (nm) • 1 nanometer = 10 -9 m or • 1 meter = 109 nm • Examples: • The atomic radius of an atom of hydrogen is 10 -10 m. • The radius of a molecule of water is 10 -9 m.

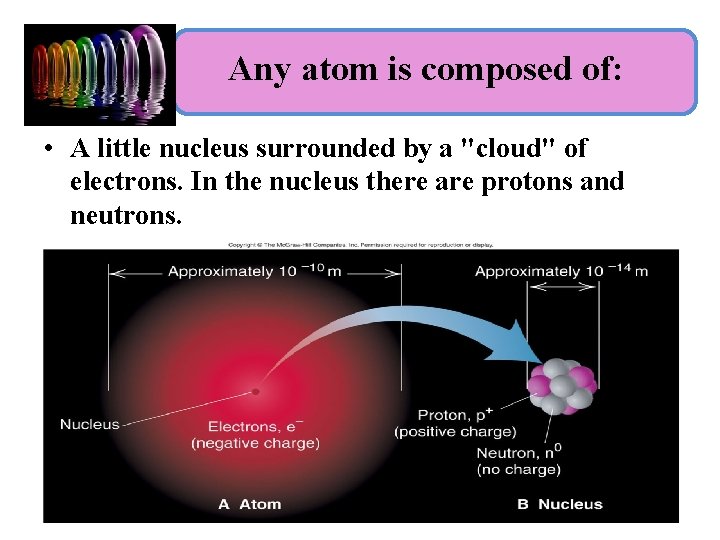
Any atom is composed of: • A little nucleus surrounded by a "cloud" of electrons. In the nucleus there are protons and neutrons.

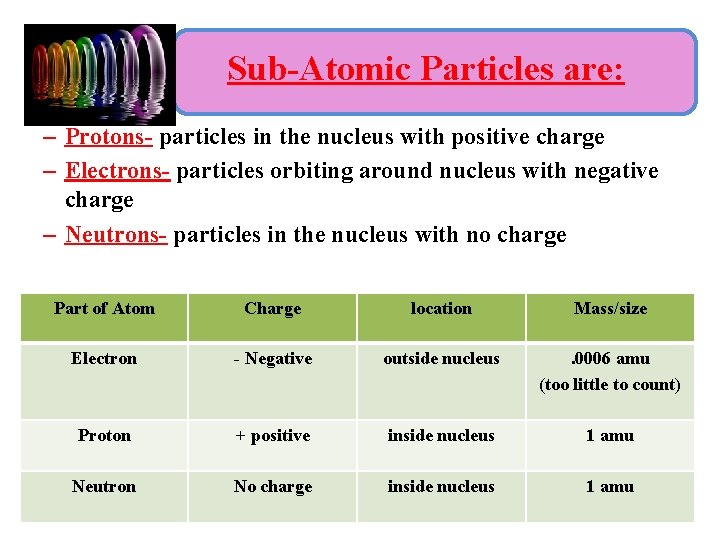
Sub-Atomic Particles are: – Protons- particles in the nucleus with positive charge – Electrons- particles orbiting around nucleus with negative charge – Neutrons- particles in the nucleus with no charge Part of Atom Charge location Mass/size Electron - Negative outside nucleus . 0006 amu (too little to count) Proton + positive inside nucleus 1 amu Neutron No charge inside nucleus 1 amu

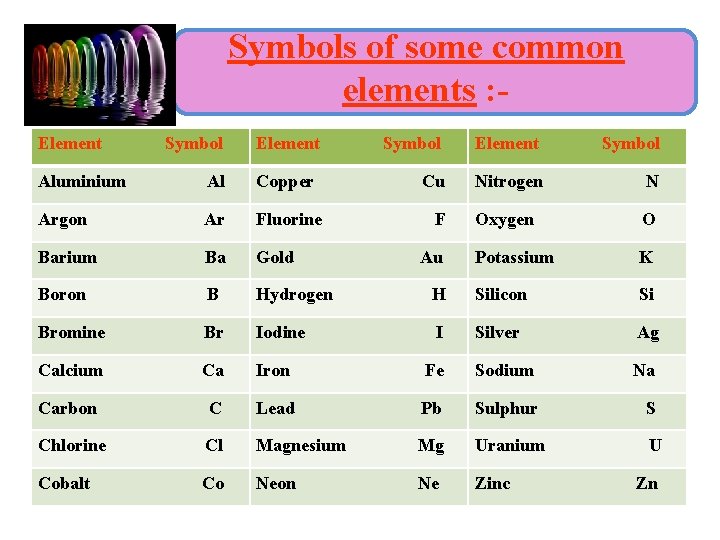
Atomic Symbols and Models • An atomic symbol is a one- or two-letter notation used to represent an element. • If the symbol has only one letter it should be written as capital letter and if the symbol has two letters then the first letter should be capital letter and the second letter should be small letter. • For example, chlorine has the symbol Cl

Symbols of some common elements : Element Symbol Cu Nitrogen N Oxygen O Potassium K Aluminium Al Copper Argon Ar Fluorine Barium Ba Gold Boron B Hydrogen H Silicon Si Bromine Br Iodine I Silver Ag Calcium Ca Iron Fe Sodium Na Carbon C Lead Pb Sulphur S Chlorine Cl Magnesium Mg Uranium U Cobalt Co Neon Ne Zinc F Au Zn

Using the Periodic Table

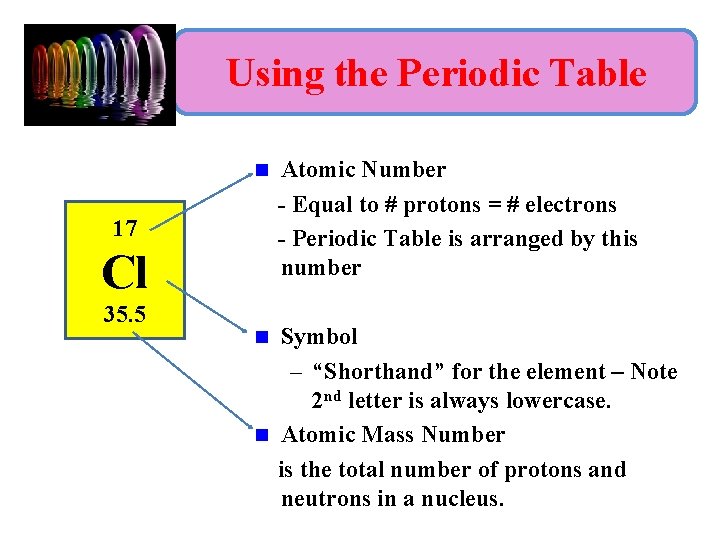
Using the Periodic Table n 17 Cl 35. 5 Atomic Number - Equal to # protons = # electrons - Periodic Table is arranged by this number Symbol – “Shorthand” for the element – Note 2 nd letter is always lowercase. n Atomic Mass Number is the total number of protons and neutrons in a nucleus. n

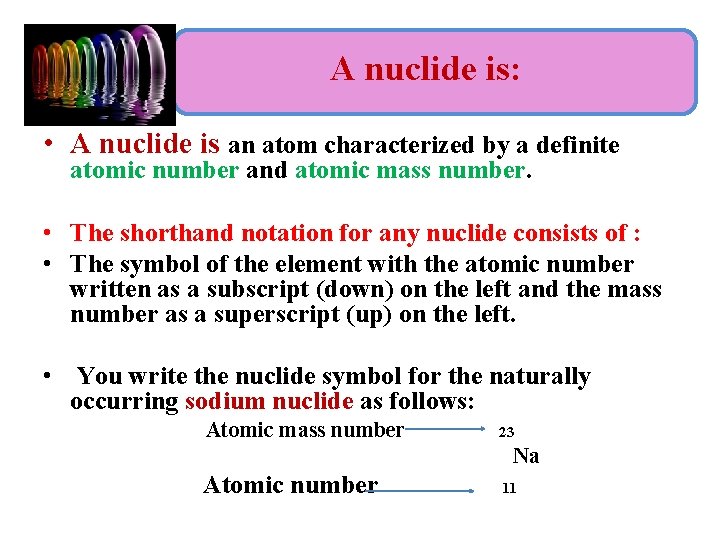
A nuclide is: • A nuclide is an atom characterized by a definite atomic number and atomic mass number. • The shorthand notation for any nuclide consists of : • The symbol of the element with the atomic number written as a subscript (down) on the left and the mass number as a superscript (up) on the left. • You write the nuclide symbol for the naturally occurring sodium nuclide as follows: Atomic mass number 23 Na Atomic number 11

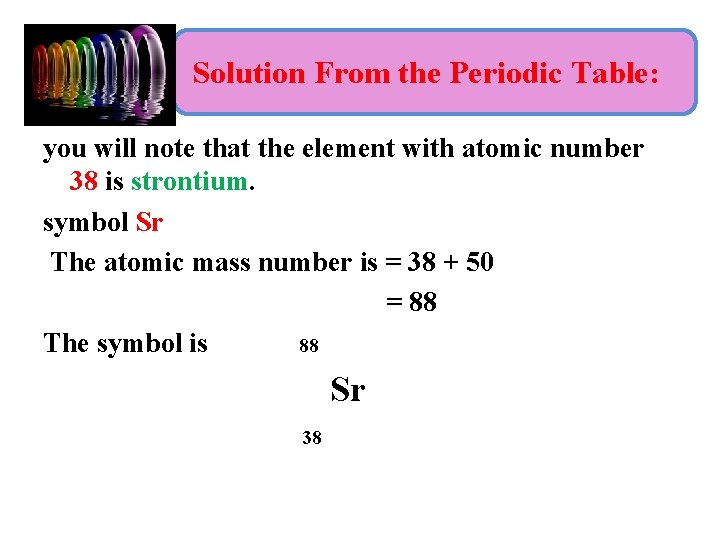
Example 1: • What is the nuclide symbol for a nucleus that contains 38 protons and 50 neutrons? • Problem Strategy: To solve this problem, we need to keep in mind that the number of protons in the nucleus (the atomic number) is what uniquely identifies an element, and that the mass number is the sum of the protons and neutrons.

Solution From the Periodic Table: you will note that the element with atomic number 38 is strontium. symbol Sr The atomic mass number is = 38 + 50 = 88 The symbol is 88 Sr 38

Example 2: • What is the nuclide symbol for a nucleus that contains 24 protons and 28 neutrons? • What is the nuclide symbol for a nucleus that has the atomic number 53 and 74 neutrons?

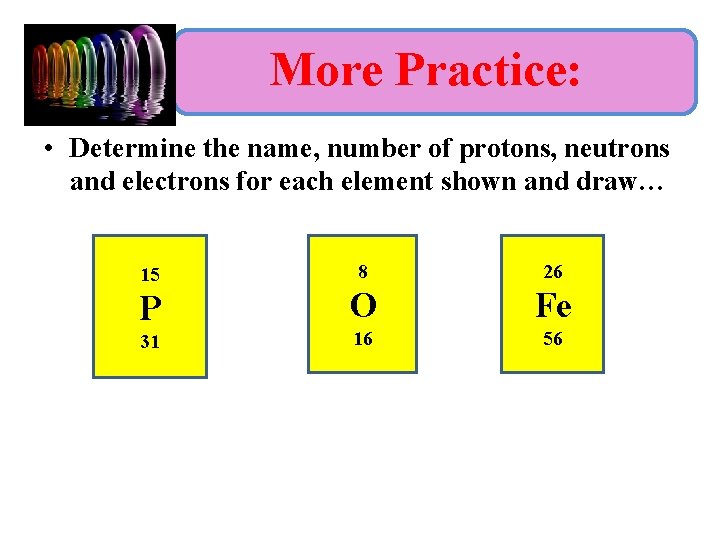
More Practice: • Determine the name, number of protons, neutrons and electrons for each element shown and draw… 15 8 26 P O Fe 16 56 31


Atomic mass : • Since atoms are very small in size its mass is very small and determining its mass is very difficult. • So the mass of an atom is compared with the mass of a standard atom. • The atom which is considered as a standard atom for comparing the masses of other atoms is carbon – 12 atom whose atomic mass is 12 amu (atomic mass unit)or (u). • One atomic mass unit (amu) is the mass of 1/12 th the mass of a carbon – 12 atom.

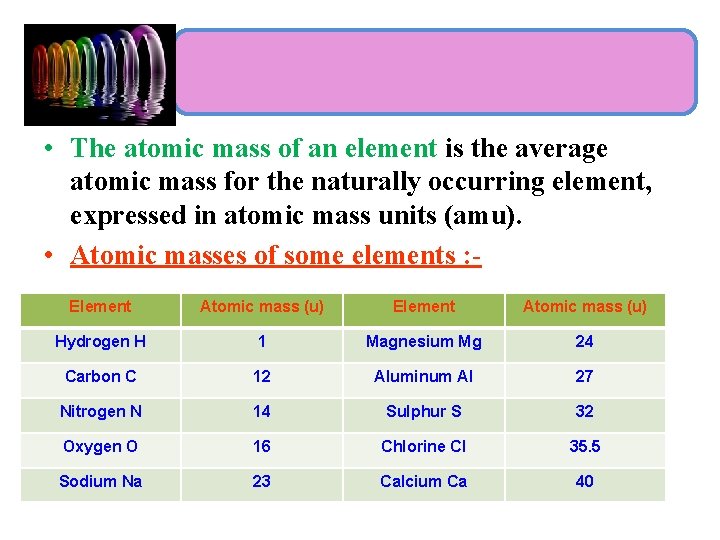
• The atomic mass of an element is the average atomic mass for the naturally occurring element, expressed in atomic mass units (amu). • Atomic masses of some elements : Element Atomic mass (u) Hydrogen H 1 Magnesium Mg 24 Carbon C 12 Aluminum Al 27 Nitrogen N 14 Sulphur S 32 Oxygen O 16 Chlorine Cl 35. 5 Sodium Na 23 Calcium Ca 40

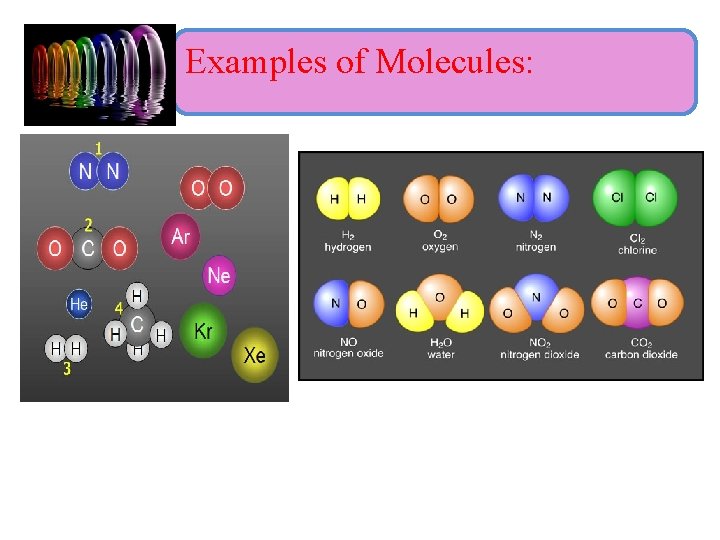
Molecule : • A molecule is the smallest particle of an element or compound which exists independently and shows all the properties of that substance. • A molecule is a definite group of atoms that are chemically bonded together—that is, tightly connected by attractive forces. • Atoms of the same element or different elements can join together to form molecules.

Examples of Molecules:

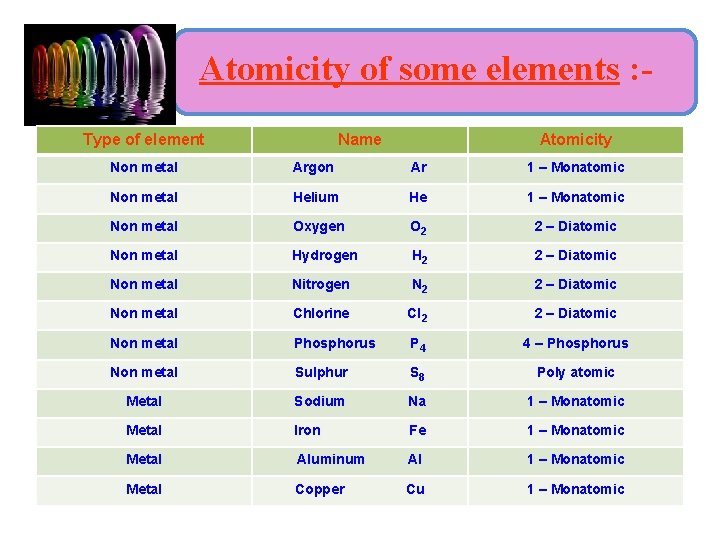
i) Molecule of elements : • Molecule of an element contains atoms of the same element, called homoatomic molecule (O 2). • Molecules of some elements contain only one atom and molecules of some elements contain two or more atoms. • Atomicity of an element : - is the number of atoms present in one molecule of the element.

Atomicity of some elements : Type of element Name Atomicity Non metal Argon Ar 1 – Monatomic Non metal Helium He 1 – Monatomic Non metal Oxygen O 2 2 – Diatomic Non metal Hydrogen H 2 2 – Diatomic Non metal Nitrogen N 2 2 – Diatomic Non metal Chlorine Cl 2 2 – Diatomic Non metal Phosphorus P 4 4 – Phosphorus Non metal Sulphur S 8 Poly atomic Metal Sodium Na 1 – Monatomic Metal Iron Fe 1 – Monatomic Metal Aluminum Al 1 – Monatomic Metal Copper Cu 1 – Monatomic

ii) Molecule of compounds : • Molecule of a compound contains atoms of two or more different types of elements, it is called (heteroatomic molecule).

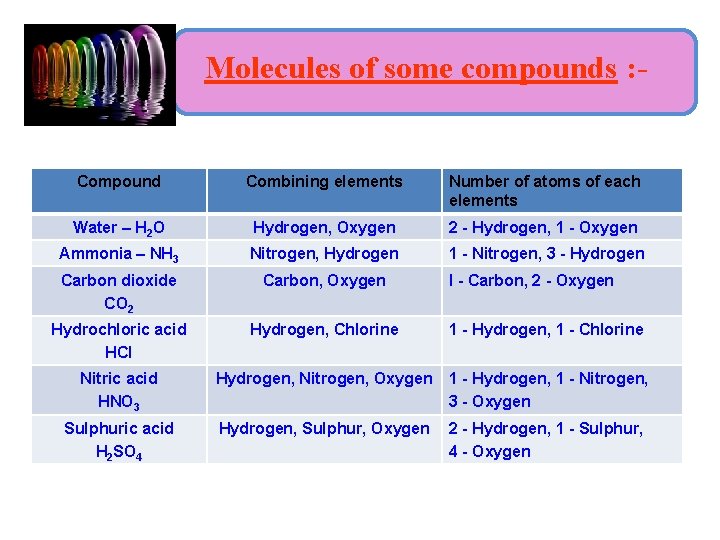
Molecules of some compounds : - Compound Combining elements Number of atoms of each elements Water – H 2 O Hydrogen, Oxygen 2 - Hydrogen, 1 - Oxygen Ammonia – NH 3 Nitrogen, Hydrogen 1 - Nitrogen, 3 - Hydrogen Carbon dioxide CO 2 Carbon, Oxygen Hydrochloric acid HCl Hydrogen, Chlorine 1 - Hydrogen, 1 - Chlorine Nitric acid HNO 3 Hydrogen, Nitrogen, Oxygen 1 - Hydrogen, 1 - Nitrogen, 3 - Oxygen Sulphuric acid H 2 SO 4 Hydrogen, Sulphur, Oxygen 2 - Hydrogen, 1 - Sulphur, 4 - Oxygen I - Carbon, 2 - Oxygen

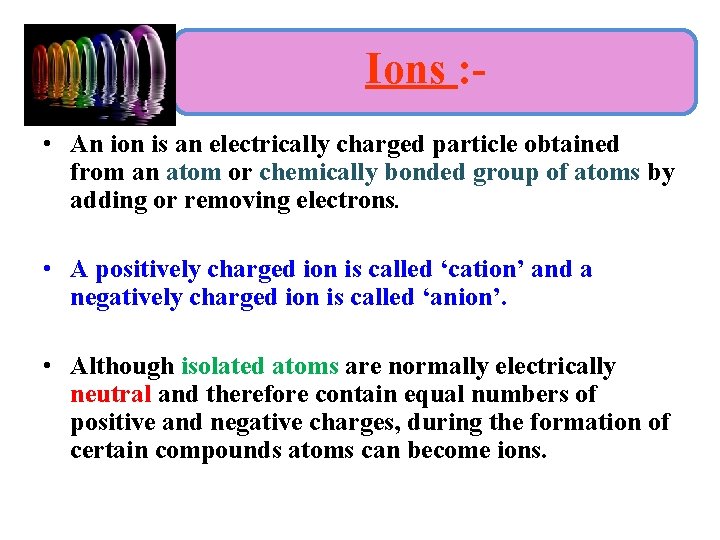
Ions : • An ion is an electrically charged particle obtained from an atom or chemically bonded group of atoms by adding or removing electrons. • A positively charged ion is called ‘cation’ and a negatively charged ion is called ‘anion’. • Although isolated atoms are normally electrically neutral and therefore contain equal numbers of positive and negative charges, during the formation of certain compounds atoms can become ions.

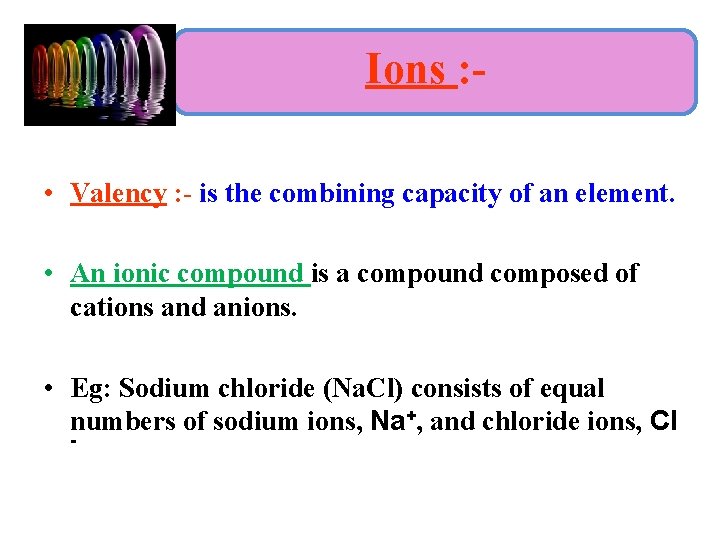
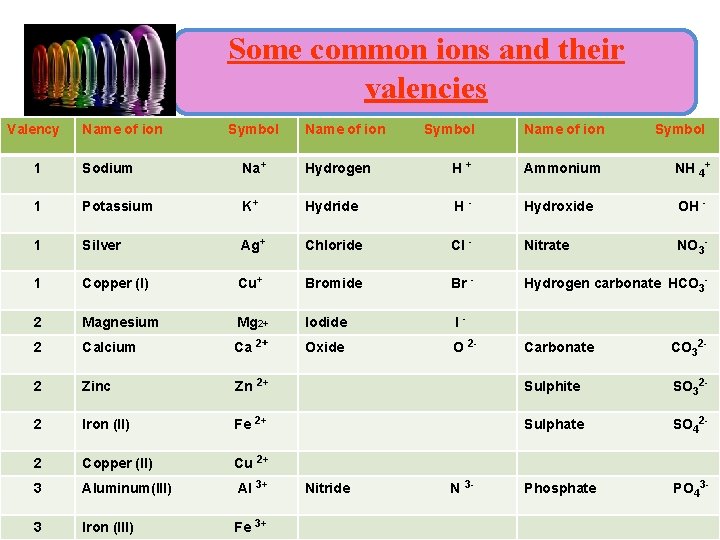
Ions : • Valency : - is the combining capacity of an element. • An ionic compound is a compound composed of cations and anions. • Eg: Sodium chloride (Na. Cl) consists of equal numbers of sodium ions, Na+, and chloride ions, Cl -

Some common ions and their valencies Valency Name of ion • : - Symbol Name of ion Symbol Ammonium NH 4+ Sodium Na+ Hydrogen H+ 1 Potassium K+ Hydride H - Hydroxide OH 1 Silver Ag+ Chloride Cl - Nitrate NO 3 - 1 Copper (I) Cu+ Bromide Br - Hydrogen carbonate HCO 3 - 2 Magnesium Mg 2+ Iodide I 2 Calcium Ca 2+ Oxide O 2 - Carbonate CO 32 - 2 Zinc Zn 2+ Sulphite SO 32 - 2 Iron (II) Fe 2+ Sulphate SO 42 - 2 Copper (II) Cu 2+ 3 Aluminum(III) Al 3+ Phosphate PO 43 - 3 Iron (III) Fe 3+ 1 Nitride N - - 3 -

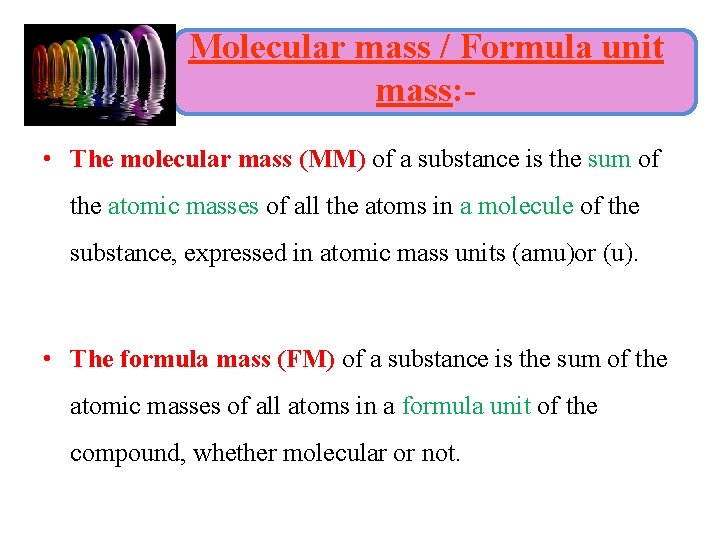
Molecular mass / Formula unit mass: • The molecular mass (MM) of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance, expressed in atomic mass units (amu)or (u). • The formula mass (FM) of a substance is the sum of the atomic masses of all atoms in a formula unit of the compound, whether molecular or not.

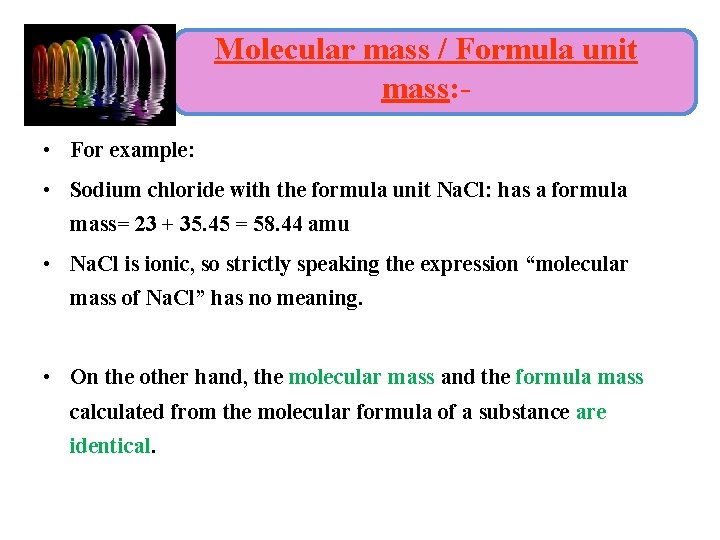
Molecular mass / Formula unit mass: • For example: • Sodium chloride with the formula unit Na. Cl: has a formula mass= 23 + 35. 45 = 58. 44 amu • Na. Cl is ionic, so strictly speaking the expression “molecular mass of Na. Cl” has no meaning. • On the other hand, the molecular mass and the formula mass calculated from the molecular formula of a substance are identical.

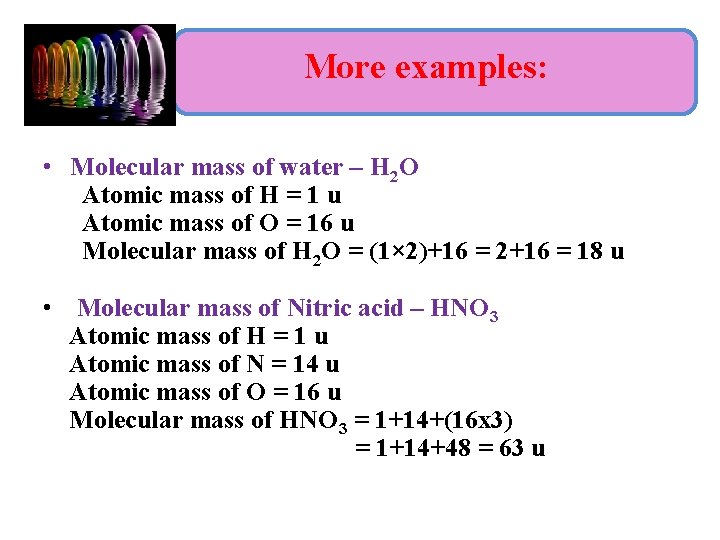
More examples: • Molecular mass of water – H 2 O Atomic mass of H = 1 u Atomic mass of O = 16 u Molecular mass of H 2 O = (1× 2)+16 = 2+16 = 18 u • Molecular mass of Nitric acid – HNO 3 Atomic mass of H = 1 u Atomic mass of N = 14 u Atomic mass of O = 16 u Molecular mass of HNO 3 = 1+14+(16 x 3) = 1+14+48 = 63 u

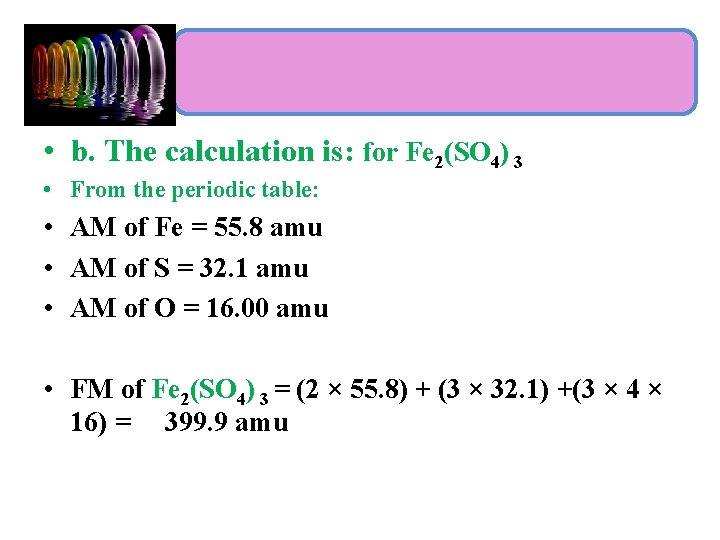
More examples: • Calculate the formula mass of each of the following using a table of atomic masses (AM): a. chloroform, CHCl 3 b. iron(III) sulfate, Fe 2(SO 4)3 Problem Strategy: • 1 - Identify the number and type of atoms in the chemical formula. • 2 - Use the periodic table to obtain the atomic mass of each of the elements present in the compounds. • 3 -Taking into account the number of each atom present in the formula, sum the masses.

• 4 - If atoms in the formula are enclosed within parentheses as in part b, the number of each element within the parentheses should be multiplied by the subscript that follows the final, or closing, parentheses. Solution: a. The calculation for CHCl 3 is: Atomic mass of C = 12. 0 amu Atomic mass of H = 1. 0 amu Atomic mass of Cl = 35. 45 amu FM of CHCl 3 = 12 + 1 + (3 × 35. 45)= 119. 4 amu

• b. The calculation is: for Fe 2(SO 4) 3 • From the periodic table: • AM of Fe = 55. 8 amu • AM of S = 32. 1 amu • AM of O = 16. 00 amu • FM of Fe 2(SO 4) 3 = (2 × 55. 8) + (3 × 32. 1) +(3 × 4 × 16) = 399. 9 amu

• Home. Work

Homework • Calculate the formula masses of the following compounds, using a table of atomic masses. • a. nitrogen dioxide, NO 2 • b. glucose, C 6 H 12 O 6

Thank you