Molecular Geometry Lewis Structures VSEPR Theory Lewis Structures









- Slides: 41

Molecular Geometry Lewis Structures VSEPR Theory

Lewis Structures • Count up the total number of valence electrons • Draw single bonds between central atom and surrounding atoms • Place remaining electrons, in pairs around appropriate atoms – Start with outer atoms • Make sure all atoms that need octets have octets • Make double bonds if necessary • Draw resonance structures (if applicable)


Lewis Structures Examples

Lewis Structures • Ex: H 2 O • 1. # valence electrons • 2. Draw single bond between atoms. • 3. Place remaining electrons around appropriate atoms.

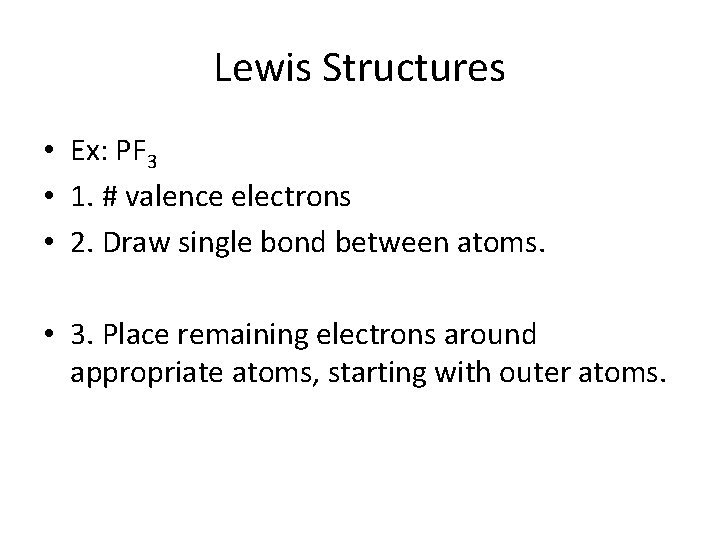
Lewis Structures • Ex: PF 3 • 1. # valence electrons • 2. Draw single bond between atoms. • 3. Place remaining electrons around appropriate atoms, starting with outer atoms.

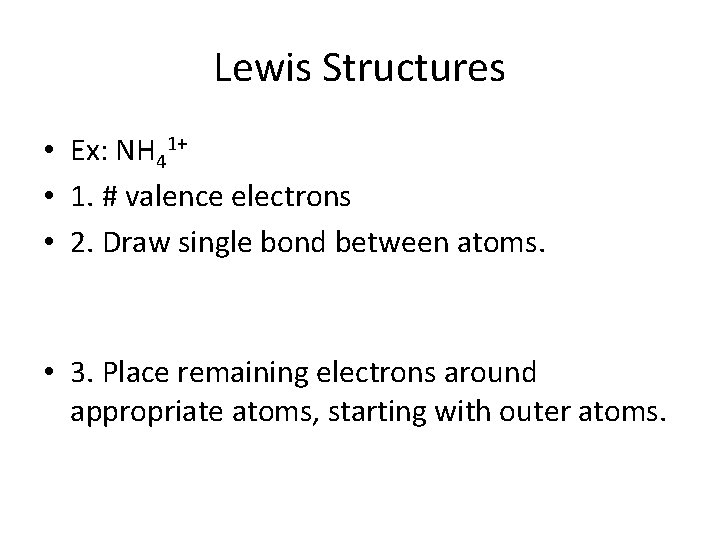
Lewis Structures • Ex: NH 41+ • 1. # valence electrons • 2. Draw single bond between atoms. • 3. Place remaining electrons around appropriate atoms, starting with outer atoms.

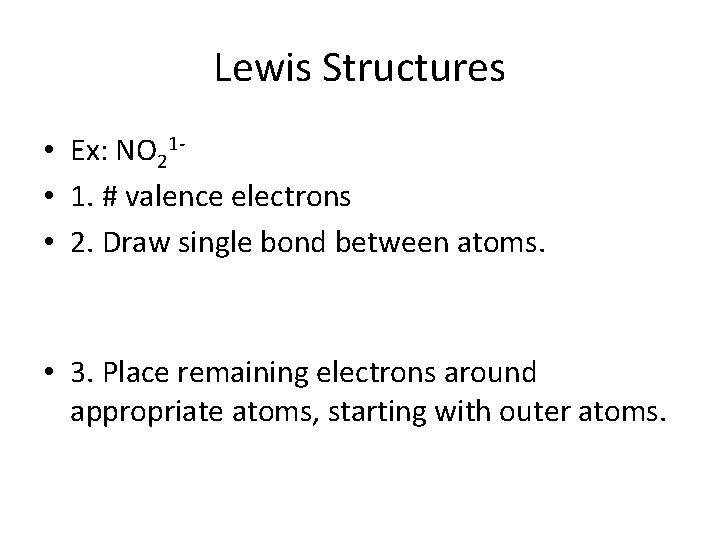
Lewis Structures • Ex: NO 21 • 1. # valence electrons • 2. Draw single bond between atoms. • 3. Place remaining electrons around appropriate atoms, starting with outer atoms.

Lewis Structures • Check octets of each atom.

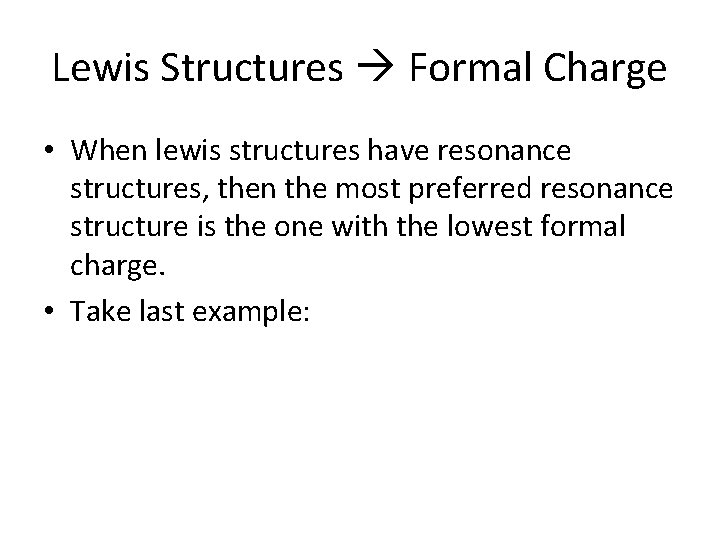
Lewis Structures • Nitrate can have two resonance structures because the valence electrons are free to move over the entire structure of the polyatomic.

Lewis Structures Formal Charge • When lewis structures have resonance structures, then the most preferred resonance structure is the one with the lowest formal charge. • Take last example:

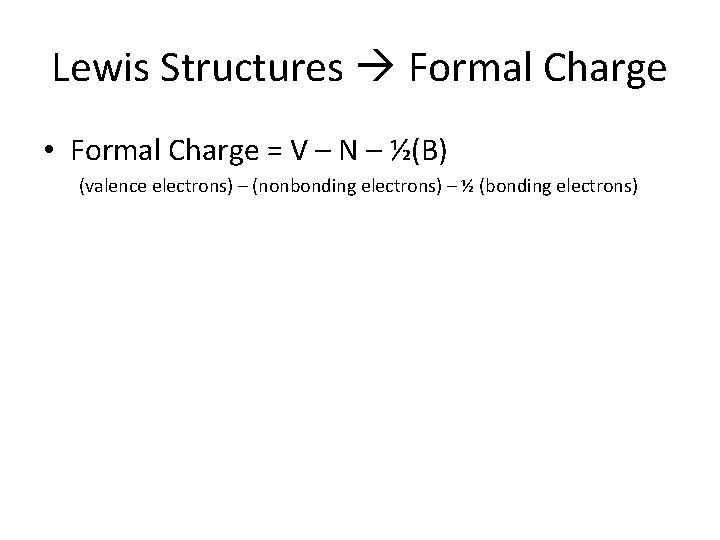
Lewis Structures Formal Charge • Formal Charge = V – N – ½(B) (valence electrons) – (nonbonding electrons) – ½ (bonding electrons)

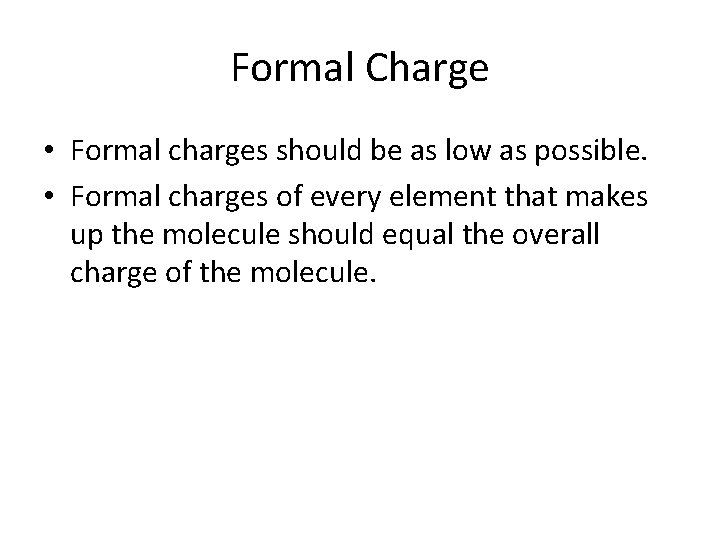
Formal Charge • Formal charges should be as low as possible. • Formal charges of every element that makes up the molecule should equal the overall charge of the molecule.

Formal Charge • Negative formal charges should be on the more electronegative atom (elements closest to fluorine). • Positive formal charges should be on the less electronegative atoms.

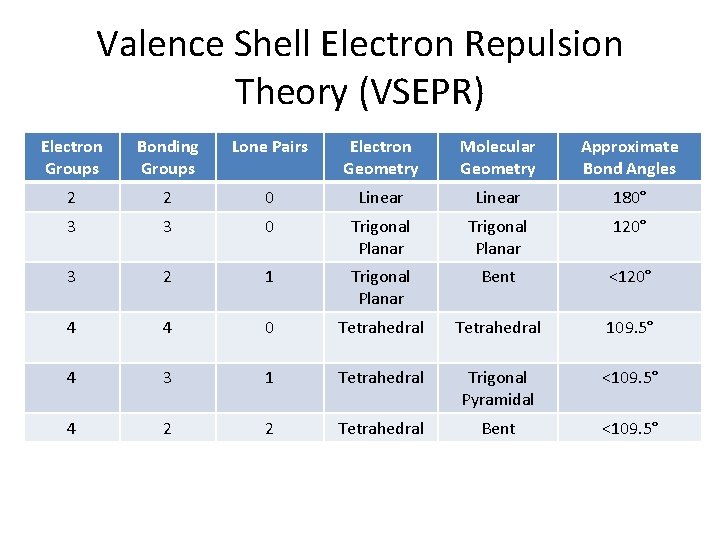
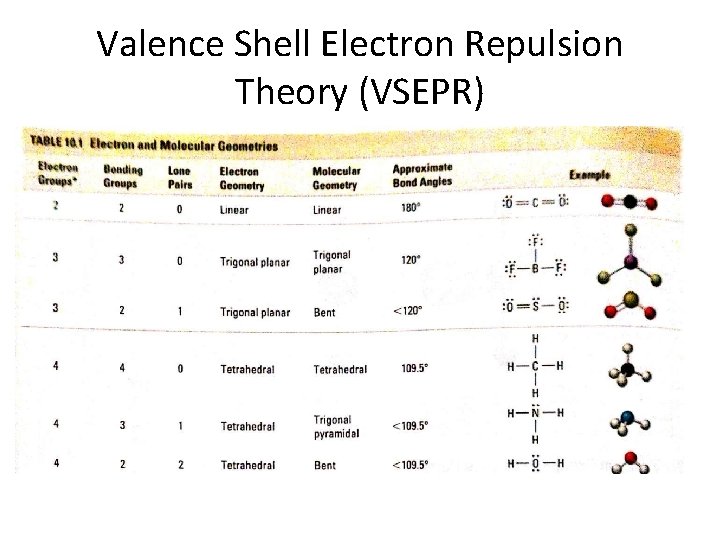
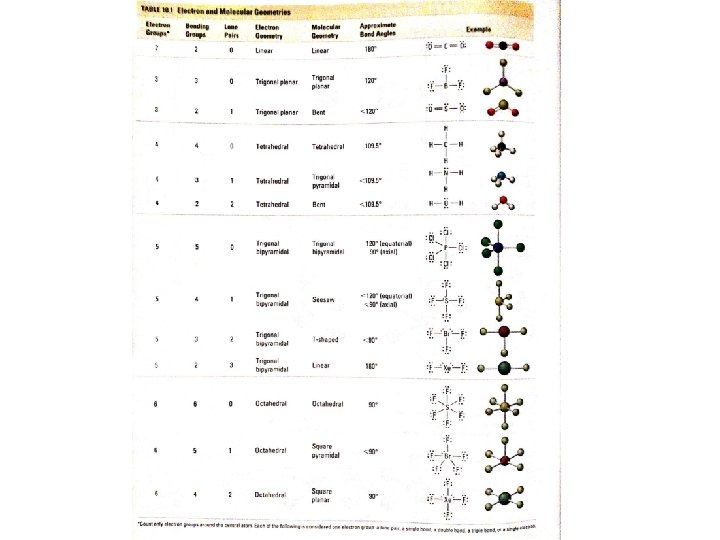
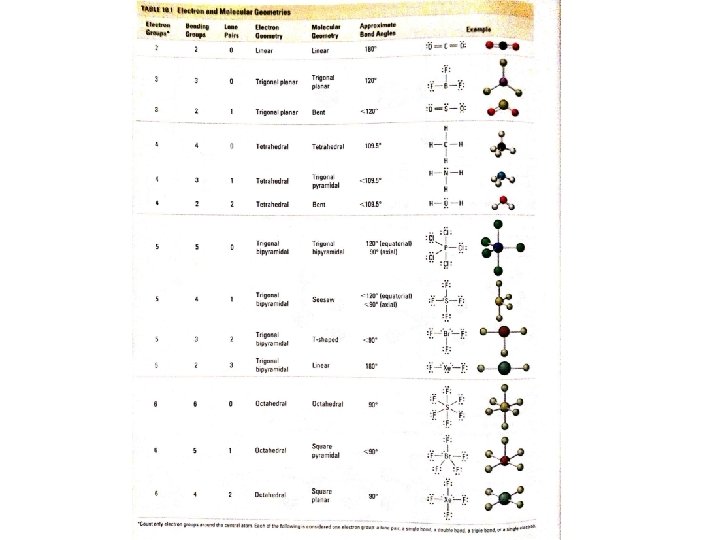
Valence Shell Electron Repulsion Theory (VSEPR) Electron Groups Bonding Groups Lone Pairs Electron Geometry Molecular Geometry Approximate Bond Angles 2 2 0 Linear 180° 3 3 0 Trigonal Planar 120° 3 2 1 Trigonal Planar Bent <120° 4 4 0 Tetrahedral 109. 5° 4 3 1 Tetrahedral Trigonal Pyramidal <109. 5° 4 2 2 Tetrahedral Bent <109. 5°

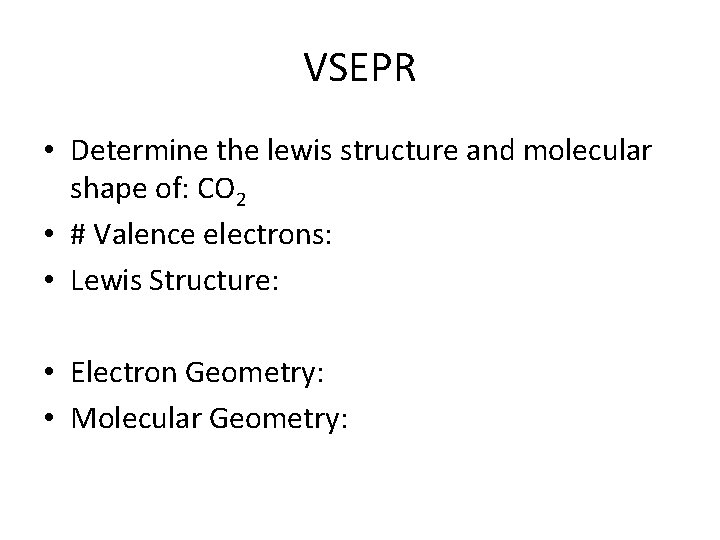
VSEPR • Determine the lewis structure and molecular shape of: CO 2 • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

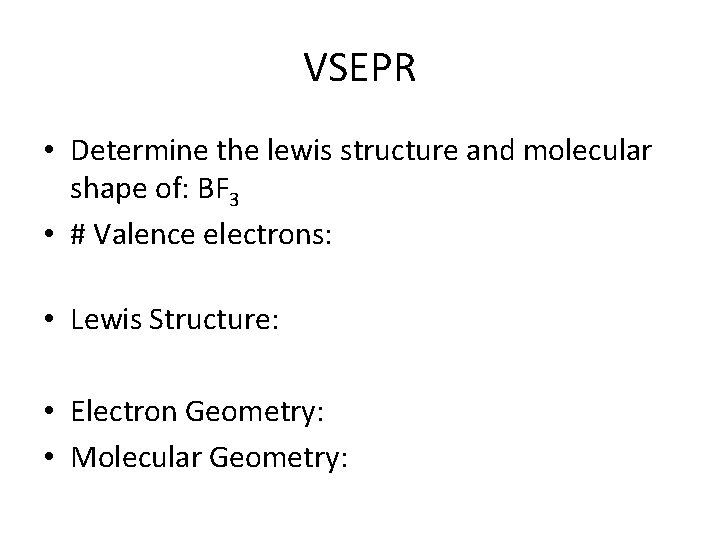
VSEPR • Determine the lewis structure and molecular shape of: BF 3 • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

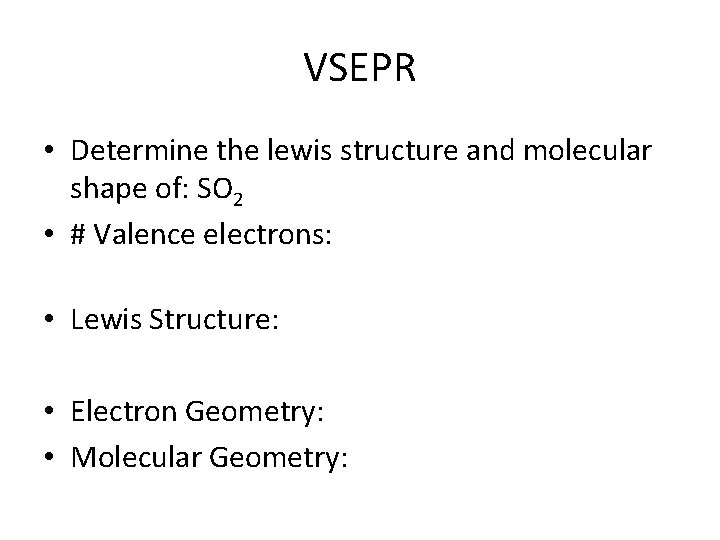
VSEPR • Determine the lewis structure and molecular shape of: SO 2 • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

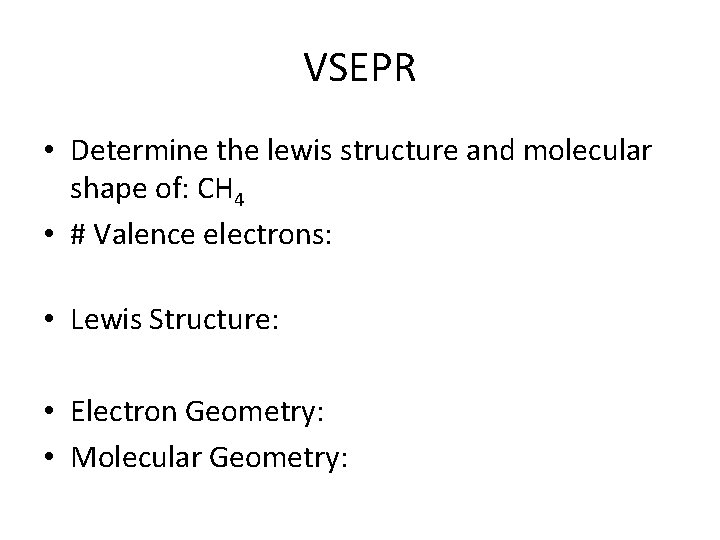
VSEPR • Determine the lewis structure and molecular shape of: CH 4 • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

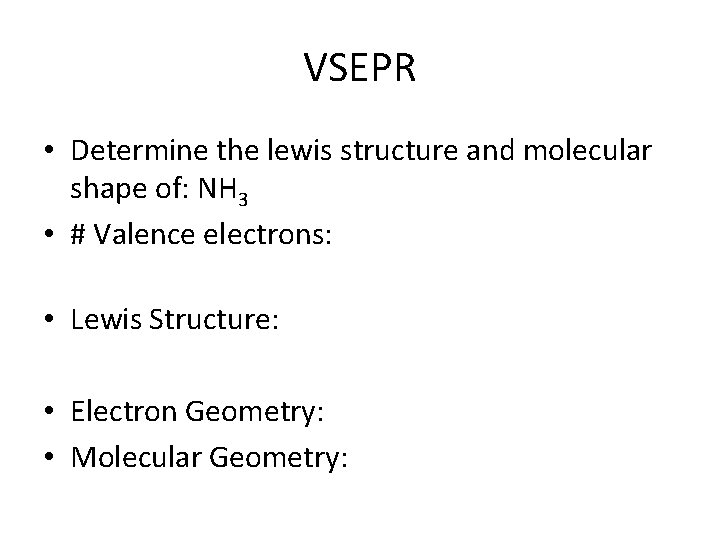
VSEPR • Determine the lewis structure and molecular shape of: NH 3 • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

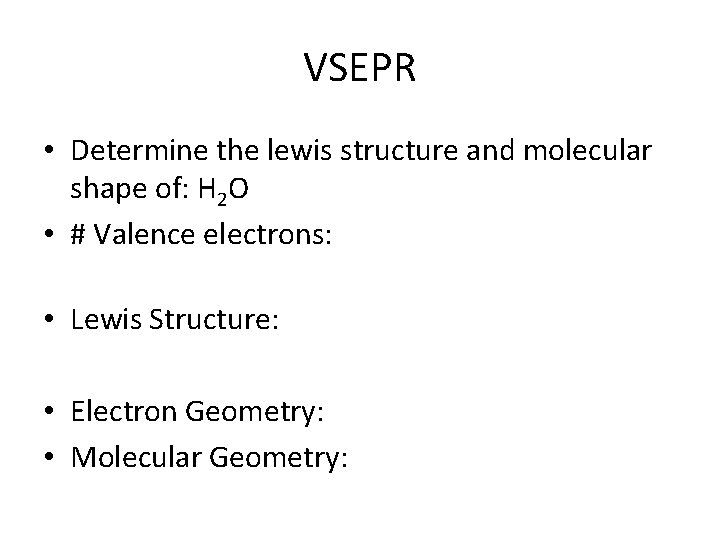
VSEPR • Determine the lewis structure and molecular shape of: H 2 O • # Valence electrons: • Lewis Structure: • Electron Geometry: • Molecular Geometry:

Valence Shell Electron Repulsion Theory (VSEPR)


Polar Bonds • Created when electrons are drawn more closely to one of the atoms in the bond – The bond is polarized • Electrons are pulled toward more electronegative atoms • Electronegative atoms: atoms that strongly attract bonding electrons • Most electronegative element: Fluorine

Polar Bonds • Symbolize polar bonds with δ • Can also symbolize by

Polar Bonds • Examples:

Polar Bonds • Examples:

Polar Bonds • Examples:

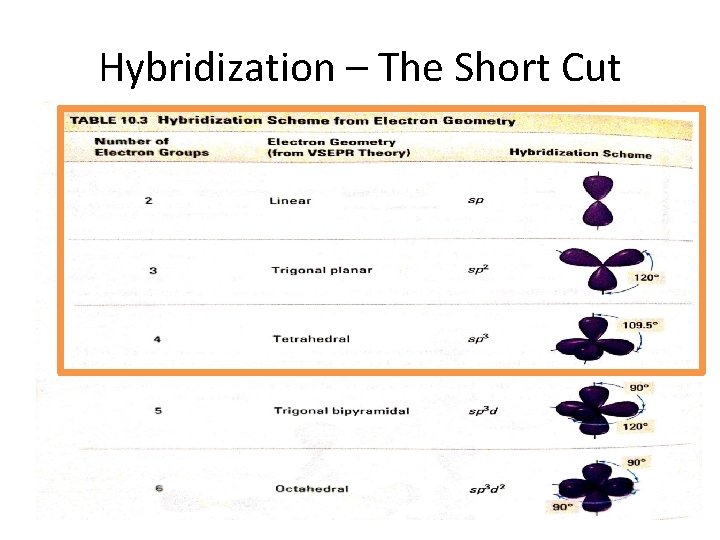
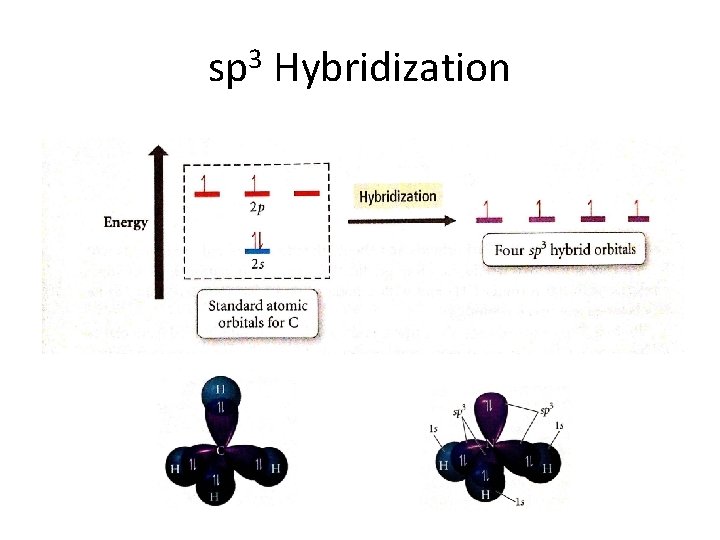
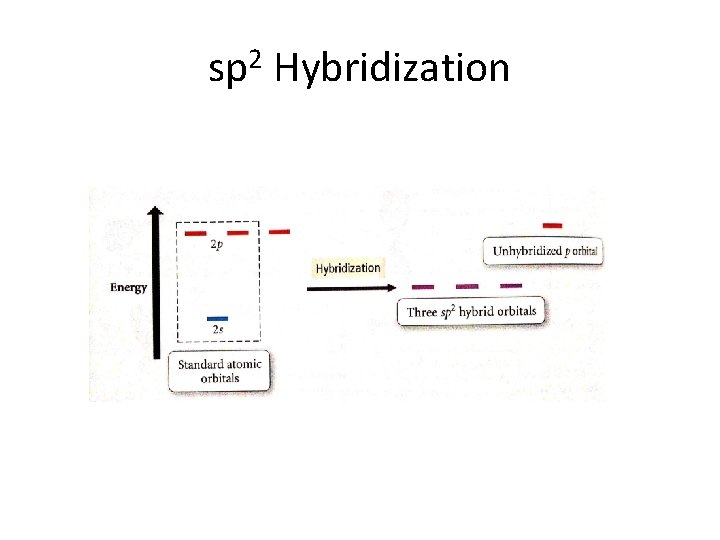
Hybridization • Hydridization occurs to allow the atoms of the molecule to spread further away from each other • S orbitals and P orbitals hybridize into sp, sp 2, and sp 3 orbitals • Anything above sp 3 does not occur because there are only 3 p orbitals – Therefore, DO NOT write sp 4 , ect. • Also, there is only one s orbital, therefore, DO NOT write s 2 p 3, ect

Hybridization • Note: We only look at hybridization in terms of the central atom.

Hybridization – The Short Cut

sp 3 Hybridization

sp 2 Hybridization

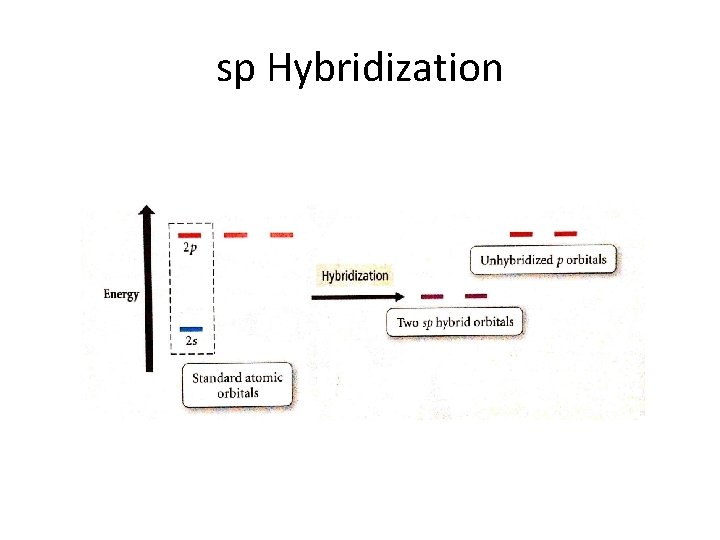
sp Hybridization

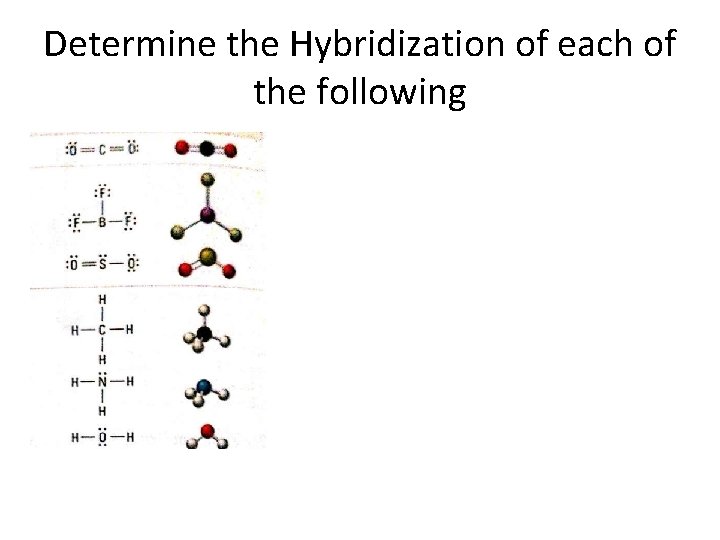
Determine the Hybridization of each of the following


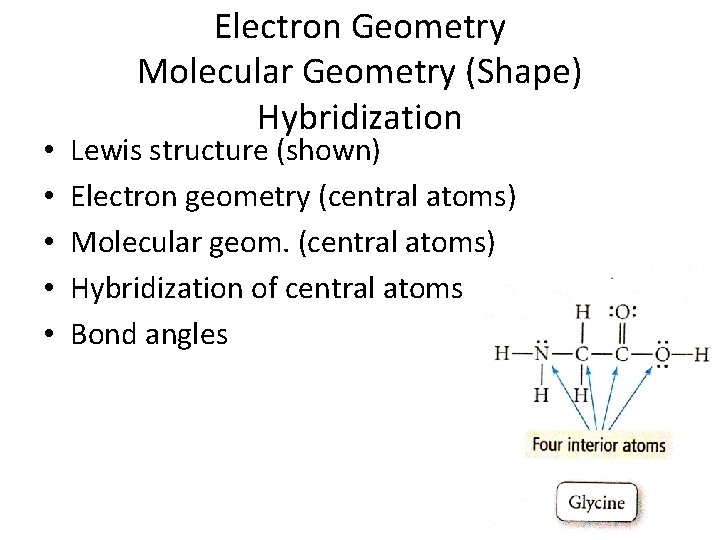
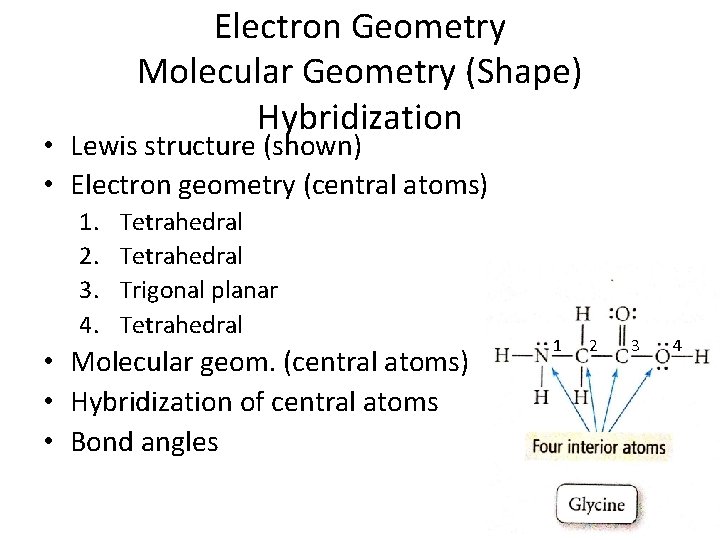
• • • Electron Geometry Molecular Geometry (Shape) Hybridization Lewis structure (shown) Electron geometry (central atoms) Molecular geom. (central atoms) Hybridization of central atoms Bond angles

Electron Geometry Molecular Geometry (Shape) Hybridization • Lewis structure (shown) • Electron geometry (central atoms) 1. 2. 3. 4. Tetrahedral Trigonal planar Tetrahedral • Molecular geom. (central atoms) • Hybridization of central atoms • Bond angles 1 2 3 4

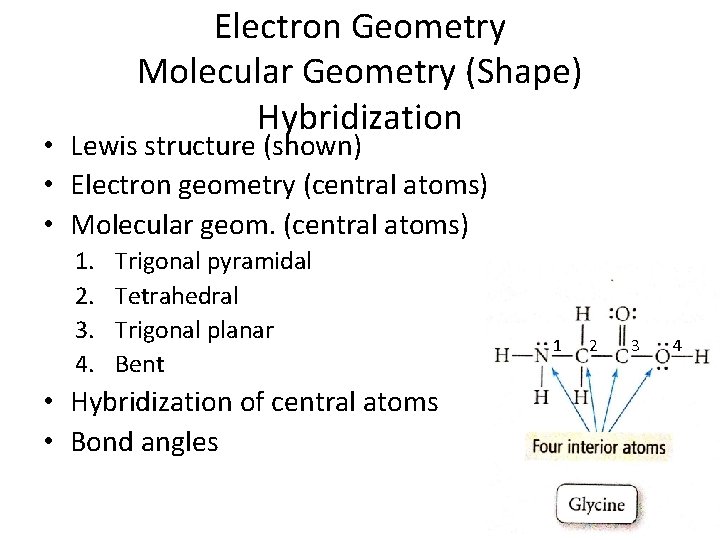
Electron Geometry Molecular Geometry (Shape) Hybridization • Lewis structure (shown) • Electron geometry (central atoms) • Molecular geom. (central atoms) 1. 2. 3. 4. Trigonal pyramidal Tetrahedral Trigonal planar Bent • Hybridization of central atoms • Bond angles 1 2 3 4

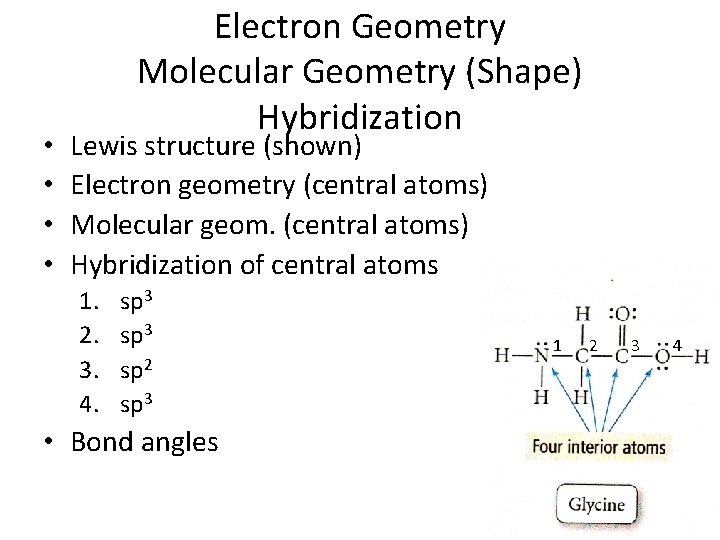
• • Electron Geometry Molecular Geometry (Shape) Hybridization Lewis structure (shown) Electron geometry (central atoms) Molecular geom. (central atoms) Hybridization of central atoms 1. 2. 3. 4. sp 3 sp 2 sp 3 • Bond angles 1 2 3 4

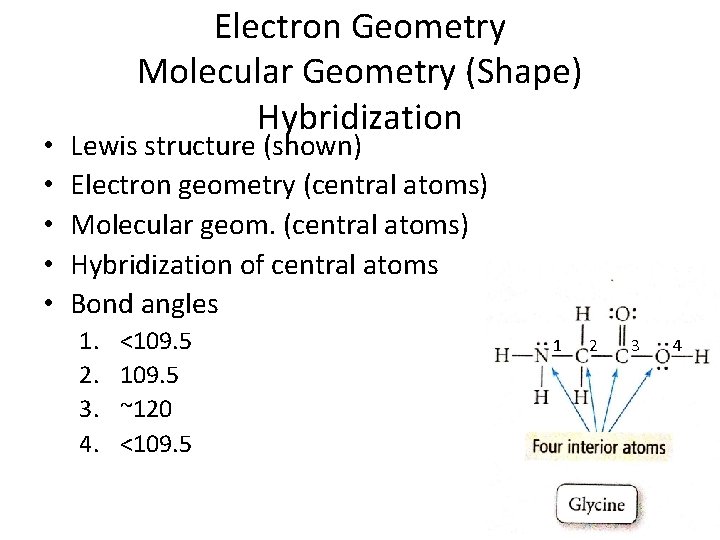
• • • Electron Geometry Molecular Geometry (Shape) Hybridization Lewis structure (shown) Electron geometry (central atoms) Molecular geom. (central atoms) Hybridization of central atoms Bond angles 1. 2. 3. 4. <109. 5 ~120 <109. 5 1 2 3 4