Chemistry A Molecular Approach 1 st Ed Nivaldo





















- Slides: 70

Chemistry: A Molecular Approach, 1 st Ed. Nivaldo Tro Chapter 22 Chemistry of the Nonmetals Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Tro, Chemistry: A Molecular Approach 2008, Prentice Hall

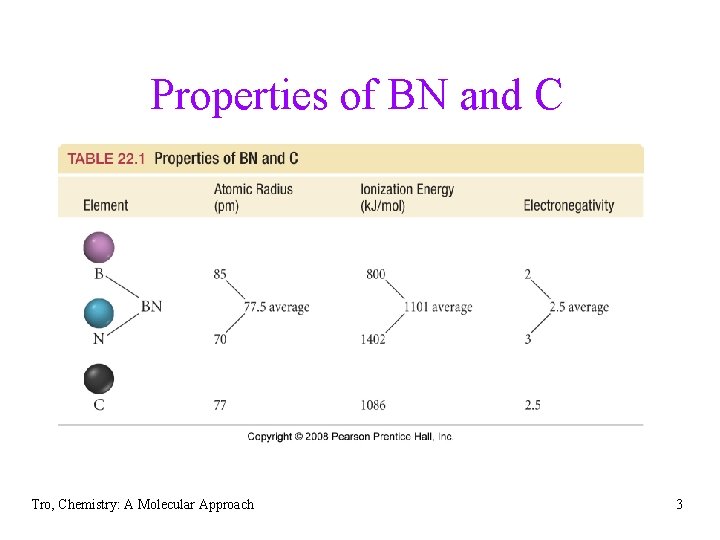
Nanotubes • nanotubes – long, thin, hollow cylinders of atoms • carbon nanotube = sp 2 C in fused hexagonal rings ü electrical conductors • boron-nitride nanotubes = rings of alternating B and N atoms ü isoelectronic with C ü similar size to C ü average electronegativity of B & N about the same as C ü electrical insulators Tro, Chemistry: A Molecular Approach 2

Properties of BN and C Tro, Chemistry: A Molecular Approach 3

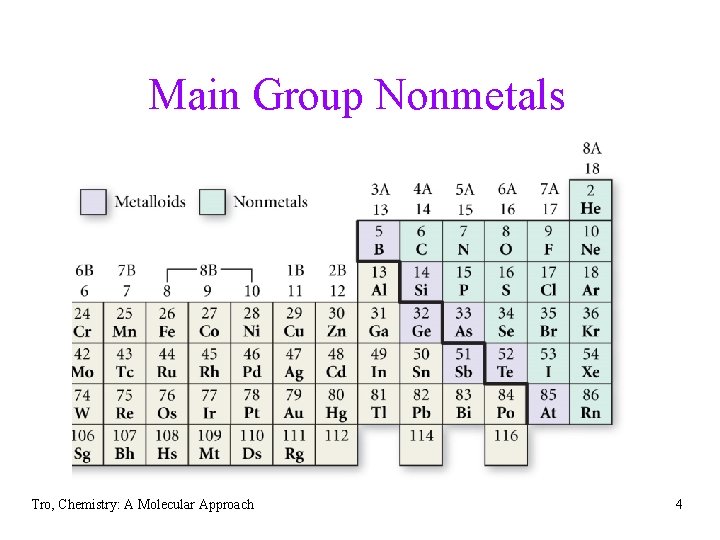
Main Group Nonmetals Tro, Chemistry: A Molecular Approach 4

Atomic Radius and Bonding • atomic radius decreases across the period • electronegativity, ionization energy increase across the • period nonmetals on right of p block form anions in ionic compounds ü often reduced in chemical reactions Ø making them oxidizing agents • nonmetals on left of p block can form cations and • • electron-deficient species in covalent bonding nonmetals near the center of the p block tend to use covalent bonding to complete their octets bonding tendency changes across the period for nonmetals from cation and covalent; to just covalent; to anion and covalent Tro, Chemistry: A Molecular Approach 5

Insulated Nanowire Tro, Chemistry: A Molecular Approach 6

Silicates • the most abundant elements of the Earth’s crust • are O and Si silicates are covalent atomic solids of Si and O üand minor amounts of other elements üfound in rocks, soils, and clays üsilicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Chemistry: A Molecular Approach 7

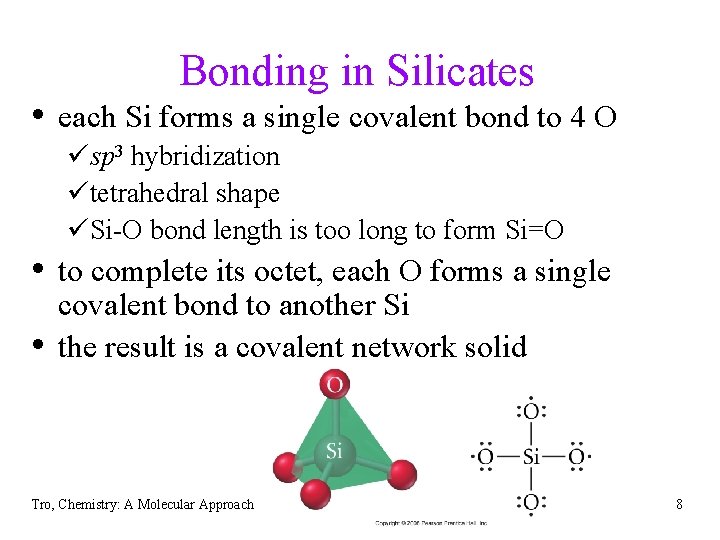
Bonding in Silicates • each Si forms a single covalent bond to 4 O üsp 3 hybridization ütetrahedral shape üSi-O bond length is too long to form Si=O • to complete its octet, each O forms a single • covalent bond to another Si the result is a covalent network solid Tro, Chemistry: A Molecular Approach 8

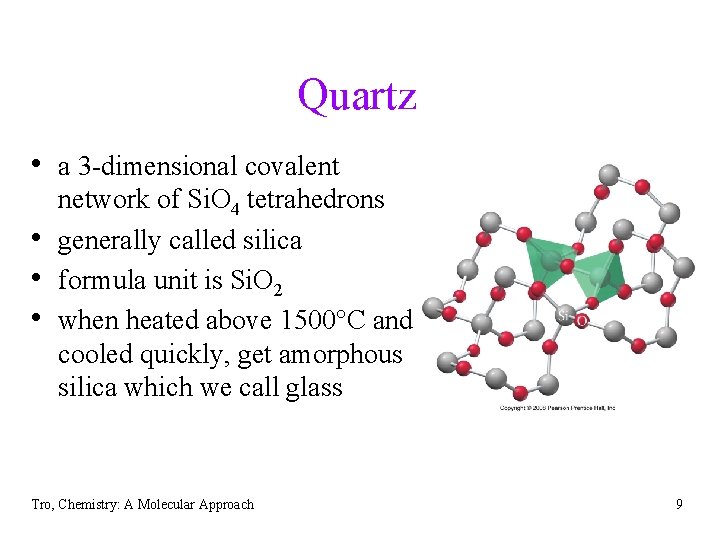
Quartz • a 3 -dimensional covalent • • • network of Si. O 4 tetrahedrons generally called silica formula unit is Si. O 2 when heated above 1500 C and cooled quickly, get amorphous silica which we call glass Tro, Chemistry: A Molecular Approach 9

Aluminosilicates • Al substitutes for Si in some of the lattice sites • Si. O 2 becomes Al. O 2− • the negative charge is countered by the inclusion of a cation üAlbite = ¼ of Si replaced by Al; Na(Al. O 2)(Si. O 2)3 üAnorthite = ½ of Si replaced by Al; Ca(Al. O 2)2(Si. O 2)2 Tro, Chemistry: A Molecular Approach 10

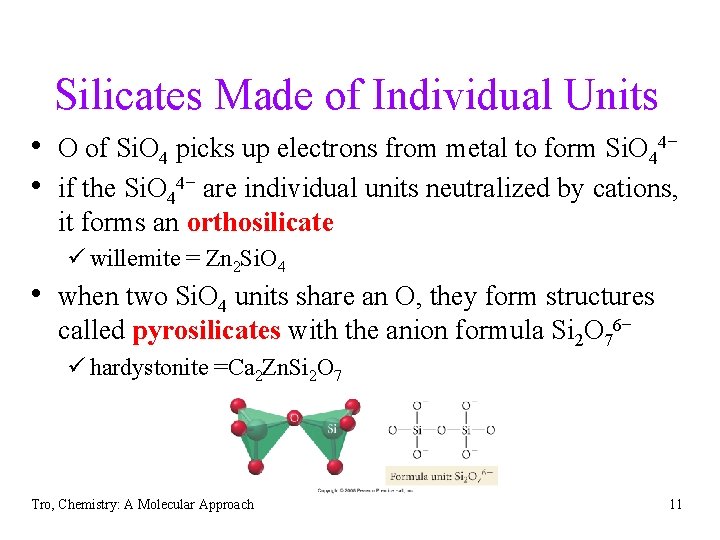
Silicates Made of Individual Units • O of Si. O 4 picks up electrons from metal to form Si. O 44− • if the Si. O 44− are individual units neutralized by cations, it forms an orthosilicate ü willemite = Zn 2 Si. O 4 • when two Si. O 4 units share an O, they form structures called pyrosilicates with the anion formula Si 2 O 76− ü hardystonite =Ca 2 Zn. Si 2 O 7 Tro, Chemistry: A Molecular Approach 11

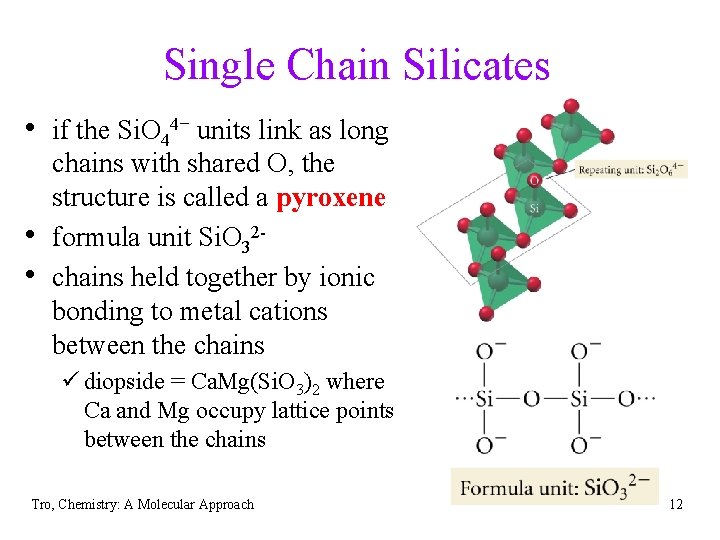
Single Chain Silicates • if the Si. O 44− units link as long • • chains with shared O, the structure is called a pyroxene formula unit Si. O 32 chains held together by ionic bonding to metal cations between the chains ü diopside = Ca. Mg(Si. O 3)2 where Ca and Mg occupy lattice points between the chains Tro, Chemistry: A Molecular Approach 12

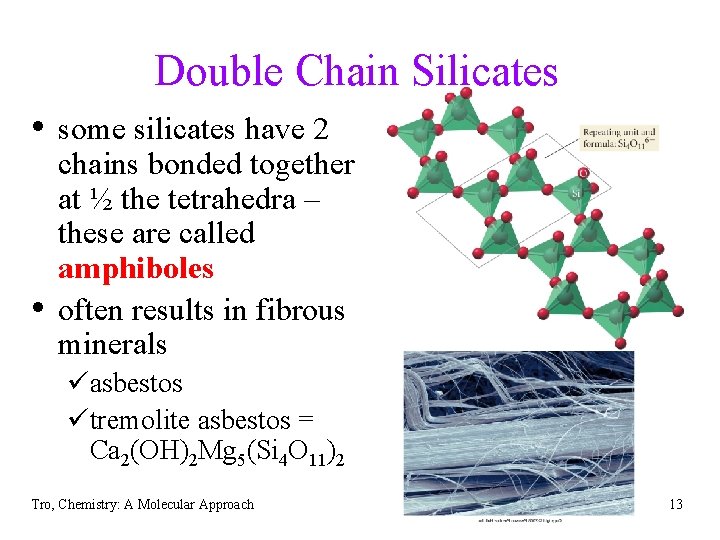
Double Chain Silicates • some silicates have 2 • chains bonded together at ½ the tetrahedra – these are called amphiboles often results in fibrous minerals üasbestos ütremolite asbestos = Ca 2(OH)2 Mg 5(Si 4 O 11)2 Tro, Chemistry: A Molecular Approach 13

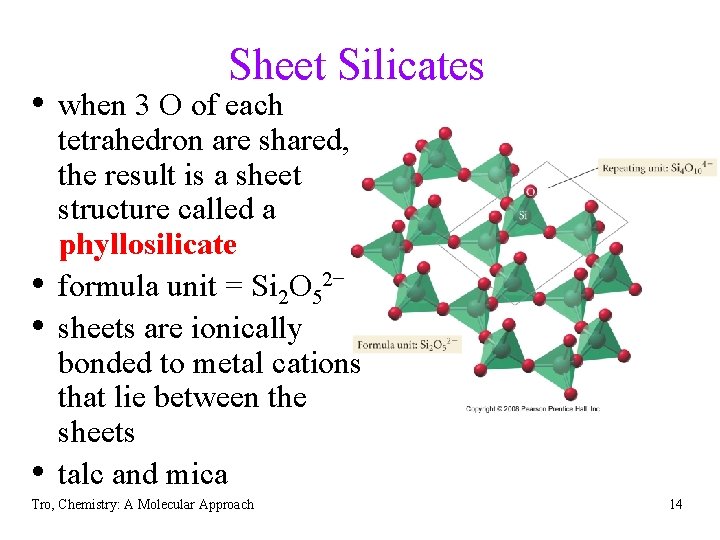
Sheet Silicates • when 3 O of each • • • tetrahedron are shared, the result is a sheet structure called a phyllosilicate formula unit = Si 2 O 52− sheets are ionically bonded to metal cations that lie between the sheets talc and mica Tro, Chemistry: A Molecular Approach 14

Mica: a Phyllosilicate Tro, Chemistry: A Molecular Approach 15

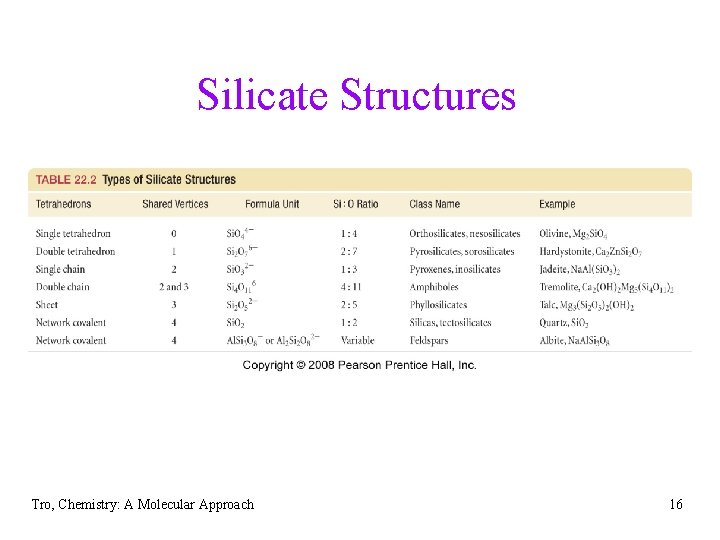
Silicate Structures Tro, Chemistry: A Molecular Approach 16

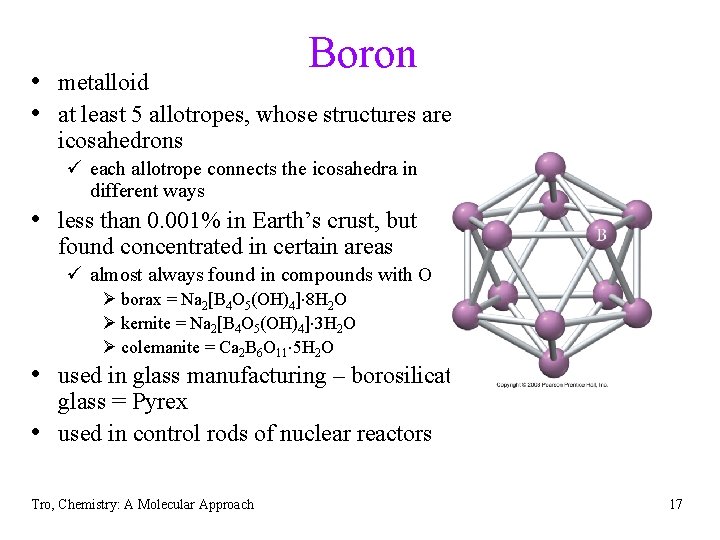
Boron • metalloid • at least 5 allotropes, whose structures are icosahedrons ü each allotrope connects the icosahedra in different ways • less than 0. 001% in Earth’s crust, but found concentrated in certain areas ü almost always found in compounds with O Ø borax = Na 2[B 4 O 5(OH)4] 8 H 2 O Ø kernite = Na 2[B 4 O 5(OH)4] 3 H 2 O Ø colemanite = Ca 2 B 6 O 11 5 H 2 O • used in glass manufacturing – borosilicate • glass = Pyrex used in control rods of nuclear reactors Tro, Chemistry: A Molecular Approach 17

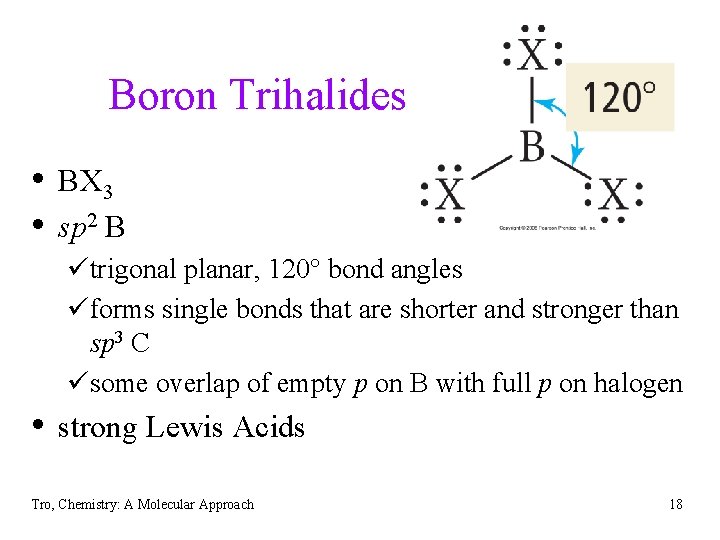
Boron Trihalides • BX 3 • sp 2 B ütrigonal planar, 120 bond angles üforms single bonds that are shorter and stronger than sp 3 C üsome overlap of empty p on B with full p on halogen • strong Lewis Acids Tro, Chemistry: A Molecular Approach 18

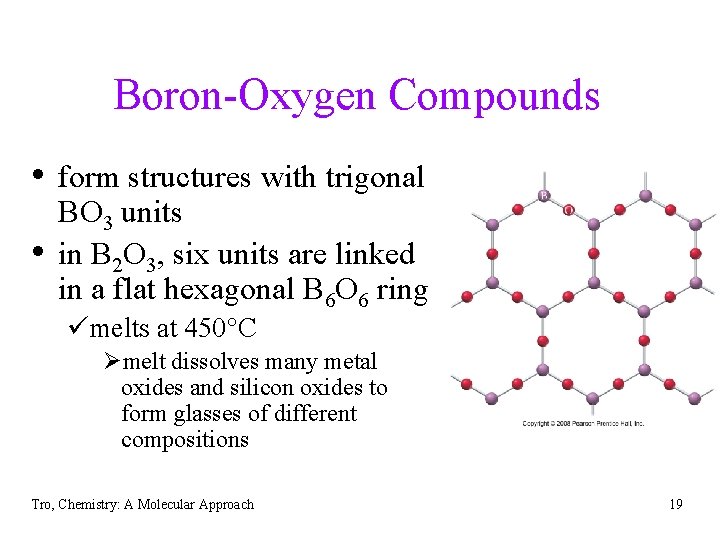
Boron-Oxygen Compounds • form structures with trigonal • BO 3 units in B 2 O 3, six units are linked in a flat hexagonal B 6 O 6 ring ümelts at 450 C Ømelt dissolves many metal oxides and silicon oxides to form glasses of different compositions Tro, Chemistry: A Molecular Approach 19

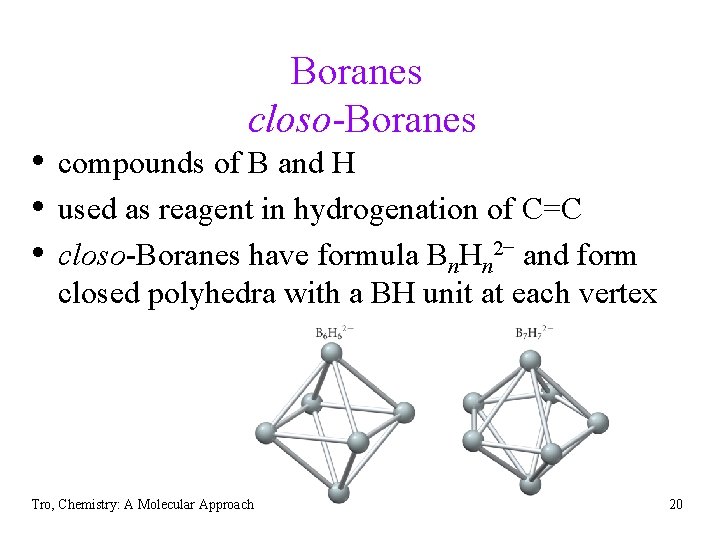
Boranes closo-Boranes • compounds of B and H • used as reagent in hydrogenation of C=C • closo-Boranes have formula Bn. Hn 2− and form closed polyhedra with a BH unit at each vertex Tro, Chemistry: A Molecular Approach 20

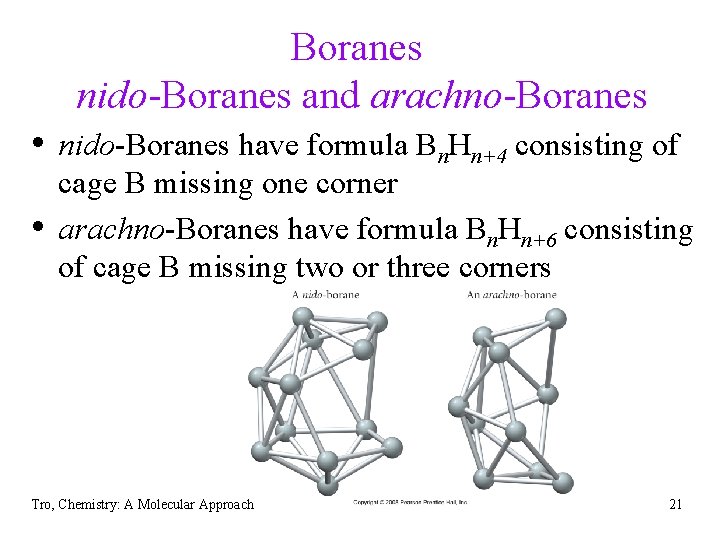
Boranes nido-Boranes and arachno-Boranes • nido-Boranes have formula Bn. Hn+4 consisting of • cage B missing one corner arachno-Boranes have formula Bn. Hn+6 consisting of cage B missing two or three corners Tro, Chemistry: A Molecular Approach 21

Carbon • exhibits the most versatile bonding of all the • • elements diamond structure consists of tetrahedral sp 3 carbons in a 3 -dimensional array graphite structures consist of trigonal planar sp 2 carbons in a 2 -dimensional array üsheets attracted by weak dispersion forces • fullerenes consist of 5 and 6 member carbon rings fused into icosahedral spheres of at least 60 C Tro, Chemistry: A Molecular Approach 22

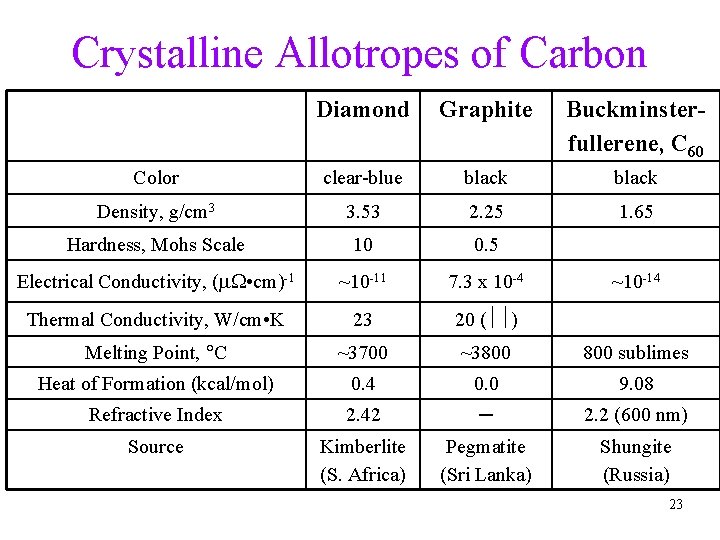
Crystalline Allotropes of Carbon Diamond Graphite Buckminsterfullerene, C 60 Color clear-blue black Density, g/cm 3 3. 53 2. 25 1. 65 Hardness, Mohs Scale 10 0. 5 Electrical Conductivity, (m • cm)-1 ~10 -11 7. 3 x 10 -4 Thermal Conductivity, W/cm • K 23 20 ( ) Melting Point, C ~3700 ~3800 sublimes Heat of Formation (kcal/mol) 0. 4 0. 0 9. 08 Refractive Index 2. 42 ─ 2. 2 (600 nm) Source Kimberlite (S. Africa) Pegmatite (Sri Lanka) Shungite (Russia) ~10 -14 23

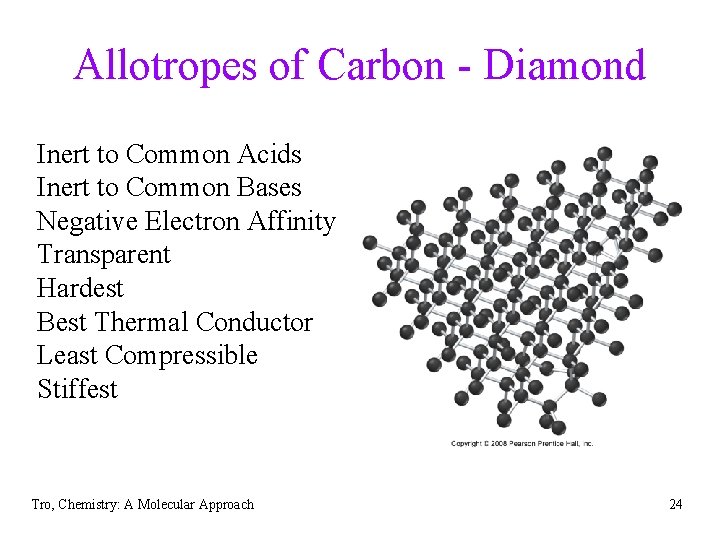
Allotropes of Carbon - Diamond Inert to Common Acids Inert to Common Bases Negative Electron Affinity Transparent Hardest Best Thermal Conductor Least Compressible Stiffest Tro, Chemistry: A Molecular Approach 24

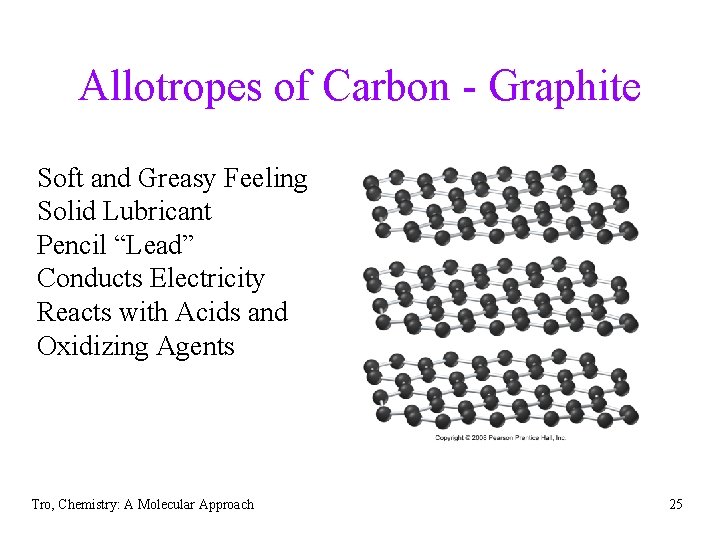
Allotropes of Carbon - Graphite Soft and Greasy Feeling Solid Lubricant Pencil “Lead” Conducts Electricity Reacts with Acids and Oxidizing Agents Tro, Chemistry: A Molecular Approach 25

Noncrystalline Forms of Carbon • coal is a mixture of hydrocarbons and carbon-rich particles ü the product of carbonation of ancient plant material Ø carbonation removes H and O from organic compounds in the form of volatile hydrocarbons and water • anthracite coal has highest C content • bituminous coal has high C, but high S • heating coal in the absence of air forms coke ü carbon and ash • heating wood in the absence of air forms charcoal ü activated carbon is charcoal used to adsorb other molecules • soot is composed of hydrocarbons from incomplete combustion ü carbon black is finely divided form of carbon that is a component of soot Ø used as rubber strengthener Tro, Chemistry: A Molecular Approach 26

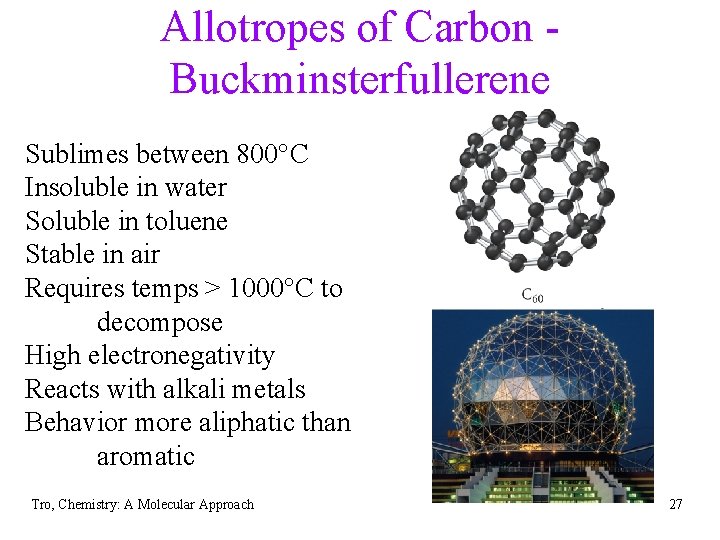
Allotropes of Carbon Buckminsterfullerene Sublimes between 800°C Insoluble in water Soluble in toluene Stable in air Requires temps > 1000°C to decompose High electronegativity Reacts with alkali metals Behavior more aliphatic than aromatic Tro, Chemistry: A Molecular Approach 27

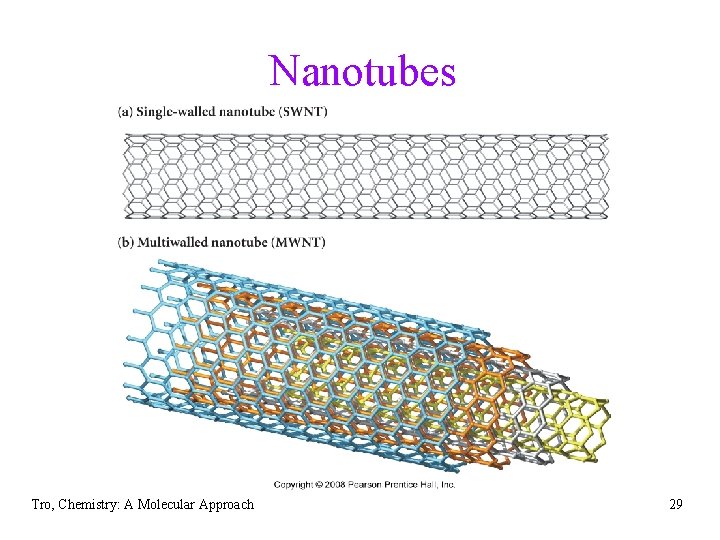
Nanotubes • long hollow tubes constructed of fused C 6 rings • electrical conductors • can incorporate metals and other small molecules and elements üused to stabilize unstable molecules • single-walled nanotubes (SWNT) have one layer • of fused rings multi-walled nanotubes (MWNT) have concentric layers of fused rings Tro, Chemistry: A Molecular Approach 28

Nanotubes Tro, Chemistry: A Molecular Approach 29

Nanocars Tro, Chemistry: A Molecular Approach 30

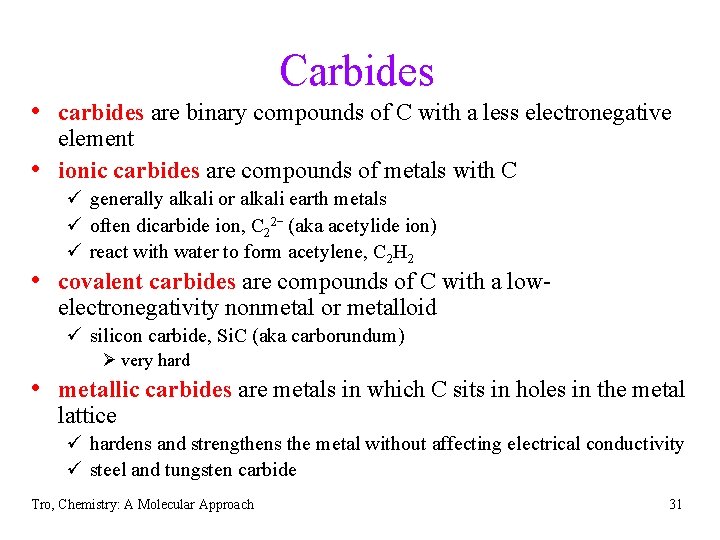
Carbides • carbides are binary compounds of C with a less electronegative • element ionic carbides are compounds of metals with C ü generally alkali or alkali earth metals ü often dicarbide ion, C 22− (aka acetylide ion) ü react with water to form acetylene, C 2 H 2 • covalent carbides are compounds of C with a lowelectronegativity nonmetal or metalloid ü silicon carbide, Si. C (aka carborundum) Ø very hard • metallic carbides are metals in which C sits in holes in the metal lattice ü hardens and strengthens the metal without affecting electrical conductivity ü steel and tungsten carbide Tro, Chemistry: A Molecular Approach 31

Calcium Carbide Tro, Chemistry: A Molecular Approach 32

Cementite Fe 3 C regions found in steel Tro, Chemistry: A Molecular Approach 33

Carbon Oxides • CO 2 ü 0. 04% in atmosphere Ø increased by 25% over the past century ü high solubility in water Ø due to reaction with water to form HCO 3− ions ü triple point − 57 C and 5. 1 atm • CO Ø liquid CO 2 doesn’t exist at atmospheric pressure Ø solid CO 2 = dry ice ü colorless, odorless, tasteless gas ü relatively reactive Ø 2 CO + O 2 2 CO 2 – burns with a blue flame Ø reduces many nonmetals – CO + Cl 2 COCl 2 (phosgene) – CO + S COS (fungicide) Tro, Chemistry: A Molecular Approach 34

Carbonates • solubility of CO 2 in H 2 O due to carbonate formation ü CO 2 + H 2 O H 2 CO 3 ü H 2 CO 3 + H 2 O H 3 O+ + HCO 3− ü HCO 3− + H 2 O H 3 O+ + CO 32− • washing soda = Na 2 CO 3 10 H 2 O ü doesn’t decompose on heating • all carbonate solutions are basic in water ü due to CO 32− + H 2 O OH− + HCO 32− • baking soda = Na. HCO 3 ü decomposes on heating to Na 2 CO 3, H 2 O and CO 2 Tro, Chemistry: A Molecular Approach 35

Elemental Nitrogen • N 2 ü 78% of atmosphere üpurified by distillation of liquid air, or filtering air through zeolites üvery stable, very unreactive ØN N Tro, Chemistry: A Molecular Approach 36

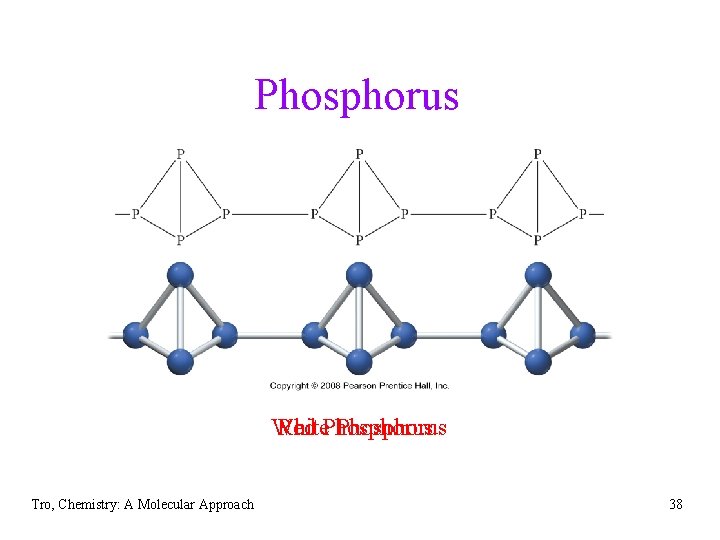
Elemental Phosphorus • P ü white phosphorus Ø white, soft, waxy solid that is flammable and toxic Ø stored under water to prevent spontaneous combustion Ø 2 Ca 3(PO 4)2 (apatite) + 6 Si. O 2 + 10 C P 4(g, wh) + 6 Ca. Si. O 3 + 10 CO Ø tetrahedron with small angles 60 ü red phosphorus Ø formed by heating white P to about 300 C in absence of air Ø amorphous Ø mostly linked tetrahedra Ø not as reactive or toxic as white P Ø used in match heads ü black phosphorus Ø formed by heating white P under pressure Ø most thermodynamically stable form, therefore least reactive Ø layered structure similar to graphite Tro, Chemistry: A Molecular Approach 37

Phosphorus White Red Phosphorus Tro, Chemistry: A Molecular Approach 38

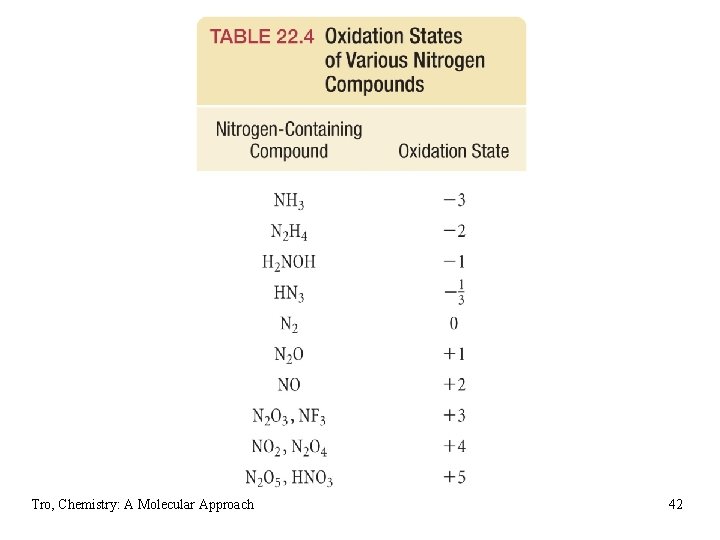
Hydrides of Nitrogen • ammonia, NH 3 ü pungent gas ü basic NH 3 + H 2 O NH 4+ + OH− Ø reacts with acids to make NH 4+ salts – used as chemical fertilizers ü made by fixing N from N 2 using the Haber-Bosch process • hydrazine, N 2 H 4 ü colorless liquid ü basic N 2 H 4 + H 2 O N 2 H 5+ + OH− ü powerful reducing agent • hydrogen azide, HN 3 ü acidic HN 3 + H 2 O H 3 O+ + N 3− ü thermodynamically unstable and decomposes explosively to its elements Tro, Chemistry: A Molecular Approach 39

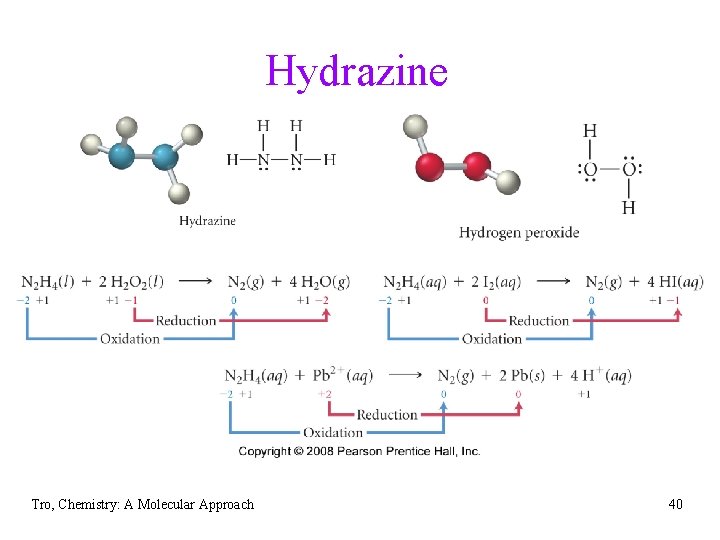
Hydrazine Tro, Chemistry: A Molecular Approach 40

Oxides of Nitrogen • formed by reaction of N 2 or NOx with O 2 • all unstable and will eventually decompose into N 2 and O 2 • NO = nitrogen monoxide = nitric oxide ü important in living systems ü free radical • NO 2 = nitrogen dioxide ü 2 NO 2 N 2 O 4 ü red-brown gas ü free radical • N 2 O = dinitrogen monoxide = nitrous oxide ü ü ü laughing gas made by heating ammonium nitrate NH 4 NO 3 N 2 O + H 2 O oxidizing agent Mg + N 2 O N 2 + Mg. O decomposes on heating 2 N 2 O 2 N 2 + O 2 pressurize food dispensers Tro, Chemistry: A Molecular Approach 41

Tro, Chemistry: A Molecular Approach 42

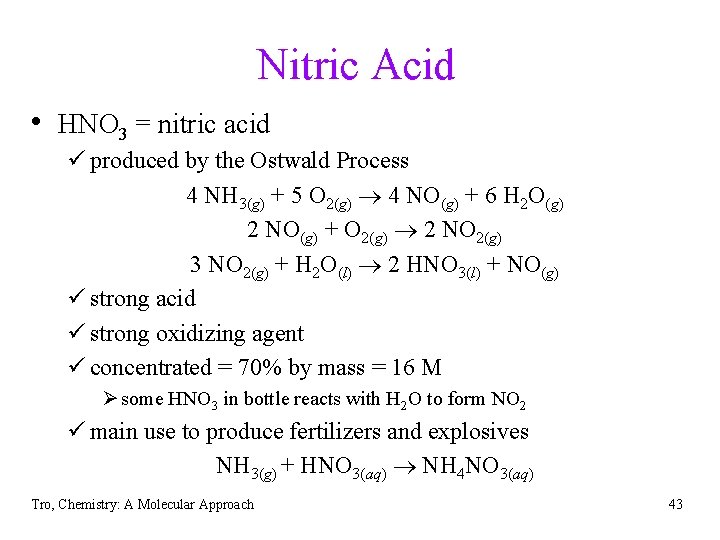
Nitric Acid • HNO 3 = nitric acid ü produced by the Ostwald Process 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) 2 NO(g) + O 2(g) 2 NO 2(g) 3 NO 2(g) + H 2 O(l) 2 HNO 3(l) + NO(g) ü strong acid ü strong oxidizing agent ü concentrated = 70% by mass = 16 M Ø some HNO 3 in bottle reacts with H 2 O to form NO 2 ü main use to produce fertilizers and explosives NH 3(g) + HNO 3(aq) NH 4 NO 3(aq) Tro, Chemistry: A Molecular Approach 43

Nitrates and Nitrites • NO 3− = nitrate ü ANFO = ammonium nitrate fuel oil Ø used as explosive in Oklahoma City ü ammonium nitrate can decompose explosively Ø and other nitrates 2 NH 4 NO 3 2 N 2 + O 2 + 4 H 2 O ü metal nitrates used to give colors to fireworks ü very soluble in water ü oxidizing agent • NO 2− = nitrite ü Na. NO 2 used as food preservative in processed meats Ø kills botulism bacteria Ø keeps meat from browning when exposed to air Ø can form nitrosamines which may increase risk of colon cancer? ? Tro, Chemistry: A Molecular Approach 44

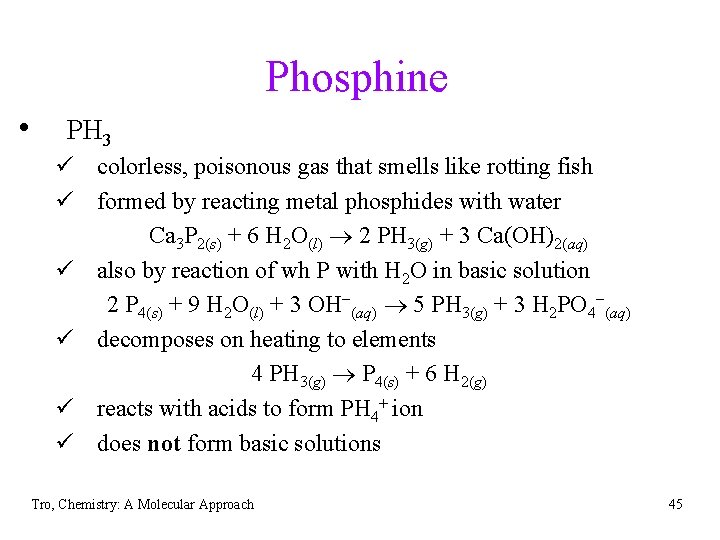
Phosphine • PH 3 ü colorless, poisonous gas that smells like rotting fish ü formed by reacting metal phosphides with water Ca 3 P 2(s) + 6 H 2 O(l) 2 PH 3(g) + 3 Ca(OH)2(aq) ü also by reaction of wh P with H 2 O in basic solution 2 P 4(s) + 9 H 2 O(l) + 3 OH−(aq) 5 PH 3(g) + 3 H 2 PO 4−(aq) ü decomposes on heating to elements 4 PH 3(g) P 4(s) + 6 H 2(g) ü reacts with acids to form PH 4+ ion ü does not form basic solutions Tro, Chemistry: A Molecular Approach 45

Phosphorus Halides • P 4 can react directly with halogens to form PX 3 and • PX 5 compounds PX 3 can react with water to form H 3 PO 3 ü PX 5 can react with water to form H 3 PO 4 PCl 3(l) + 3 H 2 O(l) H 3 PO 3(aq) + 3 HCl(aq) • PCl 3 reacts with O 2 to form POCl 3(l) ü phosphorus oxychloride ü other oxyhalides made by substitution on POCl 3 • phosphous halide and oxyhalides are key starting materials in the production of many P compounds ü fertilizers, pesticides, oil-additives, fire-retardants, surfactants Tro, Chemistry: A Molecular Approach 46

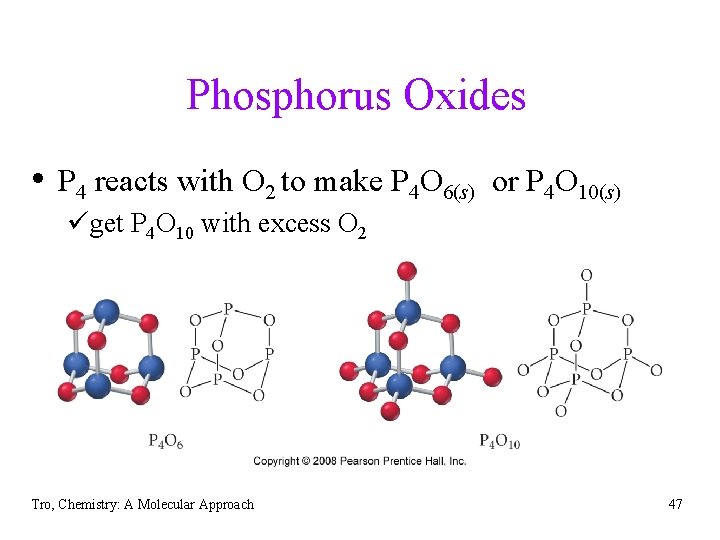
Phosphorus Oxides • P 4 reacts with O 2 to make P 4 O 6(s) or P 4 O 10(s) üget P 4 O 10 with excess O 2 Tro, Chemistry: A Molecular Approach 47

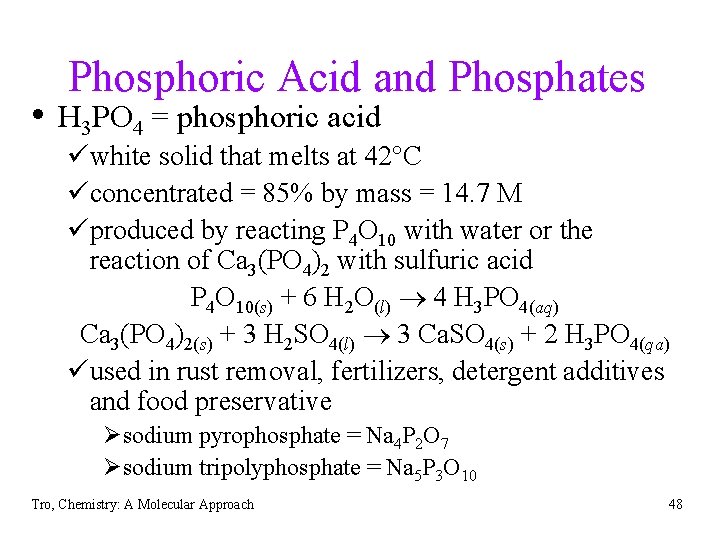
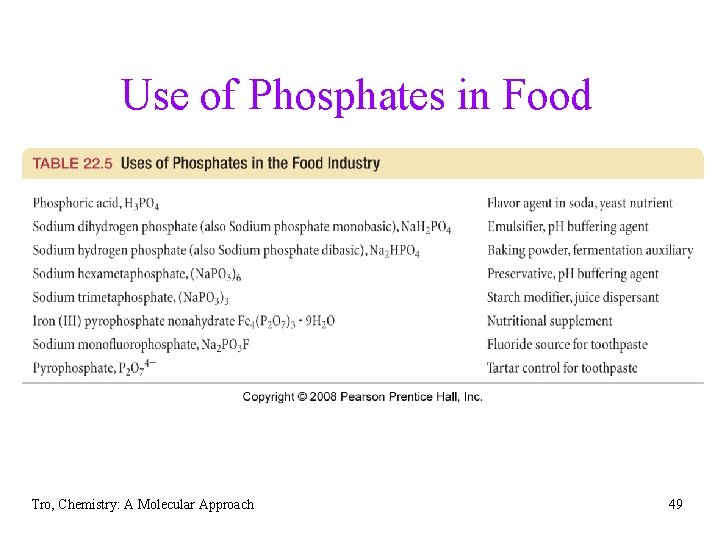
Phosphoric Acid and Phosphates • H 3 PO 4 = phosphoric acid üwhite solid that melts at 42 C üconcentrated = 85% by mass = 14. 7 M üproduced by reacting P 4 O 10 with water or the reaction of Ca 3(PO 4)2 with sulfuric acid P 4 O 10(s) + 6 H 2 O(l) 4 H 3 PO 4(aq) Ca 3(PO 4)2(s) + 3 H 2 SO 4(l) 3 Ca. SO 4(s) + 2 H 3 PO 4(qa) üused in rust removal, fertilizers, detergent additives and food preservative Øsodium pyrophosphate = Na 4 P 2 O 7 Øsodium tripolyphosphate = Na 5 P 3 O 10 Tro, Chemistry: A Molecular Approach 48

Use of Phosphates in Food Tro, Chemistry: A Molecular Approach 49

Oxygen • 2 s 22 p 4 ü 6 valence electrons • stronger oxidizing agent than other 6 A elements üused by living system to acquire energy • second highest electronegativity (3. 5) • very high abundance in crust, and highest • abundance of any element on Earth found in most common compounds Tro, Chemistry: A Molecular Approach 50

Elemental Oxygen • O 2 ü nonpolar, colorless, odorless gas ü freezing point − 183 C at which it becomes a pale blue liquid ü slightly soluble in water Ø 0. 04 g/L ü mainly produced by fractional distillation of air Ø also by the electrolysis of water ü can be synthesized by heating metal oxides, chlorates, or nitrates Hg. O(s) Hg(l) + O 2(g) 2 Na. NO 3(s) 2 Na. NO 2(s) + O 2(g) 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) ü used in high temperature combustion Ø blast furnace, oxyacetylene torch ü used to create artificial atmospheres Ø divers, high-altitude flight ü medical treatment Ø lung disease, hyperbaric O 2 to treat skin wounds Tro, Chemistry: A Molecular Approach 51

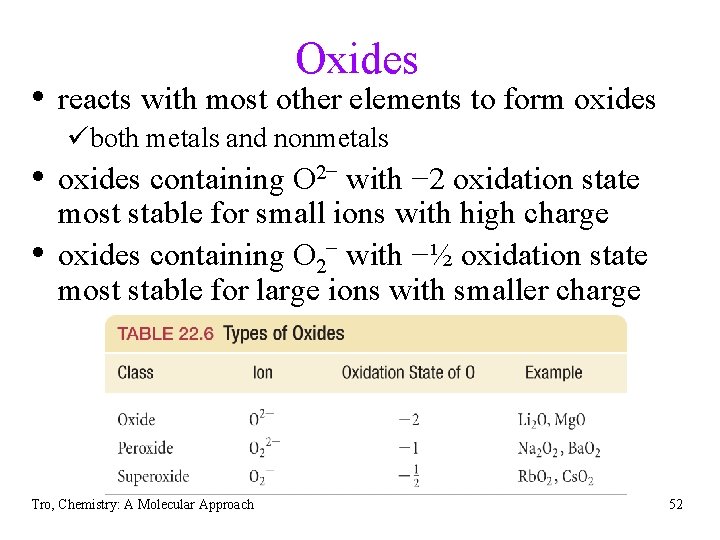
Oxides • reacts with most other elements to form oxides üboth metals and nonmetals • oxides containing O 2− with − 2 oxidation state • most stable for small ions with high charge oxides containing O 2− with −½ oxidation state most stable for large ions with smaller charge Tro, Chemistry: A Molecular Approach 52

Ozone • O 3 ü toxic, pungent, blue, diamagnetic gas ü denser than O 2 ü freezing point − 112 C, where it becomes a blue liquid ü synthesized naturally from O 2 through the activation by ultraviolet light Ø mainly in the stratosphere Ø protecting the living Earth from harmful UV rays ü spontaneously decomposes into O 2 ü commercial use as a strong oxidizing agent and disinfectant ü formed in the troposphere by interaction of UV light and auto exhaust Ø oxidation damages skin, lungs, eyes, and cracks plastics and rubbers Tro, Chemistry: A Molecular Approach 53

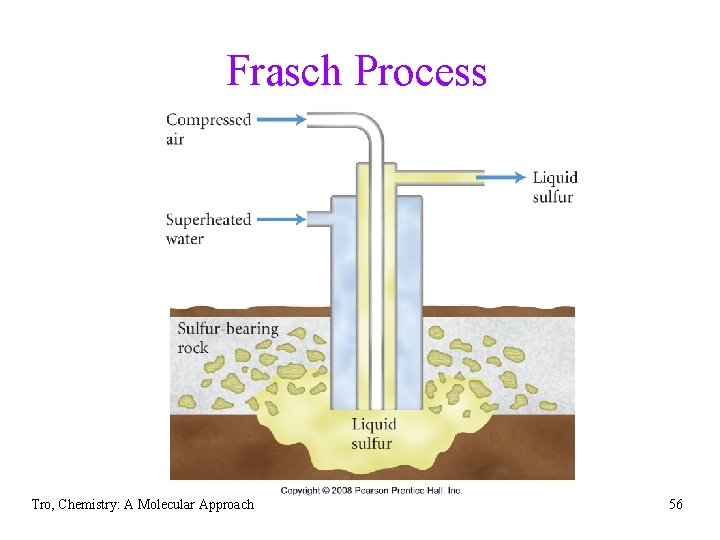
Sulfur • large atom and weaker oxidizer than oxygen • often shows +2, +4, or +6 oxidation numbers in its • • compounds, as well as − 2 composes 0. 06% of Earth’s crust elemental sulfur found in a few natural deposits ü some on the surface • below ground recovered by the Frasch Process ü superheated water pumped down into deposit, melting the sulfur and forcing it up the recovery pipe with the water • also obtained from byproducts of several industrial processes Tro, Chemistry: A Molecular Approach 54

Natural Sulfur Deposit Tro, Chemistry: A Molecular Approach 55

Frasch Process Tro, Chemistry: A Molecular Approach 56

Allotropes of Sulfur • several crystalline forms • the most common naturally occurring allotrope has S 8 rings ü most others also ring structures of various sizes • when heated to 112 C, S 8 melts to a yellow liquid with low • viscosity when heated above 150 C, rings start breaking and a dark brown viscous liquid forms ü darkest at 180 C ü above 180 C the liquid becomes less viscous • if the hot liquid is quenched in cold water, a plastic amorphous solid forms that becomes brittle and hard on cooling Tro, Chemistry: A Molecular Approach 57

sulfur at ~150 C Tro, Chemistry: A Molecular Approach sulfur at ~180 C 58

Amorphous Sulfur Tro, Chemistry: A Molecular Approach 59

Other Sources of Sulfur • H 2 S(g) from oil and natural gas deposits ü ü ü toxic gas (death > 100 ppm), smells like rotten eggs bond angle only 92. 5 nonpolar S-H bond weaker and longer than O-H bond oxidized to elemental S through the Claus Process 2 H 2 S(g) + 2 O 2(g) 2 SO 2(g) + 2 H 2 O(g) 4 H 2 S(g) + 2 SO 2(g) 6 S(s) + 4 H 2 O(g) • Fe. S 2 (iron pyrite) ü roasted in absence of air forming Fe. S(s) and S 2(g) • metal sulfides ü ü roasted in air to make SO 2(g), which is later reduced react with acids to make H 2 S most insoluble in water used as bactericide and stop dandruff in shampoo Tro, Chemistry: A Molecular Approach 60

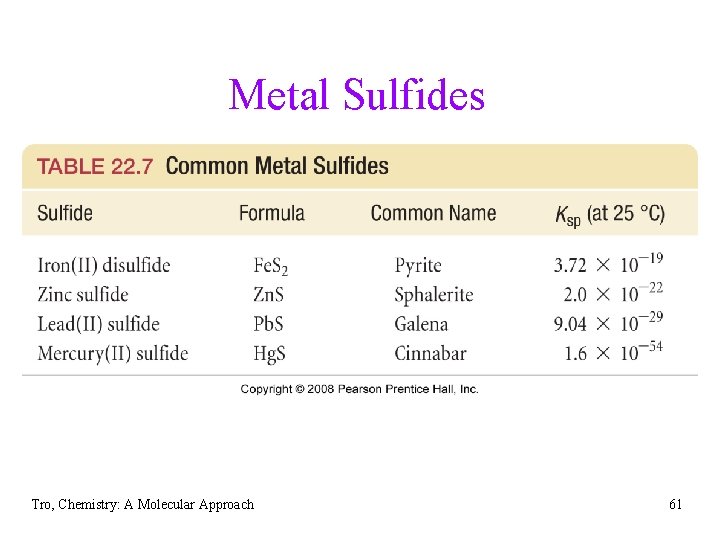
Metal Sulfides Tro, Chemistry: A Molecular Approach 61

• SO 2 Sulfur Dioxide ü colorless, dense, acrid gas that is toxic ü produced naturally by volcanic action and as a byproduct of industrial processes Ø including electrical generation by burning oil and coal, as well as metal extraction ü acidic SO 2(g) + H 2 O(l) H 2 SO 3(aq) ü forms acid rain in the air 2 SO 2(g) + 2 H 2 O(l) 2 H 2 SO 4(aq) ü removed from stack by scrubbing with limestone Ca. CO 3(s) Ca. O(s) + O 2(g) 2 Ca. O(g) + 2 SO 2(g) + O 2(g) 2 Ca. SO 4(g) ü used to treat fruits and vegetables as a preservative Tro, Chemistry: A Molecular Approach 62

Sulfuric Acid • most produced chemical in the world • strong acid, good oxidizing agent, dehydrating agent • used in production of fertilizers, dyes, petrochemicals, • paints, plastics, explosives, batteries, steel, and detergents melting point 10. 4 C, boiling point 337 C ü oily, dense liquid at room temperature • reacts vigorously and exothermically with water ü “you always oughter(sic) add acid to water” Tro, Chemistry: A Molecular Approach 63

Dehydration of Sucrose C 12 H 22 O 11(s) + H 2 SO 4(l) 12 C(s) + 11 H 2 O(g) + H 2 SO 4(aq) Tro, Chemistry: A Molecular Approach 64

Production of H 2 SO 4 • contact process • step 1: combustion of elemental S ü complete using V 2 O 5 catalyst S(g) + O 2(g) SO 2(g) 2 SO 2(g) + O 2(g) 2 SO 3(g) • step 2: absorbing the SO 2 into conc. H 2 SO 4 to form oleum, H 2 S 2 O 7 SO 3(g) + H 2 SO 4(l) H 2 S 2 O 7(l) • step 3: dissolve the oleum in water H 2 S 2 O 7(l) + H 2 O(l) 2 H 2 SO 4(aq) Tro, Chemistry: A Molecular Approach 65

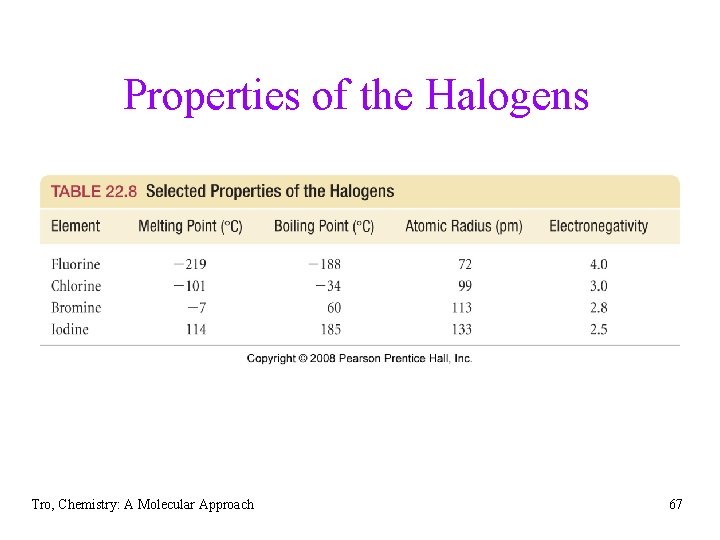
Halogens • most reactive nonmetal group, never found in elemental • form in nature come from dissolved salts in seawater ü except fluorine, which comes from minerals fluorospar (Ca. F 2) and fluoroapatite [Ca 10 F 2(PO 4)6] • atomic radius increases down the column • most electronegative element in its period, decreasing • down the column fluorine only has oxidation states of -1 or 0, others have oxidation states ranging from -1 to +7 Tro, Chemistry: A Molecular Approach 66

Properties of the Halogens Tro, Chemistry: A Molecular Approach 67

Fluorine • F 2 is a yellow-green toxic gas • F 2 is the most reactive nonmetal and forms binary compounds with every element except He, Ne, and Ar ü including Xe. F 2, Xe. F 6, Xe. OF 4, Kr. F 2 ü so reactive it reacts with other elements of low reactivity resulting in flames ü even reacts with the very unreactive asbestos and glass Ø stored in Fe, Cu, or Ni containers because the metal fluoride that forms coats the surface protecting the rest of the metal • F 2 bond weakest of the X 2 bonds, allowing reactions to be more • • exothermic small ion size of F− leads to large lattice energies in ionic compounds produced by the electrolysis of HF Tro, Chemistry: A Molecular Approach 68

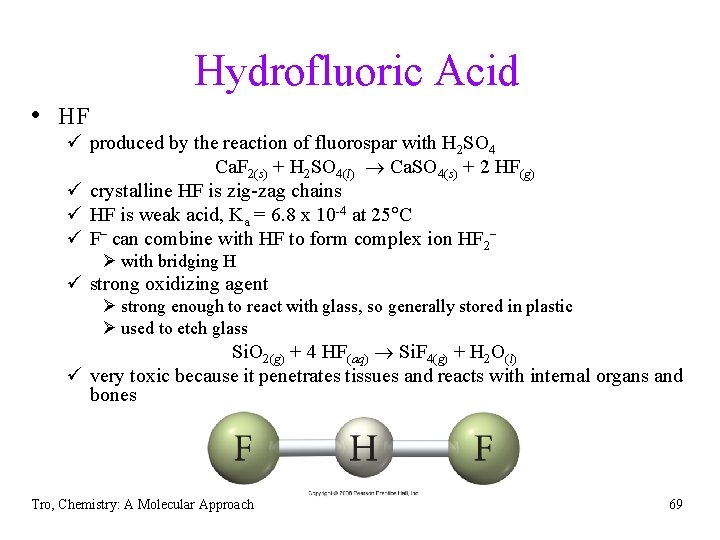
Hydrofluoric Acid • HF ü produced by the reaction of fluorospar with H 2 SO 4 Ca. F 2(s) + H 2 SO 4(l) Ca. SO 4(s) + 2 HF(g) ü crystalline HF is zig-zag chains ü HF is weak acid, Ka = 6. 8 x 10 -4 at 25 C ü F− can combine with HF to form complex ion HF 2− Ø with bridging H ü strong oxidizing agent Ø strong enough to react with glass, so generally stored in plastic Ø used to etch glass Si. O 2(g) + 4 HF(aq) Si. F 4(g) + H 2 O(l) ü very toxic because it penetrates tissues and reacts with internal organs and bones Tro, Chemistry: A Molecular Approach 69

Halogen Compounds • form ionic compounds with metals and molecular compounds • having covalent bonds with nonmetals halogens can also form compounds with other halogens – called interhalides ü for interhalides, the larger has lower electronegativity – so it is central in the molecule; with a number of more electronegative halides attached ü general formula ABn where n can be 1, 3, 5, or 7 Ø most common AB or AB 3; only AB 5 has B = F, IF 7 only known n = 7 ü only Cl. F 3 used industrially Ø to produce UF 6 in nuclear fuel enrichment • most halogen oxides are unstable ü tend to be explosive ü OF 2 only compound with O = +2 oxidation state ü Cl. O 2(g) is strong oxidizer used to bleach flour and wood pulp Ø explosive – so diluted with CO 2 and N 2 Ø produced by oxidation of Na. Cl. O 2 with Cl 2 or the reduction of Na. Cl. O 3 with HCl 2 Na. Cl. O 2 + Cl 2 2 Na. Cl + 2 Cl. O 2 2 Na. Cl. O 3 + 4 HCl 2 Cl. O 2 + 2 H 2 O + 2 Na. Cl Tro, Chemistry: A Molecular Approach 70
 Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Melting and boiling point of oxygen
Melting and boiling point of oxygen Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Is chalk natural or manmade
Is chalk natural or manmade Virtual circuit tables
Virtual circuit tables Theoretical models of counseling
Theoretical models of counseling Waterfall strategy marketing
Waterfall strategy marketing Multiple conflict
Multiple conflict Bandura's reciprocal determinism
Bandura's reciprocal determinism Process of research definition
Process of research definition Traditional approach vs object oriented approach
Traditional approach vs object oriented approach Tony wagner's seven survival skills
Tony wagner's seven survival skills Vsepr
Vsepr Examples of ab2e type molecules
Examples of ab2e type molecules Ax3e molecular shape
Ax3e molecular shape Molecular biology crash course
Molecular biology crash course Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Ab6 molecular geometry
Ab6 molecular geometry Ideal gas law examples
Ideal gas law examples Covalent molecular and covalent network
Covalent molecular and covalent network Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids What is the significance of a chemical formula
What is the significance of a chemical formula Criterios de amsterdam cancer de colon
Criterios de amsterdam cancer de colon Molecular geometry chart
Molecular geometry chart Palindromic sequence
Palindromic sequence Molecular rebar
Molecular rebar Covalent molecular substances
Covalent molecular substances Empirical formula vs molecular formula
Empirical formula vs molecular formula Number molecular weight
Number molecular weight Dicots
Dicots Empirical formula from percentages
Empirical formula from percentages Empirical formula from percent composition
Empirical formula from percent composition Empirical formula to percent composition
Empirical formula to percent composition Empirical formula to percent composition
Empirical formula to percent composition Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Explain homologous series with example
Explain homologous series with example Naming compounds and writing formulas
Naming compounds and writing formulas Binary molecular
Binary molecular N srinivasan iisc passed away
N srinivasan iisc passed away Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Molecular shape for ch2o
Molecular shape for ch2o Kahoot shapes
Kahoot shapes Co shape and polarity
Co shape and polarity Patterson function
Patterson function Molecular replacement method
Molecular replacement method Carbon dioxide orbital diagram
Carbon dioxide orbital diagram Heteronuclear diatomic molecules molecular orbital diagram
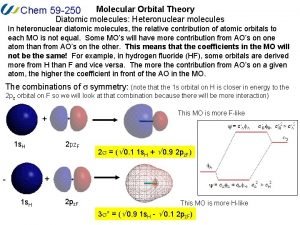
Heteronuclear diatomic molecules molecular orbital diagram B2 molecular orbital diagram
B2 molecular orbital diagram Standardised cml monitoring
Standardised cml monitoring What is the molecular shape of carbon tetrachloride
What is the molecular shape of carbon tetrachloride Molecular microbiology definition
Molecular microbiology definition Molecular geometry chart
Molecular geometry chart Non polar molecule
Non polar molecule Theory of structures
Theory of structures Scl molecular geometry
Scl molecular geometry Molecular geometry and bonding theories
Molecular geometry and bonding theories Pf3 electron pair geometry
Pf3 electron pair geometry Molecular gastronomy restaurants
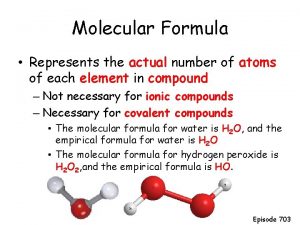
Molecular gastronomy restaurants Molecular formula
Molecular formula Molecular farming definition
Molecular farming definition Molecular ecological network analyses
Molecular ecological network analyses