Formula Weights Molecular Mass and Percent Composition Molecular

- Slides: 11

Formula Weights (Molecular Mass) and Percent Composition

Molecular Mass � The molecular mass is the same numeric value as the atomic mass but the unit is different � Atomic mass is measured in atomic mass units (amu) and molecular mass is measured in grams � So molecular mass is the atomic mass with the unit “grams” or “g”

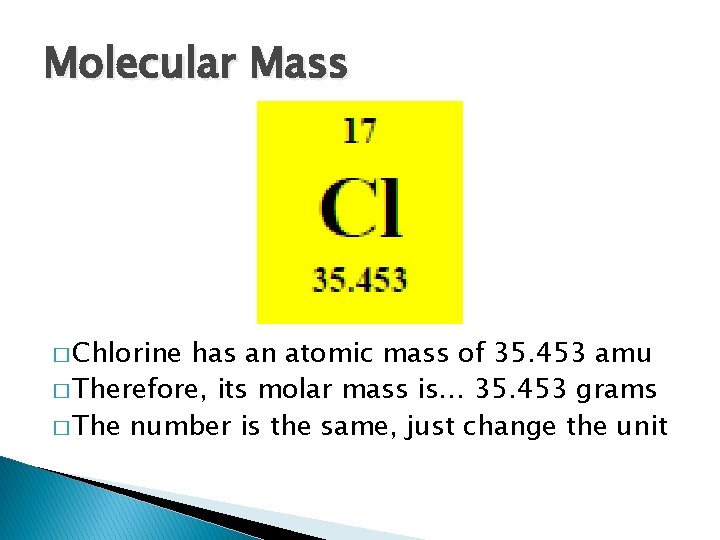
Molecular Mass � Chlorine has an atomic mass of 35. 453 amu � Therefore, its molar mass is… 35. 453 grams � The number is the same, just change the unit

Formula Weight � Formula weight of a compound is the sum of the atomic masses of the atoms as given in the formula of the compound. ◦ Sum of molecular masses

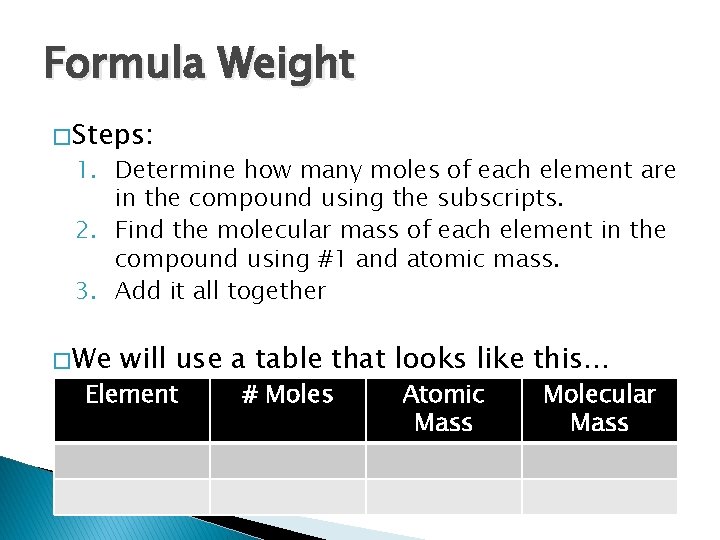
Formula Weight � Steps: 1. Determine how many moles of each element are in the compound using the subscripts. 2. Find the molecular mass of each element in the compound using #1 and atomic mass. 3. Add it all together � We will use a table that looks like this… Element # Moles Atomic Mass Molecular Mass

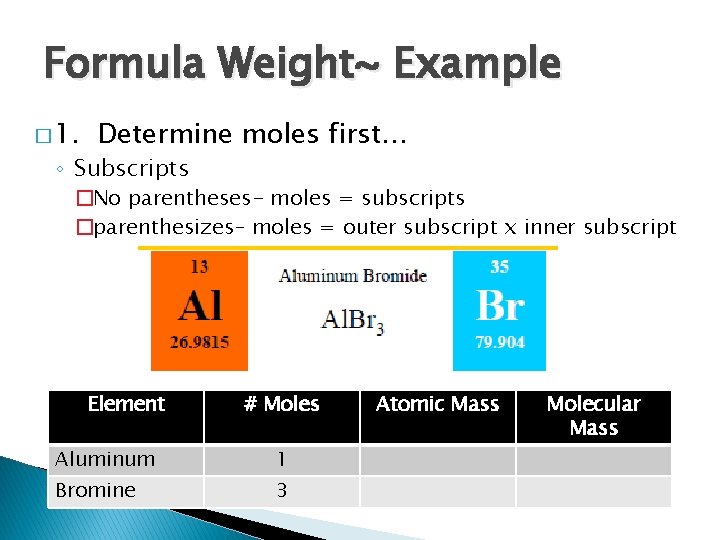
Formula Weight~ Example � 1. Determine moles first… ◦ Subscripts �No parentheses- moles = subscripts �parenthesizes– moles = outer subscript x inner subscript Element # Moles Aluminum 1 Bromine 3 Atomic Mass Molecular Mass

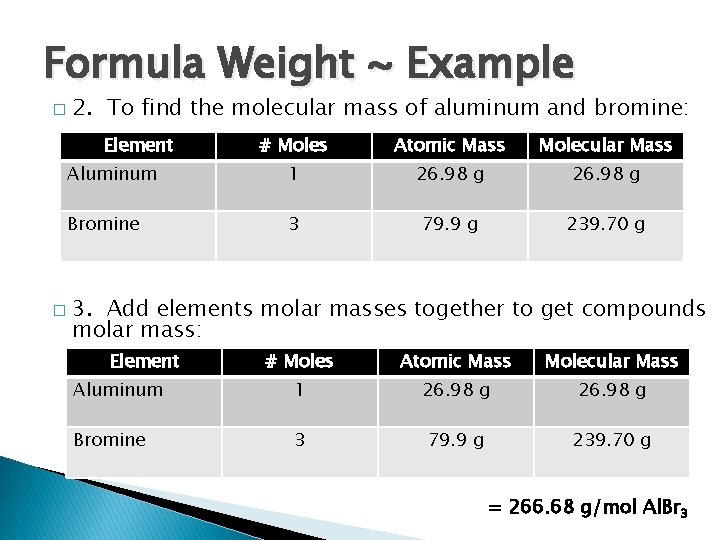
Formula Weight ~ Example � 2. To find the molecular mass of aluminum and bromine: Element # Moles Atomic Mass Molecular Mass Aluminum 1 26. 98 g Bromine 3 79. 9 g 239. 70 g � 3. Add elements molar masses together to get compounds molar mass: Element # Moles Atomic Mass Molecular Mass Aluminum 1 26. 98 g Bromine 3 79. 9 g 239. 70 g = 266. 68 g/mol Al. Br 3

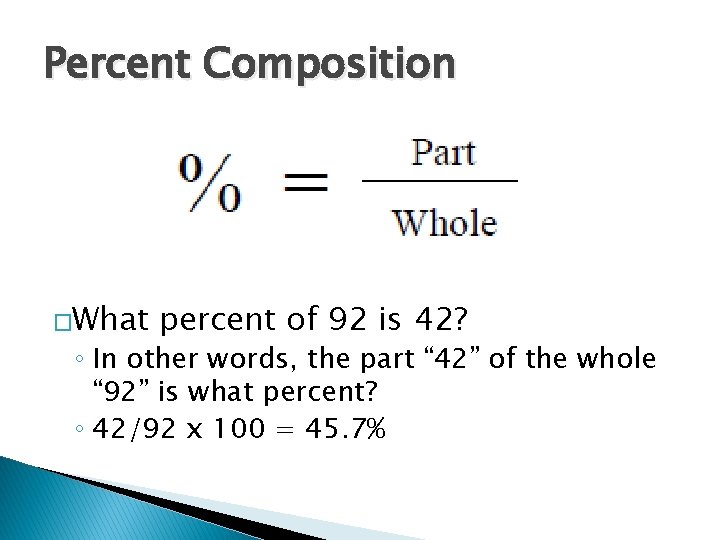
Percent Composition � Percent composition is the percent that you find of any ONE element in a compound of elements. � Percent is found by taking the PART that you are looking for and dividing it by the WHOLE. ◦ Then multiply by 100 to move decimal.

Percent Composition �What percent of 92 is 42? ◦ In other words, the part “ 42” of the whole “ 92” is what percent? ◦ 42/92 x 100 = 45. 7%

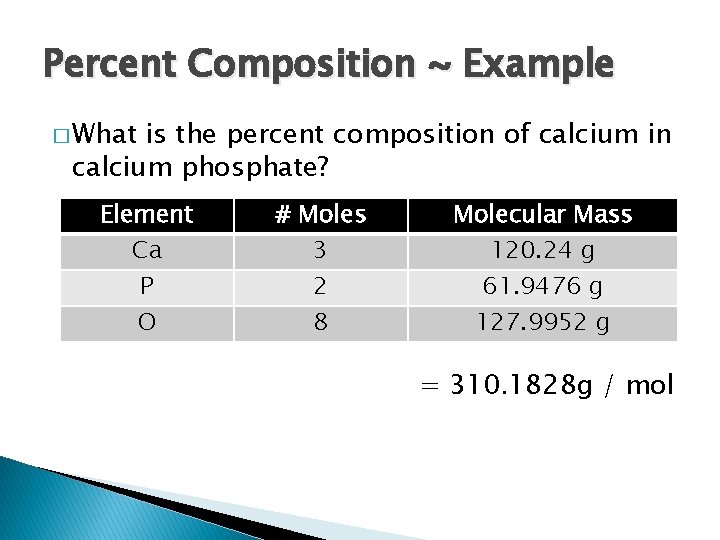
Percent Composition ~ Example � What is the percent composition of calcium in calcium phosphate? Element Ca P O # Moles 3 2 8 Molecular Mass 120. 24 g 61. 9476 g 127. 9952 g = 310. 1828 g / mol

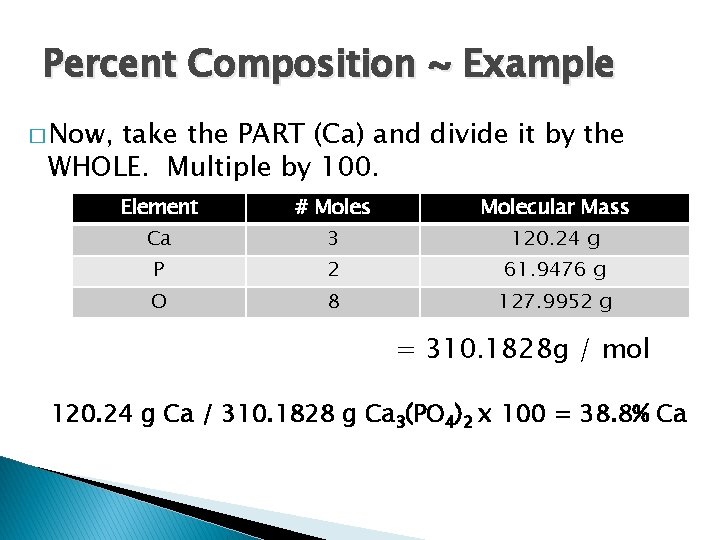
Percent Composition ~ Example � Now, take the PART (Ca) and divide it by the WHOLE. Multiple by 100. Element # Moles Molecular Mass Ca 3 120. 24 g P 2 61. 9476 g O 8 127. 9952 g = 310. 1828 g / mol 120. 24 g Ca / 310. 1828 g Ca 3(PO 4)2 x 100 = 38. 8% Ca